Relationships of Telomere Homeostasis with Oxidative Stress and Cardiac Dysfunction in Human Ischaemic Hearts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Sample Collection
2.2. RNA Extraction and Quality Assessment
2.3. mRNA Sequencing
2.4. Gene Functional Enrichment
2.5. ncRNA Sequencing
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
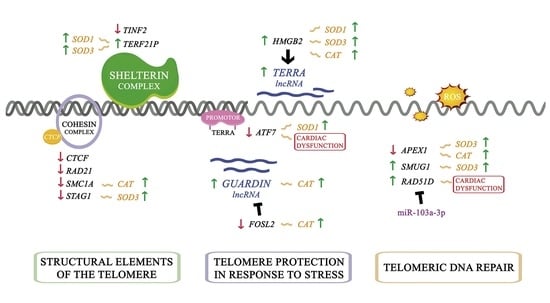
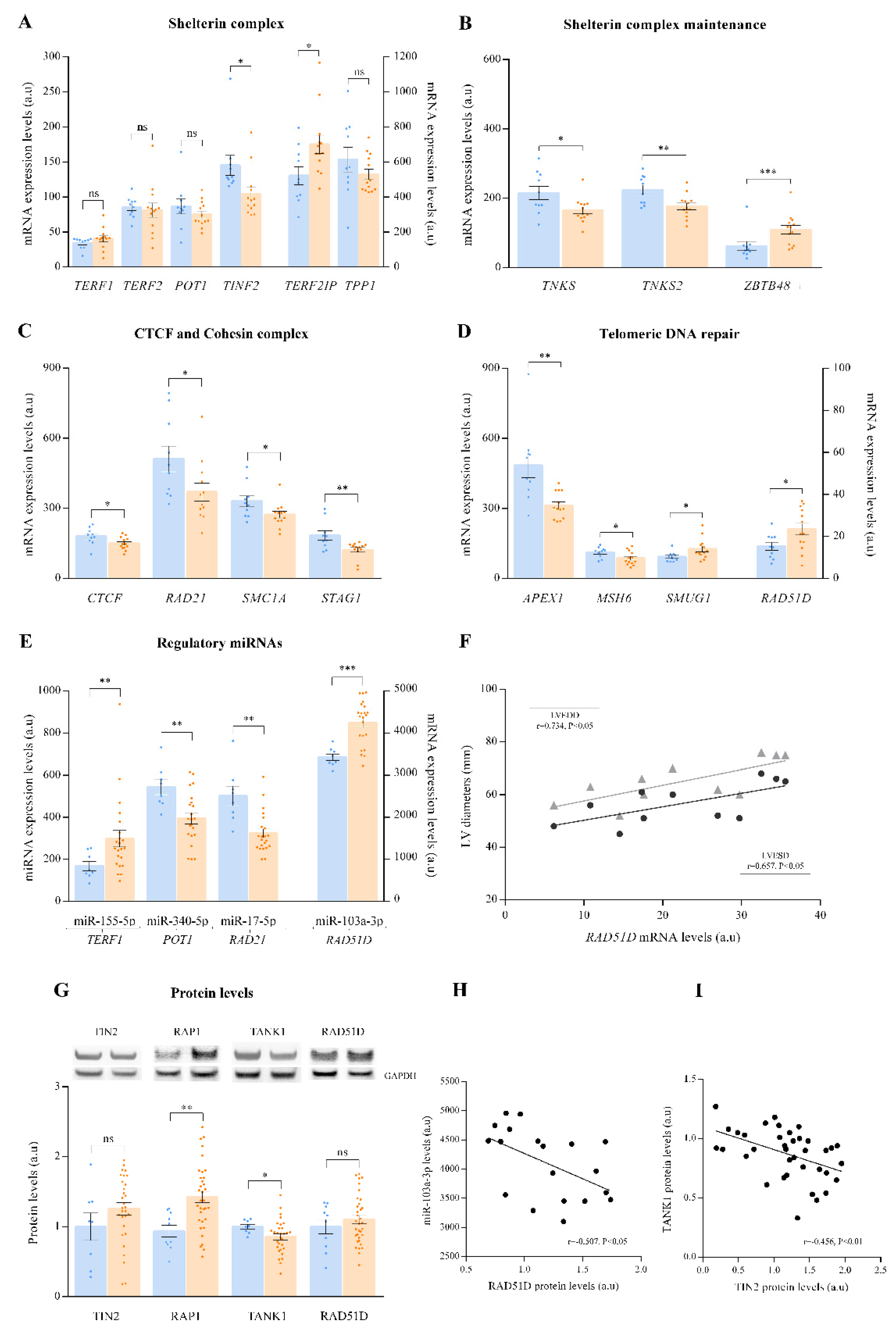
3.2. Telomere Homeostasis Alterations in ICM Patients. Relationship with Cardiac Function Parameters
3.3. TERRA and GUARDIN Regulation. Relationship with Cardiac Function Parameters
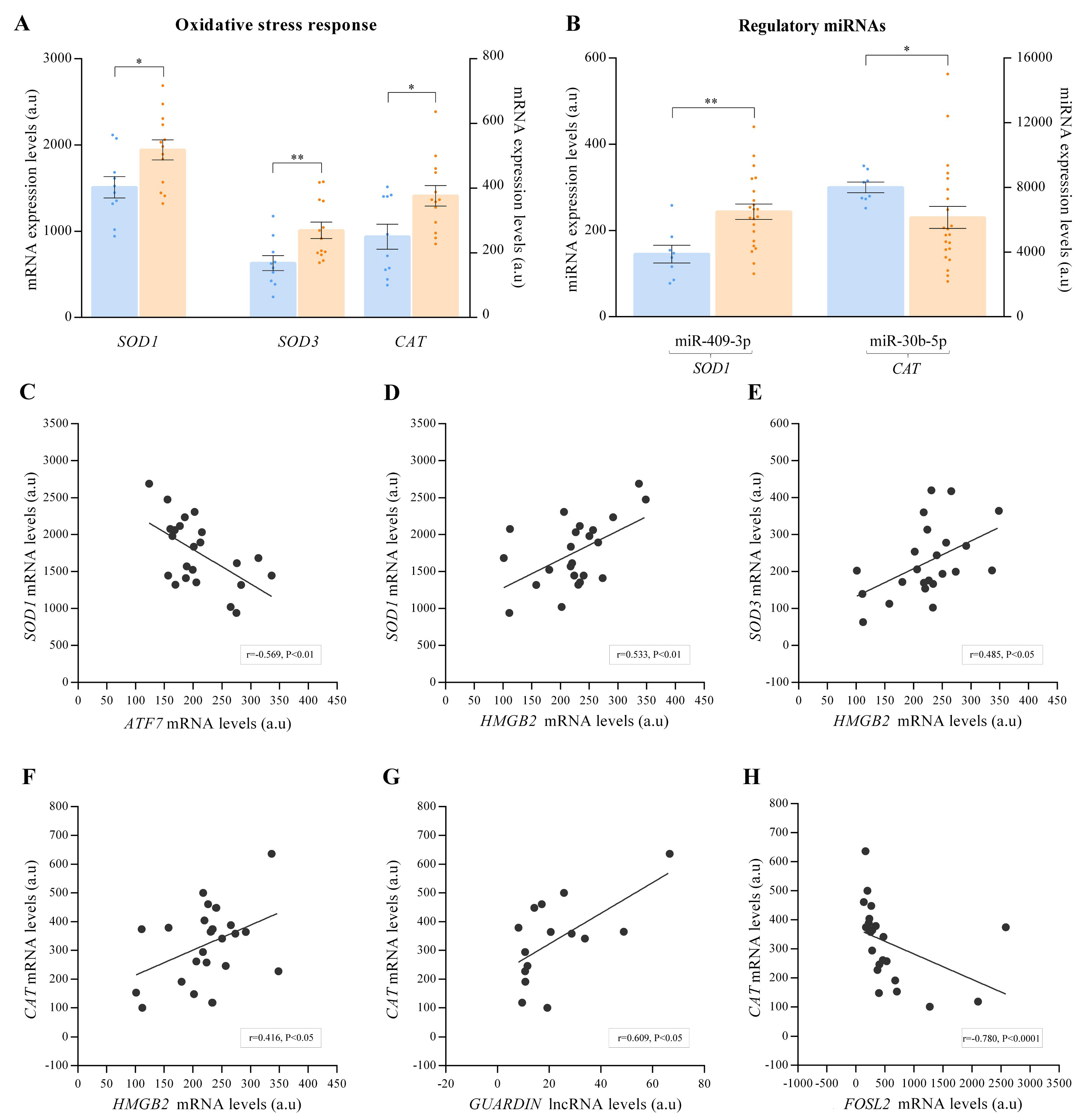
3.4. Regulation of Oxidative State in ICM and Relationship with Telomere Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Martinez, P.; Blasco, M.A. Heart-Breaking Telomeres. Circ. Res. 2018, 123, 787–802. [Google Scholar] [CrossRef]
- Wong, L.S.; Oeseburg, H.; de Boer, R.A.; van Gilst, W.H.; van Veldhuisen, D.J.; van der Harst, P. Telomere biology in cardiovascular disease: The TERC-/- mouse as a model for heart failure and ageing. Cardiovasc. Res. 2009, 81, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Diotti, R.; Loayza, D. Shelterin complex and associated factors at human telomeres. Nucleus 2011, 2, 119–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusanelli, E.; Chartrand, P. Telomeric repeat-containing RNA TERRA: A noncoding RNA connecting telomere biology to genome integrity. Front. Genet. 2015, 6, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebeid, D.E.; Khalafalla, F.G.; Broughton, K.M.; Monsanto, M.M.; Esquer, C.Y.; Sacchi, V.; Hariharan, N.; Korski, K.I.; Moshref, M.; Emathinger, J.; et al. Pim1 maintains telomere length in mouse cardiomyocytes by inhibiting TGFbeta signalling. Cardiovasc. Res. 2021, 117, 201–211. [Google Scholar] [CrossRef] [PubMed]
- O′Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Teubel, I.; Elchinova, E.; Roura, S.; Fernandez, M.A.; Galvez-Monton, C.; Moliner, P.; de Antonio, M.; Lupon, J.; Bayes-Genis, A. Telomere attrition in heart failure: A flow-FISH longitudinal analysis of circulating monocytes. J. Transl. Med. 2018, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Sanjani, M.; Oyster, N.M.; Tichy, E.D.; Bedi, K.C., Jr.; Harel, O.; Margulies, K.B.; Mourkioti, F. Cardiomyocyte-Specific Telomere Shortening is a Distinct Signature of Heart Failure in Humans. J. Am. Heart Assoc. 2017, 6, e005086. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Booth, S.A.; Charchar, F.J. Cardiac telomere length in heart development, function, and disease. Physiol. Genomics 2017, 49, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Richardson, G.D.; Passos, J.F. Mechanisms driving the ageing heart. Exp. Gerontol. 2018, 109, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Aging Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Rossi, M.; Gorospe, M. Noncoding RNAs Controlling Telomere Homeostasis in Senescence and Aging. Trends Mol. Med. 2020, 26, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Pu, W.T. Recounting Cardiac Cellular Composition. Circ. Res. 2016, 118, 368–370. [Google Scholar] [CrossRef]
- Macrae, D.J. The Council for International Organizations and Medical Sciences (CIOMS) guidelines on ethics of clinical trials. Proc. Am. Thorac. Soc. 2007, 4, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Rosello-Lleti, E.; Carnicer, R.; Tarazon, E.; Ortega, A.; Gil-Cayuela, C.; Lago, F.; Gonzalez-Juanatey, J.R.; Portoles, M.; Rivera, M. Human Ischemic Cardiomyopathy Shows Cardiac Nos1 Translocation and its Increased Levels are Related to Left Ventricular Performance. Sci. Rep. 2016, 6, 24060. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic. Acids. Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 11 December 2019).
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for occurrences of a given motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feretzaki, M.; Renck Nunes, P.; Lingner, J. Expression and differential regulation of human TERRA at several chromosome ends. RNA 2019, 25, 1470–1480. [Google Scholar] [CrossRef]
- Liu, B.; Maekawa, T.; Yoshida, K.; Ly, N.H.; Inoue, K.; Hasegawa, A.; Chatton, B.; Ogura, A.; Ishii, S. Telomere shortening by transgenerational transmission of TNF-alpha-induced TERRA via ATF7. Nucleic. Acids. Res. 2019, 47, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Bettin, N.; Oss Pegorar, C.; Cusanelli, E. The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheibe, M.; Arnoult, N.; Kappei, D.; Buchholz, F.; Decottignies, A.; Butter, F.; Mann, M. Quantitative interaction screen of telomeric repeat-containing RNA reveals novel TERRA regulators. Genome. Res. 2013, 23, 2149–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Thorne, R.F.; Zhang, X.D.; He, M.; Li, J.; Feng, S.; Liu, X.; Wu, M. LncRNA GUARDIN suppresses cellular senescence through a LRP130-PGC1alpha-FOXO4-p21-dependent signaling axis. EMBO Rep. 2020, 21, e48796. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Telomeres and disease. EMBO. J. 2009, 28, 2532–2540. [Google Scholar] [CrossRef]
- Sellami, M.; Al-Muraikhy, S.; Al-Jaber, H.; Al-Amri, H.; Al-Mansoori, L.; Mazloum, N.A.; Donati, F.; Botre, F.; Elrayess, M.A. Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants 2021, 10, 1035. [Google Scholar] [CrossRef]
- Berby, B.; Bichara, C.; Rives-Feraille, A.; Jumeau, F.; Pizio, P.D.; Setif, V.; Sibert, L.; Dumont, L.; Rondanino, C.; Rives, N. Oxidative Stress Is Associated with Telomere Interaction Impairment and Chromatin Condensation Defects in Spermatozoa of Infertile Males. Antioxidants 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome. Med. 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Mir, S.M.; Samavarchi Tehrani, S.; Goodarzi, G.; Jamalpoor, Z.; Asadi, J.; Khelghati, N.; Qujeq, D.; Maniati, M. Shelterin Complex at Telomeres: Implications in Ageing. Clin. Interv. Aging 2020, 15, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.J.; Smith, S. Tankyrase function at telomeres, spindle poles, and beyond. Biochimie 2008, 90, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Z.; de Lange, T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat. Genet. 2004, 36, 618–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, A.; Rane, G.; Paszkowski-Rogacz, M.; Sayols, S.; Bluhm, A.; Han, C.T.; Draskovic, I.; Londono-Vallejo, J.A.; Kumar, A.P.; Buchholz, F.; et al. ZBTB48 is both a vertebrate telomere-binding protein and a transcriptional activator. EMBO Rep. 2017, 18, 929–946. [Google Scholar] [CrossRef]
- Lototska, L.; Yue, J.X.; Li, J.; Giraud-Panis, M.J.; Songyang, Z.; Royle, N.J.; Liti, G.; Ye, J.; Gilson, E.; Mendez-Bermudez, A. Human RAP1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 2020, 21, e49076. [Google Scholar] [CrossRef]
- Makino, N.; Maeda, T.; Oyama, J.; Sasaki, M.; Higuchi, Y.; Mimori, K.; Shimizu, T. Antioxidant therapy attenuates myocardial telomerase activity reduction in superoxide dismutase-deficient mice. J. Mol. Cell Cardiol. 2011, 50, 670–677. [Google Scholar] [CrossRef]
- Remeseiro, S.; Cuadrado, A.; Carretero, M.; Martinez, P.; Drosopoulos, W.C.; Canamero, M.; Schildkraut, C.L.; Blasco, M.A.; Losada, A. Cohesin-SA1 deficiency drives aneuploidy and tumourigenesis in mice due to impaired replication of telomeres. EMBO J. 2012, 31, 2076–2089. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Z.; Stong, N.; Plasschaert, R.; Moczan, A.; Chen, H.S.; Hu, S.; Wikramasinghe, P.; Davuluri, R.V.; Bartolomei, M.S.; et al. A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J. 2012, 31, 4165–4178. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, I.; Minamino, T. Cellular senescence in cardiac diseases. J. Cardiol. 2019, 74, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Kroustallaki, P.; Lirussi, L.; Carracedo, S.; You, P.; Esbensen, Q.Y.; Gotz, A.; Jobert, L.; Alsoe, L.; Saetrom, P.; Gagos, S.; et al. SMUG1 Promotes Telomere Maintenance through Telomerase RNA Processing. Cell Rep. 2019, 28, 1690–1702 e1610. [Google Scholar] [CrossRef] [Green Version]
- Tarsounas, M.; Munoz, P.; Claas, A.; Smiraldo, P.G.; Pittman, D.L.; Blasco, M.A.; West, S.C. Telomere maintenance requires the RAD51D recombination/repair protein. Cell 2004, 117, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Berti, M.; Teloni, F.; Mijic, S.; Ursich, S.; Fuchs, J.; Palumbieri, M.D.; Krietsch, J.; Schmid, J.A.; Garcin, E.B.; Gon, S.; et al. Sequential role of RAD51 paralog complexes in replication fork remodeling and restart. Nat. Commun. 2020, 11, 3531. [Google Scholar] [CrossRef] [PubMed]
- Somyajit, K.; Saxena, S.; Babu, S.; Mishra, A.; Nagaraju, G. Mammalian RAD51 paralogs protect nascent DNA at stalled forks and mediate replication restart. Nucleic. Acids. Res. 2015, 43, 9835–9855. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Wang, Y.; Dhillon, K.K.; Calses, P.; Villegas, E.; Mitchell, P.S.; Tewari, M.; Kemp, C.J.; Taniguchi, T. Systematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivity. Mol. Cancer Res. 2013, 11, 1564–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Li, B.; Xu, J.; Hu, S.; Zhan, N.; Wang, H.; Gao, C.; Li, J.; Xu, X. SOD1 Promotes Cell Proliferation and Metastasis in Non-small Cell Lung Cancer via an miR-409-3p/SOD1/SETDB1 Epigenetic Regulatory Feedforward Loop. Front. Cell Dev. Biol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, W.; Liao, H.; Dai, L.; Jiang, Z.; Pan, Y.; Huang, H.; Mo, Y.; Li, S.; Yang, G.; et al. The regulatory and predictive functions of miR-17 and miR-92 families on cisplatin resistance of non-small cell lung cancer. BMC Cancer 2015, 15, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, J.; Yang, Y.; Wu, Q.; Ning, H. MicroRNA-340-5p increases telomere length by targeting telomere protein POT1 to improve Alzheimer's disease in mice. Cell Biol. Int. 2021, 45, 1306–1315. [Google Scholar] [CrossRef]
- Dinami, R.; Ercolani, C.; Petti, E.; Piazza, S.; Ciani, Y.; Sestito, R.; Sacconi, A.; Biagioni, F.; le Sage, C.; Agami, R.; et al. miR-155 drives telomere fragility in human breast cancer by targeting TRF1. Cancer Res. 2014, 74, 4145–4156. [Google Scholar] [CrossRef] [Green Version]
- Haque, R.; Chun, E.; Howell, J.C.; Sengupta, T.; Chen, D.; Kim, H. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS ONE 2012, 7, e42542. [Google Scholar] [CrossRef]
- Li, B.; Hu, J.; Chen, X. MicroRNA-30b protects myocardial cell function in patients with acute myocardial ischemia by targeting plasminogen activator inhibitor-1. Exp. Ther. Med. 2018, 15, 5125–5132. [Google Scholar] [CrossRef]
- Hu, W.L.; Jin, L.; Xu, A.; Wang, Y.F.; Thorne, R.F.; Zhang, X.D.; Wu, M. GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability. Nat. Cell Biol. 2018, 20, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Bink, D.; Pham, T.P.; Hofmann, P.; Dimmeler, S.; Boon, R.A. Long non-coding RNA TERRA influences DNA damage and survival of endothelial cells and cardiomyocytes. Eur. Heart J. 2020, 41, ehaa946.3758. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Liu, Y.; Zhang, S.; Wang, Y.; Liu, B.; Liu, H.; Li, R.; Lv, C.; Song, X. Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis. BMC Pulm. Med. 2017, 17, 163. [Google Scholar] [CrossRef] [Green Version]
- Galigniana, N.M.; Charo, N.L.; Uranga, R.; Cabanillas, A.M.; Piwien-Pilipuk, G. Oxidative stress induces transcription of telomeric repeat-containing RNA (TERRA) by engaging PKA signaling and cytoskeleton dynamics. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118643. [Google Scholar] [CrossRef] [PubMed]
- Rippe, K.; Luke, B. TERRA and the state of the telomere. Nat. Struct. Mol. Biol. 2015, 22, 853–858. [Google Scholar] [CrossRef]
- Diman, A.; Boros, J.; Poulain, F.; Rodriguez, J.; Purnelle, M.; Episkopou, H.; Bertrand, L.; Francaux, M.; Deldicque, L.; Decottignies, A. Nuclear respiratory factor 1 and endurance exercise promote human telomere transcription. Sci. Adv. 2016, 2, e1600031. [Google Scholar] [CrossRef] [Green Version]
- Kuster, D.W.; Merkus, D.; Kremer, A.; van Ijcken, W.F.; de Beer, V.J.; Verhoeven, A.J.; Duncker, D.J. Left ventricular remodeling in swine after myocardial infarction: A transcriptional genomics approach. Basic. Res. Cardiol. 2011, 106, 1269–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitelli, V.; Falvo, P.; Khoriauli, L.; Smirnova, A.; Gamba, R.; Santagostino, M.; Nergadze, S.G.; Giulotto, E. More on the Lack of Correlation between Terra Expression and Telomere Length. Front. Oncol. 2013, 3, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| mRNA-seq | ncRNA-seq | Western Blot | |
|---|---|---|---|
| ICM (n = 13) | ICM (n = 18) | ICM (n = 34) | |
| Age (years) | 54 ± 8 | 55 ± 8 | 54 ± 7 |
| Gender male (%) | 100 | 100 | 97 |
| NYHA class | III-IV | III-IV | III-IV |
| BMI (kg/m2) | 27 ± 4 | 26 ± 3 | 27 ± 4 |
| Haemoglobin (mg/dL) | 14 ± 3 | 14 ± 2 | 13 ± 2 |
| Haematocrit (%) | 41 ± 6 | 41 ± 4 | 40 ± 6 |
| Total cholesterol (mg/dL) | 162 ± 41 | 175 ± 46 | 178 ± 45 |
| Prior hypertension (%) | 33 | 35 | 52 |
| Prior smoking (%) | 92 | 78 | 87 |
| Diabetes mellitus (%) | 42 | 47 | 52 |
| LVEF (%) | 24 ± 4 | 23 ± 6 | 23 ± 7 |
| LVESD (mm) | 56 ± 8 | 53 ± 8 | 55 ± 8 |
| LVEDD (mm) | 64 ± 8 | 62 ± 9 | 63 ± 8 |
| Oxidative Stress Genes | Telomere Homeostasis Genes | Main Function | r | p Value |
|---|---|---|---|---|
| CAT | APEX1 | Telomeric DNA repair | −0.555 | <0.01 |
| SMC1A | Cohesin complex | −0.678 | <0.0001 | |
| SOD1 | TERF2IP | Shelterin complex | 0.548 | <0.01 |
| TNKS | Shelterin complex maintenance | −0.419 | <0.05 | |
| SOD3 | TERF2IP | Shelterin complex | 0.520 | <0.05 |
| TNKS | Shelterin complex maintenance | −0.466 | <0.05 | |
| ZBTB48 | Shelterin complex maintenance | 0.673 | <0.01 | |
| APEX1 | Telomeric DNA repair | −0.540 | <0.01 | |
| SMUG1 | Telomeric DNA repair | 0.596 | <0.01 | |
| STAG1 | Cohesin complex | −0.548 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarazón, E.; Pérez-Carrillo, L.; Giménez-Escamilla, I.; Ramos-Castellanos, P.; Martínez-Dolz, L.; Portolés, M.; Roselló-Lletí, E. Relationships of Telomere Homeostasis with Oxidative Stress and Cardiac Dysfunction in Human Ischaemic Hearts. Antioxidants 2021, 10, 1750. https://doi.org/10.3390/antiox10111750
Tarazón E, Pérez-Carrillo L, Giménez-Escamilla I, Ramos-Castellanos P, Martínez-Dolz L, Portolés M, Roselló-Lletí E. Relationships of Telomere Homeostasis with Oxidative Stress and Cardiac Dysfunction in Human Ischaemic Hearts. Antioxidants. 2021; 10(11):1750. https://doi.org/10.3390/antiox10111750
Chicago/Turabian StyleTarazón, Estefanía, Lorena Pérez-Carrillo, Isaac Giménez-Escamilla, Pablo Ramos-Castellanos, Luis Martínez-Dolz, Manuel Portolés, and Esther Roselló-Lletí. 2021. "Relationships of Telomere Homeostasis with Oxidative Stress and Cardiac Dysfunction in Human Ischaemic Hearts" Antioxidants 10, no. 11: 1750. https://doi.org/10.3390/antiox10111750