An Overview of Physical Exercise and Antioxidant Supplementation Influences on Skeletal Muscle Oxidative Stress
Abstract
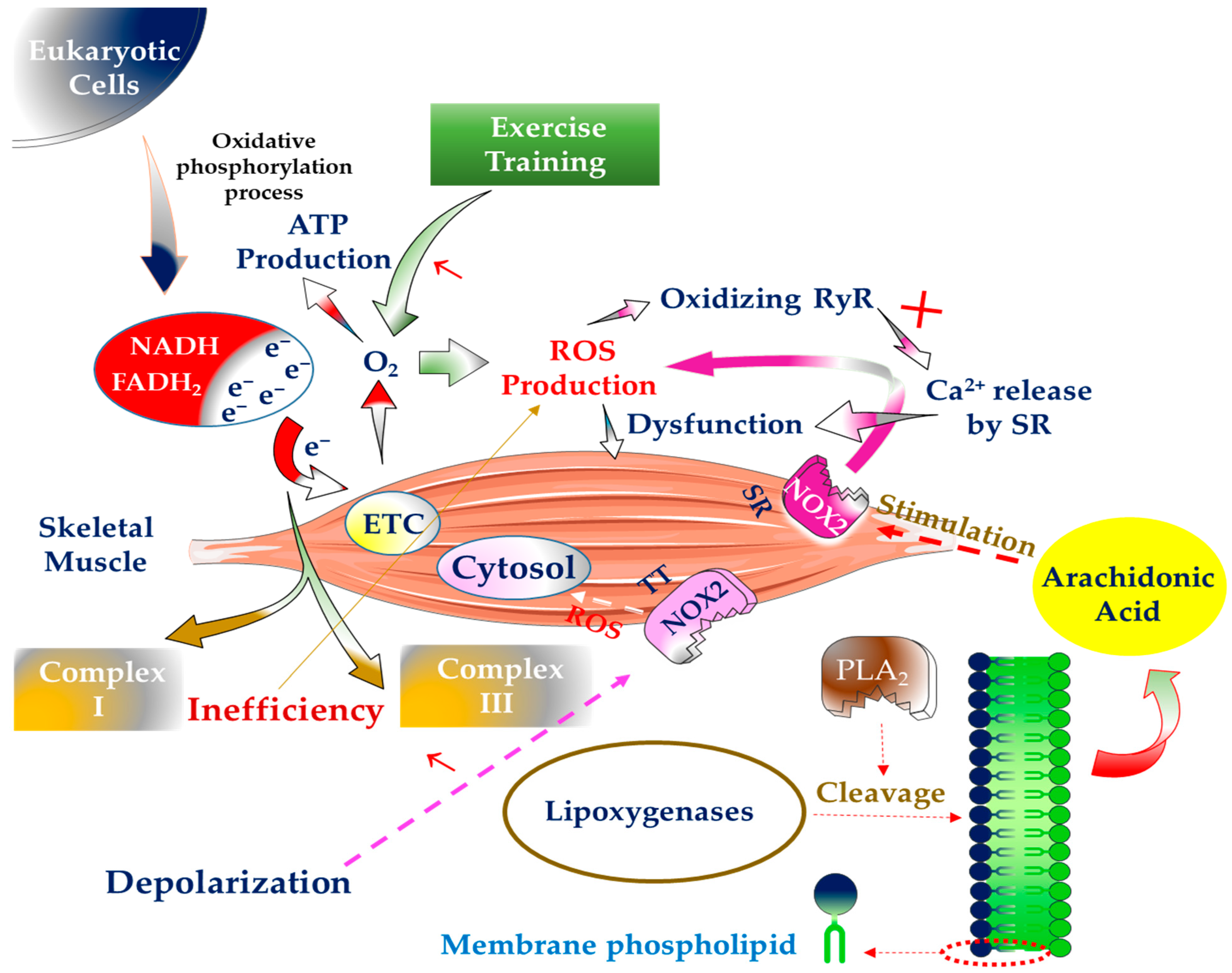
:1. Introduction
2. The Generation of Free Radicals inside SM and Way to Deal with Them
3. The Significance of Physical Exercise in the Generation of Free Radicals in SM
4. The Influence of Antioxidant Supplementation on SM
4.1. In Terms of Adaptations to Training
4.2. In Terms of Inflammation
4.3. In Terms of Muscle Damage
5. Disorders Related to Loss of Strength and SM Mass, Antioxidant Supplements and Physical Exercise
5.1. DMD, Antioxidant Supplementation, and Exercise
5.2. Sarcopenia, Antioxidant Supplementation, and Exercise
| Reference | Subjects | Antioxidant Supplementation | Exercise Training | Results |
|---|---|---|---|---|
| Paulsen et al. [55] | Strength-trained recreational men and women | Vitamins E and C (235 mg per day and 1000 mg per day, respectively) for ten weeks | Heavy-duty resistance training four times a week | No increase in muscle mass |
| Makanae et al. [56] | Wistar rats | Vitamin C (500 mg kg−1) for 14 days | Mechanical overload | Decrease of hypertrophy of overworked muscles |
| Theodorou et al. [57] | Exercised-recreational men | Vitamins E and C (400 IU and 1000 mg per day) for 11 weeks | Eccentric exercise for four weeks and twice a week | No impact on muscular function or recovery following exercise |
| Dutra et al. [58] | Healthy and non-smoking women | Vitamins E and C (400 IU and 1000 mg daily, respectively) | Ten weeks of twice-weekly strength training (ST) | Decrease of peak torque and total work due to taking antioxidant supplements |
| Cooke et al. [60] | Trained and untrained men and women | 200 mg of COQ10 every day for 14 days | WT GXT and Isokinetic tests | Higher CoQ10 levels did not affect the individuals physical endurance |
| Taub et al. [61] | Healthy and sedentary adults | 20 g of dark chocolate for three months | Stationary bike ride | Increase of VO2max |
| Bowtell et al. [65] | Trained men | 30 mL (twice daily) of Montmorency cherry juice for one week before and 48 h after exercise | Ten sets of 10 repetitions with a single knee extension at 80% of their maximal repetition | Improvement of muscular isometric strength following intensive exercise |
| McLeay et al. [66] | Healthy women | Blueberry smoothie 5 and 10 h before EIMD and immediately, 12 and 36 h afterwards | Exercise-induced muscle damage (EIMD) | Increase of isometric muscular strength recovery |
| Furlong et al. [67] | Untrained people | Proprietary herbal/botanical combination (1575 mg 2 times a day), and Aphanizomenon flos-aquae extract (1000 mg 3 times daily) | Resistance training regimen (3 times a week, two sets of 10 repetitions per movement, for 12 weeks) | No variations in the patients balance, strength, or muscular function |
| Carrera-Quintanar et al. [73] | University-level athletes | Vitamins C and E and Lippia citriodora antioxidant extract | 2000-m running test. | Protect neutrophils from oxidative injury to skeletal muscle |
| Rokitzki et al. [82] | Running athletes | 200 mg of vitamin C and 600 mg of vitamin E daily for five weeks | ------------------- | Decrease of muscular damage |
| Zoppi et al. [83] | Soccer players | 1000 mg of vitamin C and 800 mg of vitamin E for twelve weeks | ------------------- |
|
| Dawson et al. [84] | Well-trained runners | 500 to 1000 mg of vitamin C and 750 to 1500 mg of vitamin E per day | ------------------- |
|
| Nieman et al. [85] | Well-trained runners | Quercetin antioxidant supplement (500 mg/day) for three weeks | ------------------- |
|
| Kon et al. [86] | Kendo athletes | CoQ10 (300 mg/day) for 20 days | ------------------- |
|
| Orlando et al. [87] | Rugby participants | CoQ10 (200 mg/day) for 1 month | ------------------- |
|
| O’Fallen et al. [88] | Young men and women | Quercetin (100 mg daily) | ------------------- |
|
| Liang et al. [103] | Male Sprague-Dawley rats | BSP supplementation | Treadmill and grip strength tests | Improvement of grip strength, muscular mass, and muscular endurance |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Imbesi, R. Oxidative Stress and Skeletal Muscle in Exercise. Ital. J. Anat. Embryol. 2012, 117, 107–116. [Google Scholar]
- Datta, K.; Sinha, S.; Chattopadhyay, P. Reactive oxygen species in health and disease. Natl. Med. J. India 2000, 13, 304–310. [Google Scholar] [PubMed]
- Arazi, H.; Taati, B.; Suzuki, K. HMB Supplementation and Resistance Training: Current Overview on Inflammation, Oxidative Stress and Cardiovascular Risk Factors. Recent Res. Adv. Biol. 2021, 5, 155–168. [Google Scholar]
- Bassel-Duby, R.; Olson, E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, L.; Chen, Y.; Ma, Q.; Santhanam, R.K.; Xue, Z.; Gao, X.; Chen, H. Structural characterization of corn silk polysaccharides and its effect in H2O2 induced oxidative damage in L6 skeletal muscle cells. Carbohydr. Polym. 2019, 208, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Valaei, K.; Mehrabani, J.; Wong, A. Effects of L-citrulline supplementation on nitric oxide and antioxidant markers after high-intensity interval exercise in young men: A randomized controlled trial. Br. J. Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Mitochondrial Med. 2012, 942, 3–37. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, D.V.; Doust, J.H. Oxygen uptake during high-intensity running: Response following a single bout of interval training. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport: Oxidative Phosphorylation, Mitochondrial Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Lamb, G.D.; Westerblad, H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011, 589, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Boss, O.; Muzzin, P.; Giacobino, J.-P. The uncoupling proteins, a review. Eur. J. Endocrinol. 1998, 139, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014, 73, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H.; Ow, J.R.; Yang, N.D.; Taneja, R. Oxidative Stress-Mediated Skeletal Muscle Degeneration: Molecules, Mechanisms, and Therapies. Oxid. Med. Cell. Longev. 2016, 2016, 6842568. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Damle, S.; Marín-García, J. Mitochondrial uncoupler proteins. Curr. Enzym. Inhib. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical Exercise: A Novel Tool to Protect Mitochondrial Health. Front. Physiol. 2021, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taherkhani, S.; Suzuki, K.; Ruhee, R.T. A Brief Overview of Oxidative Stress in Adipose Tissue with a Therapeutic Approach to Taking Antioxidant Supplements. Antioxidants 2021, 10, 594. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Xia, R.; Webb, J.A.; Gnall, L.L.; Cutler, K.; Abramson, J.J. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am. J. Physiol. Cell Physiol. 2003, 285, C215–C221. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; García, A.; Härtel, S.; Hidalgo, C.; Jaimovich, E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J. Biol. Chem. 2009, 284, 2568–2575. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Zuo, L.; Christofi, F.L.; Wright, V.P.; Bao, S.; Clanton, T.L. Lipoxygenase-dependent superoxide release in skeletal muscle. J. Appl. Physiol. 2004, 97, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.C.; Arbogast, S.; Guo, Z.; Mathenia, J.; Su, W.; Reid, M.B. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J. Appl. Physiol. 2006, 100, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-jB. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef] [Green Version]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Balon, T.W.; Nadler, J.L. Nitric oxide release is present from incubated skeletal muscle preparations. J. Appl. Physiol. 1994, 77, 2519–2521. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Schröder, P.; Biesalski, H.K. Low molecular weight antioxidants. In Reactions, Processes; Springer: Berlin/Heidelberg, Germany, 2005; pp. 77–90. [Google Scholar]
- Lamichhane, A.; Chaudhary, V.; Sah, N.; Singh, M.; Pandey, R. Low Molecular Weight Antioxidants (lmwa) and their Orchestration. Nepal J. Med. Sci. 2013, 2, 171–180. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Kondo, H.; Miura, M.; Itokawa, Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol. Scand. 1991, 142, 527–528. [Google Scholar] [CrossRef]
- Ismaeel, A.; Holmes, M.; Papoutsi, E.; Panton, L.; Koutakis, P. Resistance Training, Antioxidant Status, and Antioxidant Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [Green Version]
- Léger, B.; Senese, R.; Al-Khodairy, A.W.; Dériaz, O.; Gobelet, C.; Giacobino, J.P.; Russell, A.P. Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 2009, 40, 69–78. [Google Scholar] [CrossRef]
- Senf, S.M.; Dodd, S.L.; Judge, A.R. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell Physiol. 2010, 298, C38–C45. [Google Scholar] [CrossRef] [Green Version]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports 2006, 16, 3–63. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Carretero, A.; Millan-Domingo, F.; Garcia-Dominguez, E.; Correas, A.G.; Olaso-Gonzalez, G.; Viña, J. Redox-related biomarkers in physical exercise. Redox Biol. 2021, 42, 101956. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N. Well-Known Antioxidants and Newcomers in Sport Nutrition: Coenzyme Q10, Quercetin, Resveratrol, Pterostilbene, Pycnogenol and Astaxanthin; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox homeostasis in sport: Do athletes really need antioxidant support? Res. Sports Med. 2019, 27, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Lamboley, C.R.; Rouffet, D.M.; Dutka, T.L.; McKenna, M.J.; Lamb, G.D. Effects of high-intensity intermittent exercise on the contractile properties of human type I and type II skeletal muscle fibers. J. Appl. Physiol. 2020, 128, 1207–1216. [Google Scholar] [CrossRef]
- Cumming, K.T.; Raastad, T.; Sørstrøm, A.; Paronetto, M.P.; Mercatelli, N.; Ugelstad, I.; Caporossi, D.; Paulsen, G. Vitamin C and E supplementation does not affect heat shock proteins or endogenous antioxidants in trained skeletal muscles during 12 weeks of strength training. BMC Nutr. 2017, 3, 70. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, G.; Hamarsland, H.; Cumming, K.; Johansen, R.; Hulmi, J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef]
- Makanae, Y.; Kawada, S.; Sasaki, K.; Nakazato, K.; Ishii, N. Vitamin C administration attenuates overload-induced skeletal muscle hypertrophy in rats. Acta Physiol. 2013, 208, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Koutsias, S.; Panayiotou, G.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am. J. Clin. Nutr. 2011, 93, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, M.T.; Alex, S.; Mota, M.R.; Sales, N.B.; Brown, L.E.; Bottaro, M. Effect of strength training combined with antioxidant supplementation on muscular performance. Appl. Physiol. Nutr. Metab. 2018, 43, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.M.; Marlin, D.J. Exercise-associated oxidative stress. Clin. Tech. Equine Pract. 2003, 2, 278–291. [Google Scholar] [CrossRef]
- Cooke, M.; Iosia, M.; Buford, T.; Shelmadine, B.; Hudson, G.; Kerksick, C.; Rasmussen, C.; Greenwood, M.; Leutholtz, B.; Willoughby, D. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J. Int. Soc. Sports Nutr. 2008, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Taub, P.R.; Ramirez-Sanchez, I.; Patel, M.; Higginbotham, E.; Moreno-Ulloa, A.; Román-Pintos, L.M.; Phillips, P.; Perkins, G.; Ceballos, G.; Villarreal, F. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: Underlying mechanisms. A double blind, randomized, placebo controlled trial. Food Funct. 2016, 7, 3686–3693. [Google Scholar] [CrossRef] [Green Version]
- Dimauro, I.; Mercatelli, N.; Caporossi, D. Exercise-induced ROS in heat shock proteins response. Free Radic. Biol. Med. 2016, 98, 46–55. [Google Scholar] [CrossRef]
- Taherkhani, S.; Suzuki, K.; Castell, L. A Short Overview of Changes in Inflammatory Cytokines and Oxidative Stress in Response to Physical Activity and Antioxidant Supplementation. Antioxidants 2020, 9, 886. [Google Scholar] [CrossRef]
- Gjøvaag, T.F.; Dahl, H.A. Effect of training and detraining on the expression of heat shock proteins in m. triceps brachii of untrained males and females. Eur. J. Appl. Physiol. 2006, 98, 310–322. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2012, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Furlong, J.; Rynders, C.A.; Sutherlin, M.; Patrie, J.; Katch, F.I.; Hertel, J.; Weltman, A. Effect of an herbal/botanical supplement on strength, balance, and muscle function following 12-weeks of resistance training: A placebo controlled study. J. Int. Soc. Sports Nutr. 2014, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef] [Green Version]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Yasui, K.; Kobayashi, N.; Yamazaki, T.; Agematsu, K.; Matsuzaki, S.; Ito, S.; Nakata, S.; Baba, A.; Koike, K. Superoxide dismutase (SOD) as a potential inhibitory mediator of inflammation via neutrophil apoptosis. Free Radic. Res. 2005, 39, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Involvement of neutrophils in exercise-induced muscle damage. Gen. Intern. Med. Clin. Innov. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in sports and exercise: Tracking health, performance, and recovery in athletes. J. Strength Cond. Res. 2017, 31, 2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera-Quintanar, L.; Funes, L.; Herranz-López, M.; Martínez-Peinado, P.; Pascual-García, S.; Sempere, J.M.; Boix-Castejón, M.; Córdova, A.; Pons, A.; Micol, V. Antioxidant Supplementation Modulates Neutrophil Inflammatory Response to Exercise-Induced Stress. Antioxidants 2020, 9, 1242. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem. Biophys. Rep. 2016, 5, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McArdle, F.; Spiers, S.; Aldemir, H.; Vasilaki, A.; Beaver, A.; Iwanejko, L.; McArdle, A.; Jackson, M. Preconditioning of skeletal muscle against contraction-induced damage: The role of adaptations to oxidants in mice. J. Physiol. 2004, 561, 233–244. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Miralda, I.; Armstrong, C.L.; Uriarte, S.M.; Bagaitkar, J. The roles of NADPH oxidase in modulating neutrophil effector responses. Mol. Oral Microbiol. 2019, 34, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. Mechanisms of exercise-induced delayed onset muscular soreness: A brief review. Med. Sci. Sports Exerc. 1984, 16, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.F.; Martinez-Ferran, M.; Vallecillo, N.; Jiménez, S.L.; Romero-Morales, C.; Pareja-Galeano, H. Supplementation with Vitamins C and E and Exercise-Induced Delayed-Onset Muscle Soreness: A Systematic Review. Antioxidants 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Barranco-Ruiz, Y.; Aragón-Vela, J.; Casals, C.; Martínez-Amat, A.; Casuso, R.A.; Huertas, J.R. Control of antioxidant supplementation through interview is not appropriate in oxidative-stress sport studies: Analytical confirmation should be required. Nutrition 2017, 33, 278–284. [Google Scholar] [CrossRef]
- Stepanyan, V.; Crowe, M.; Haleagrahara, N.; Bowden, B. Effects of vitamin E supplementation on exercise-induced oxidative stress: A meta-analysis. Appl. Physiol. Nutr. Metab. 2014, 39, 1029–1037. [Google Scholar] [CrossRef]
- Rokitzki, L.; Logemann, E.; Sagredos, A.; Murphy, M.; Wetzel-Roth, W.; Keul, J. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiol. Scand. 1994, 151, 149–158. [Google Scholar] [CrossRef]
- Zoppi, C.C.; Hohl, R.; Silva, F.C.; Lazarim, F.L.; Neto, J.A.; Stancanneli, M.; Macedo, D.V. Vitamin C and e supplementation effects in professional soccer players under regular training. J. Int. Soc. Sports Nutr. 2006, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Dawson, B.; Henry, G.; Goodman, C.; Gillam, I.; Beilby, J.; Ching, S.; Fabian, V.; Dasig, D.; Morling, P.; Kakulus, B. Effect of Vitamin C and E supplementation on biochemical and ultrastructural indices of muscle damage after a 21 km run. Int. J. Sports Med. 2002, 23, 10–15. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Dumke, C.L.; Gross, S.J.; Jenkins, D.P.; Murphy, E.A.; Carmichael, M.D.; Quindry, J.C.; McAnulty, S.R. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States Endurance Run. J. Interferon Cytokine Res. 2007, 27, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Kon, M.; Tanabe, K.; Akimoto, T.; Kimura, F.; Tanimura, Y.; Shimizu, K.; Okamoto, T.; Kono, I. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q 10. Br. J. Nutr. 2008, 100, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Orlando, P.; Silvestri, S.; Galeazzi, R.; Antonicelli, R.; Marcheggiani, F.; Cirilli, I.; Bacchetti, T.; Tiano, L. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep. 2018, 23, 136–145. [Google Scholar] [CrossRef]
- O’Fallon, K.S.; Kaushik, D.; Michniak-Kohn, B.; Dunne, C.P.; Zambraski, E.J.; Clarkson, P.M. Effects of quercetin supplementation on markers of muscle damage and inflammation after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Verhaart, I.E.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Frinchi, M.; Morici, G.; Mudó, G.; Bonsignore, M.R.; Di Liberto, V. Beneficial Role of Exercise in the Modulation of mdx Muscle Plastic Remodeling and Oxidative Stress. Antioxidants 2021, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, C.; Farini, A.; Colleoni, F.; Fortunato, F.; Razini, P.; Erratico, S.; Tavelli, A.; Fabrizi, F.; Belicchi, M.; Meregalli, M. Improvement of endurance of DMD animal model using natural polyphenols. BioMed Res. Int. 2015, 2015, 680615. [Google Scholar] [CrossRef] [PubMed]
- Rando, T.A. Oxidative stress and the pathogenesis of muscular dystrophies. Am. J. Phys. Med. Rehabil. 2002, 81, S175–S186. [Google Scholar] [CrossRef]
- Boccanegra, B.; Verhaart, I.E.; Cappellari, O.; Vroom, E.; De Luca, A. Safety issues and harmful pharmacological interactions of nutritional supplements in Duchenne muscular dystrophy: Considerations for Standard of Care and emerging virus outbreaks. Pharmacol. Res. 2020, 158, 104917. [Google Scholar] [CrossRef]
- Chahbouni, M.; Escames, G.; Venegas, C.; Sevilla, B.; García, J.A.; López, L.C.; Muñoz-Hoyos, A.; Molina-Carballo, A.; Acuña-Castroviejo, D. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J. Pineal Res. 2010, 48, 282–289. [Google Scholar] [CrossRef]
- Terrill, J.R.; Radley-Crabb, H.G.; Grounds, M.D.; Arthur, P.G. N-Acetylcysteine treatment of dystrophic mdx mice results in protein thiol modifications and inhibition of exercise induced myofibre necrosis. Neuromuscul. Disord. 2012, 22, 427–434. [Google Scholar] [CrossRef]
- Salehi, F.; Zeinaloo, A.; Riasi, H.R.; Shamloo, A.S. Effectiveness of Coenzyme Q10 on echocardiographic parameters of patients with Duchenne muscular dystrophy. Electron. Physician 2017, 9, 3896. [Google Scholar] [CrossRef] [Green Version]
- De Lateur, B.J.; Giaconi, R.M. Effect on maximal strength of submaximal exercise in Duchenne muscular dystrophy. Am. J. Phys. Med. 1979, 58, 26–36. [Google Scholar]
- Cerullo, F.; Gambassi, G.; Cesari, M. Rationale for antioxidant supplementation in sarcopenia. J. Aging Res. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.; Harvey, N.C.; Kanis, J.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.; Bruyère, O. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, S.H.; Kwak, H.-B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-J.; Hong, J.-Y.; Yang, I.; Zhou, X.-R.; Lin, Y.-W.; Lin, T.-C.; Hou, C.-H.; Lin, F.-H. To synthesize hydroxyapatite by modified low temperature method loaded with bletilla striata polysaccharide as antioxidant for the prevention of sarcopenia by intramuscular administration. Antioxidants 2021, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217. [Google Scholar] [PubMed] [Green Version]
- Pansarasa, O.; Bertorelli, L.; Vecchiet, J.; Felzani, G.; Marzatico, F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic. Biol. Med. 1999, 27, 617–622. [Google Scholar] [CrossRef]
- Cesare, M.M.; Felice, F.; Santini, V.; Di Stefano, R. Antioxidants in Sport Sarcopenia. Nutrients 2020, 12, 2869. [Google Scholar] [CrossRef]
- Jakemanl, P.; Maxwell, S. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 67, 426–430. [Google Scholar] [CrossRef]
- Shafat, A.; Butler, P.; Jensen, R.; Donnelly, A. Effects of dietary supplementation with vitamins C and E on muscle function during and after eccentric contractions in humans. Eur. J. Appl. Physiol. 2004, 93, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Marzani, B.; Balage, M.; Vénien, A.; Astruc, T.; Papet, I.; Dardevet, D.; Mosoni, L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J. Nutr. 2008, 138, 2205–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011, 43, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Williams, C.; Betts, J.A.; Thompson, D.; Hurst, T.L. Oxidative stress, inflammation and recovery of muscle function after damaging exercise: Effect of 6-week mixed antioxidant supplementation. Eur. J. Appl. Physiol. 2011, 111, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Bori, Z.; Zhao, Z.; Koltai, E.; Fatouros, I.G.; Jamurtas, A.Z.; Douroudos, I.I.; Terzis, G.; Chatzinikolaou, A.; Sovatzidis, A.; Draganidis, D. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp. Gerontol. 2012, 47, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leduc-Gaudet, J.-P.; Hussain, S.N.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Lee, M.; Jun, W.; Lee, M. Effects of a 12-week circuit exercise program on fall-related fitness in elderly women with sarcopenia. Korean J. Sports Sci. 2017, 26, 1123–1135. [Google Scholar] [CrossRef]
- Heo, J.-W.; No, M.-H.; Min, D.-H.; Kang, J.-H.; Kwak, H.-B. Aging-induced Sarcopenia and Exercise. Off. J. Korean Acad. Kinesiol. 2017, 19, 43–59. [Google Scholar]
- Takeshima, N.; Rogers, M.E.; Islam, M.M.; Yamauchi, T.; Watanabe, E.; Okada, A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur. J. Appl. Physiol. 2004, 93, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Konopka, A.R.; Undem, M.K.; Hinkley, J.M.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 2012, 113, 1495–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Hockemeyer, J.; Sedlock, D. Does combined antioxidant vitamin supplementation blunt repeated bout effect? Int. J. Sports Med. 2015, 36, 407–413. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taherkhani, S.; Valaei, K.; Arazi, H.; Suzuki, K. An Overview of Physical Exercise and Antioxidant Supplementation Influences on Skeletal Muscle Oxidative Stress. Antioxidants 2021, 10, 1528. https://doi.org/10.3390/antiox10101528
Taherkhani S, Valaei K, Arazi H, Suzuki K. An Overview of Physical Exercise and Antioxidant Supplementation Influences on Skeletal Muscle Oxidative Stress. Antioxidants. 2021; 10(10):1528. https://doi.org/10.3390/antiox10101528
Chicago/Turabian StyleTaherkhani, Shima, Kosar Valaei, Hamid Arazi, and Katsuhiko Suzuki. 2021. "An Overview of Physical Exercise and Antioxidant Supplementation Influences on Skeletal Muscle Oxidative Stress" Antioxidants 10, no. 10: 1528. https://doi.org/10.3390/antiox10101528








