Leaf Development Monitoring and Early Detection of Water Deficiency by Low Field Nuclear Magnetic Resonance Relaxation in Nicotiana tabacum Plants
Abstract
:1. Introduction
2. Materials and Methods
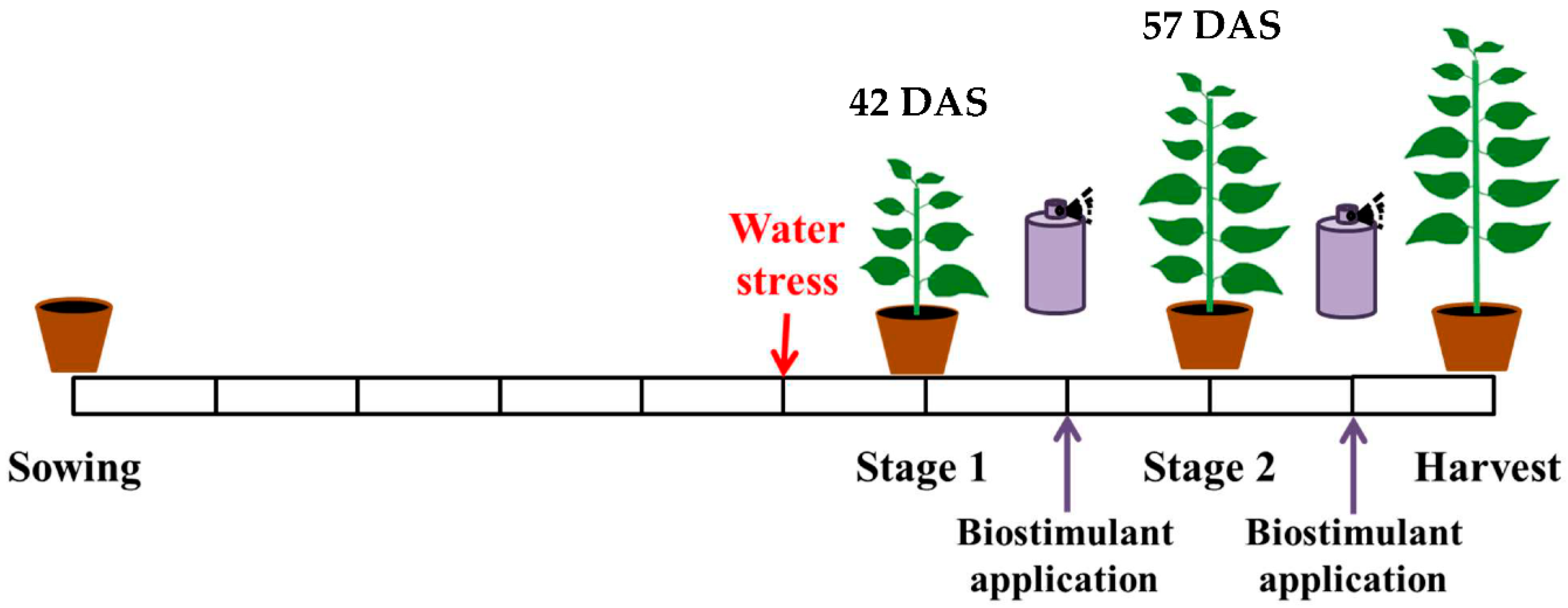
2.1. Experimental Design
2.2. Plant Material
2.3. Treatment
2.4. SPAD and PSII Activity
2.5. Sampling
2.6. NMR Relaxometry
2.7. Water Relations
2.8. Nitrogen and Free Amino Acids Assay
2.9. Data Analysis
3. Results
3.1. Leaf Development and Impact of Mild Drought Stress
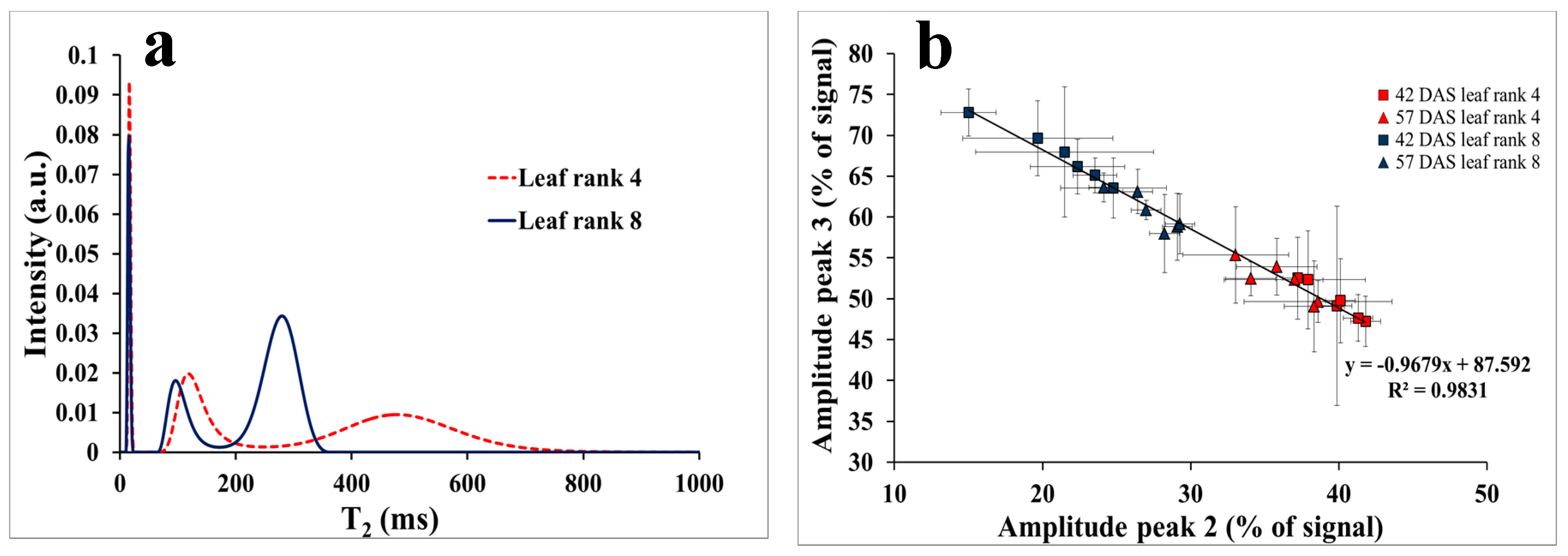
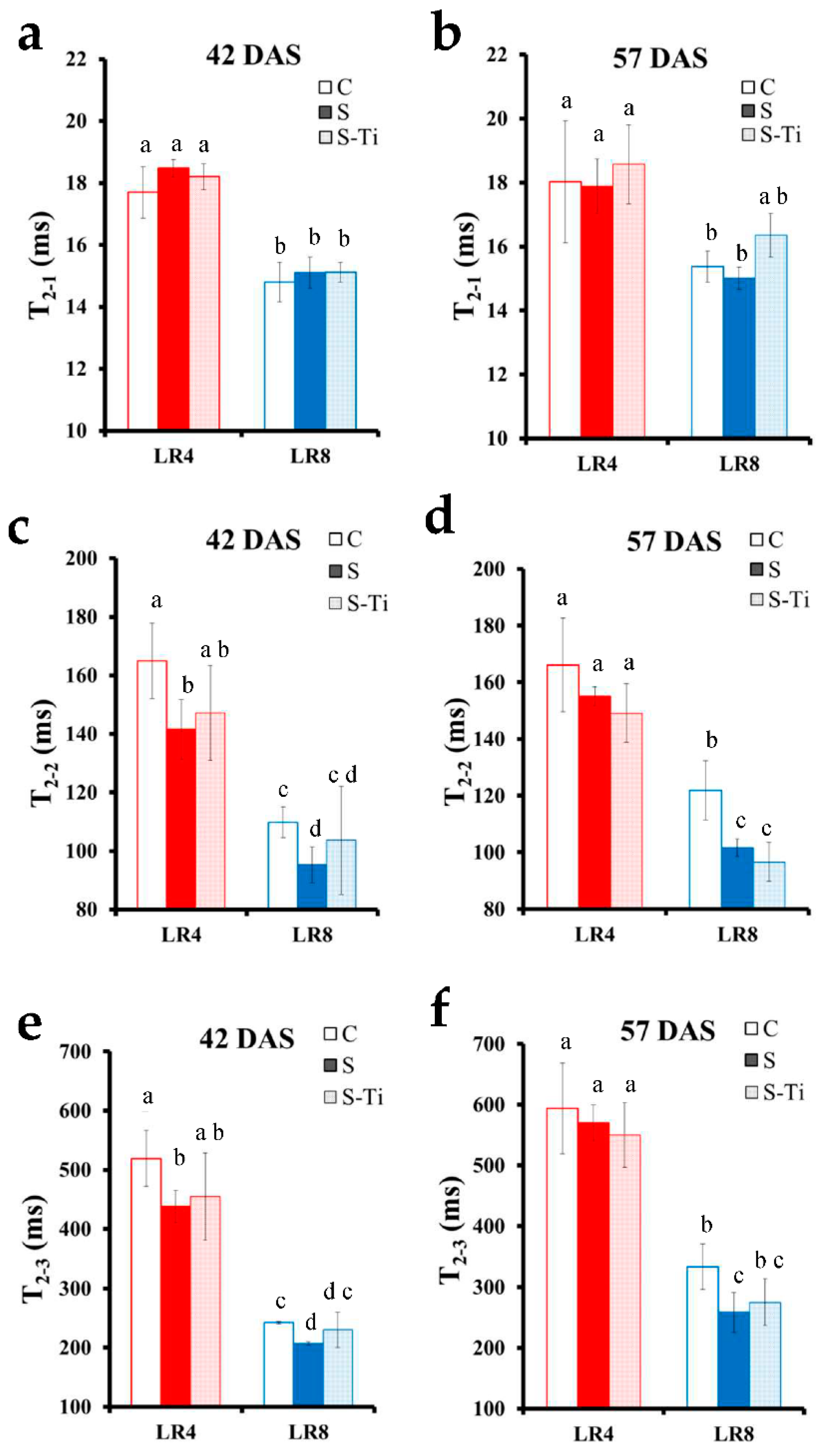
3.2. NMR Monitoring of Mild Drought Stress
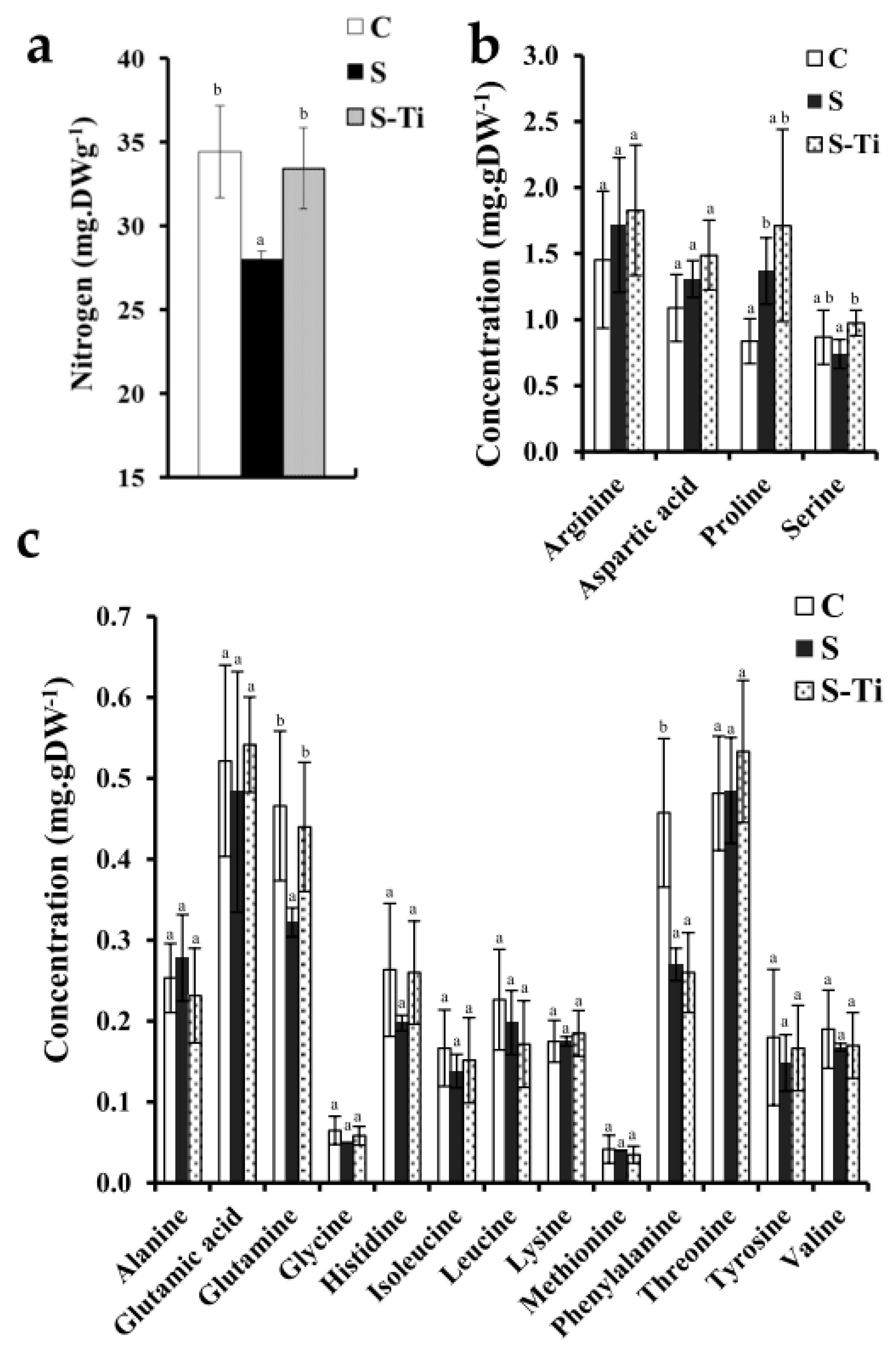
3.3. Impact at Harvest
4. Discussion
4.1. Leaves at Two Developmental Stages Monitored through NMR
4.2. Impact of Mild Drought Stress
4.3. Effects of Biostimulant
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z.L. ROS Regulation during Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; De Bodt, S.; Obata, T.; De Clercq, I.; Claeys, H.; De Rycke, R.; Andriankaja, M.; Van Aken, O.; Van Breusegem, F.; Fernie, A.R.; et al. Developmental Stage Specificity and the Role of Mitochondrial Metabolism in the Response of Arabidopsis Leaves to Prolonged Mild Osmotic Stress. Plant Phys. 2010, 152, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Clauw, P.; Coppens, F.; De Beuf, K.; Dhondt, S.; Van Daele, T.; Maleux, K.; Storme, V.; Clement, L.; Gonzalez, N.; Inze, D. Leaf Responses to Mild Drought Stress in Natural Variants of Arabidopsis. Plant Phys. 2015, 167, 800–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Des Marais, D.L.; McKay, J.K.; Richards, J.H.; Sen, S.; Wayne, T.; Juenger, T.E. Physiological Genomics of Response to Soil Drying in Diverse Arabidopsis Accessions. Plant Cell 2012, 24, 893–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchabke, O.; Chang, F.Q.; Simon, M.; Voisin, R.; Pelletier, G.; Durand-Tardif, M. Natural Variation in Arabidopsis thaliana as a Tool for Highlighting Differential Drought Responses. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and Physiological Analysis of Drought Stress in Arabidopsis Reveals Early Responses Leading to Acclimation in Plant Growth. Plant Phys. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Wiriya-Alongkorn, W.; Spreer, W.; Ongprasert, S.; Spohrer, K.; Pankasemsuk, T.; Muller, J. Detecting drought stress in longan tree using thermal imaging. Maejo Int. J. Sci. Technol. 2013, 7, 166–180. [Google Scholar]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Sepulcre-Canto, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Available online: http://www.biostimulants.eu/ (accessed on 27 September 2017).
- Przybysz, A.; Gawronska, H.; Gajc-Wolska, J. Biological mode of action of a nitrophenolates-based biostimulant: Case study. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Baldo, R.; Scalon, S.D.Q.; Rosa, Y.; Mussury, R.M.; Betoni, R.; Barreto, W.D. Cotton plant cultivar delta opal behavior under water stress with or without biostimulant. Cienc. E Agrotecnol. 2009, 33, 1804–1812. [Google Scholar] [CrossRef]
- Kocira, A.; Kocira, S.; Swieca, M.; Zlotek, U.; Jakubczyk, A.; Kapela, K. Effect of foliar application of a nitrophenolate-based biostimulant on the yield and quality of two bean cultivars. Sci. Hortic. 2017, 214, 76–82. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wilczewski, E.; Poberezny, J.; Wszelaczynska, E.; Keutgen, A.; Ochmian, I. Effect of biostimulants and storage on the content of macroelements in storage roots of carrot. J. Elementol. 2015, 20, 1021–1031. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-Sequencing Analysis Reveals Transcriptional Changes in Root of Maize Seedlings Treated with Two Increasing Concentrations of a New Biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum lycopersicon L. in Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goni, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative Transcriptome Analysis of Two Ascophyllum nodosum Extract Biostimulants: Same Seaweed but Different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Salleo, S. Leafminers help us understand leaf hydraulic design. Plant Cell Environ. 2010, 33, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaibi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Lecoeur, J.; Wery, J.; Turc, O.; Tardieu, F. Expansion of pea leaves subjected to short water-deficit-cell number and cell-size are sensitive to stress at different periods of leaf development. J. Exp. Bot. 1995, 46, 1093–1101. [Google Scholar] [CrossRef]
- Krishnan, P.; Singh, R.; Verma, A.P.S.; Joshi, D.K.; Singh, S. Changes in seed water status as characterized by NMR in developing soybean seed grown under moisture stress conditions. Biochem. Biophys. Res. Commun. 2014, 444, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.; Clark, C. Effect of postharvest water loss on ‘Hayward’ kiwifruit water status. Postharvest Biol. Technol. 2001, 22, 215–225. [Google Scholar] [CrossRef]
- Windt, C.W.; Vergeldt, F.J.; De Jager, P.A.; Van As, H. MRI of long-distance water transport: A comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ. 2006, 29, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- McCain, D.C. Nuclear Magnetic Resonance study of spin and magnetic-field gradient in maple leaves. Biophys. J. 1995, 69, 1111–1116. [Google Scholar] [CrossRef]
- Musse, M.; De Franceschi, L.; Cambert, M.; Sorin, C.; Le Caherec, F.; Burel, A.; Bouchereau, A.; Mariette, F.; Leport, L. Structural changes in senescing oilseed rape leaves at tissue and subcellular levels monitored by Nuclear Magnetic Resonance relaxometry through water status. Plant Phys. 2013, 163, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Joshi, D.K.; Ahand, A.; Verma, A.P.S.; Pathak, P.C. Proton NMR transverse relaxation time and membrane stability in wheat leaves exposed to high temperature shock. Indian J. Biochem. Biophys. 2005, 42, 122–126. [Google Scholar] [PubMed]
- Windt, C.W.; Soltner, H.; van Dusschoten, D.; Blumler, P. A portable Halbach magnet that can be opened and closed without force: The NMR-CUFF. J. Magn. Reson. 2011, 208, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Snaar, J.E.M.; Van, A.H. Probing water compartments and membrane permeability in plant cells by 1H NMR relaxation measurements. Biophys. J. 1992, 63, 1654–1658. [Google Scholar] [CrossRef]
- Henk, V.S. Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. J. Exp. Bot. 2007, 58, 743–756. [Google Scholar] [CrossRef]
- Sorin, C.; Musse, M.; Mariette, F.; Bouchereau, A.; Leport, L. Assessment of nutrient remobilization through structural changes of palisade and spongy parenchyma in oilseed rape leaves during senescence. Planta 2015, 241, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, M.; Joshi, D.K.; Saha, R.; Nagarajan, S.; Gambhir, P.N. Transverse relaxation time of leaf water protons and membrane injury in wheat (Triticum aestivum L.) in response to high temperature. Ann. Bot. 1999, 84, 741–745. [Google Scholar] [CrossRef]
- Capitani, D.; Brilli, F.; Mannina, L.; Proietti, N.; Loreto, F. In Situ Investigation of Leaf Water Status by Portable Unilateral Nuclear Magnetic Resonance. Plant Phys. 2009, 149, 1638–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambhir, P.N.; Shantha, N.; Kumari, P.; Kumar, S.; Pande, P.C.; Nagarajan, S. Proton NMR study of water in leaf and seed tissues of wheat varieties differing in tolerance to water stress. Indian J. Exp. Biol. 1997, 35, 304–306. [Google Scholar]
- Mariette, F.; Guillement, J.P.; Tellier, C.; Marchal, P. Continuous relaxation time distribution decomposition by MEM. In Signal Treatment and Signal Analysis in NMR; Rutledge, D.N., Ed.; Elsevier: Paris, France, 1996; pp. 218–234. [Google Scholar]
- Singh, U. Integrated nitrogen fertilization for intensive and sustainable agriculture. J. Crop Improv. 2005, 15, 259–288. [Google Scholar] [CrossRef]
- Albert, B.; Le Caherec, F.; Niogret, M.F.; Faes, P.; Avice, J.C.; Leport, L.; Bouchereau, A. Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions. Planta 2012, 236, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Aasamaa, K.; Niinemets, U.; Sober, A. Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny. Tree Phys. 2005, 25, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Masclaux, C.; Valadier, M.H.; Brugiere, N.; Morot-Gaudry, J.F.; Hirel, B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 2000, 211, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.P.; Duce, S.L. The influence of chemical and diffusive exchange on water proton transverse relaxation in plant-tissue. Magn. Reson. Imaging 1990, 8, 321–331. [Google Scholar] [CrossRef]
- Snaar, J.E.M.; Van As, H. A method for the simultaneous measurement of NMR spin-lattice and spin-spin relaxation times in compartmentalized systems. J. Magn. Reson. 1992, 99, 139–148. [Google Scholar] [CrossRef]
- Song, Z.P.; Wang, J.N.; Sun, M.; Wu, J.C.; Gong, C.R.; Liu, G.S. Effects of organic fertilizer applications on starch changes in tobacco (Nicotiana tabacum L.) leaves during maturation. Soil Sci. Plant Nutr. 2016, 62, 173–179. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Bonato, C.M.; de Souza, L.A.; Nanni, M.R.; Antunes, W.C. Distinct growth light and gibberellin regimes alter leaf anatomy and reveal their influence on leaf optical properties. Environ. Exp. Bot. 2017, 140, 86–95. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C-3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Phys. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, Z.; Kovacik, J.; Klejdus, B.; Maglovski, M.; Kuna, R.; Hauptvogel, P.; Matusikova, I. Drought-Induced Responses of Physiology, Metabolites, and PR Proteins in Triticum aestivum. J. Agric. Food Chem. 2015, 63, 8125–8133. [Google Scholar] [CrossRef] [PubMed]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of Bread Wheat Genotypes for Drought Tolerance Using Phenotypic and Proline Analyses. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Sarda, X.; Tousch, D.; Ferrare, K.; Cellier, F.; Alcon, C.; Dupuis, J.M.; Casse, F.; Lamaze, T. Characterization of closely related delta-TIP genes encoding aquaporins which are differentially expressed in sunflower roots upon water deprivation through exposure to air. Plant Mol. Biol. 1999, 40, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Nelson, D.E.; Ley, E.; Marquez, S.; Bohnert, H.J. The expression of an aquaporin promoter from Mesembryanthemum crystallinum in tobacco. Plant Cell Phys. 1997, 38, 1326–1332. [Google Scholar] [CrossRef]
- Johansson, I.; Karlsson, M.; Shukla, V.K.; Chrispeels, M.J.; Larsson, C.; Kjellbom, P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 1998, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kjellbom, P.; Larsson, C.; Johansson, I.; Karlsson, M.; Johanson, U. Aquaporins and water homeostasis in plants. Trends Plant Sci. 1999, 4, 308–314. [Google Scholar] [CrossRef]
- Lu, S.Y.; Zhao, H.Y.; Des Marais, D.L.; Parsons, E.P.; Wen, X.X.; Xu, X.J.; Bangarusamy, D.K.; Wang, G.C.; Rowland, O.; Juenger, T.; et al. Arabidopsis ECERIFERUM9 Involvement in Cuticle Formation and Maintenance of Plant Water Status. Plant Physiol. 2012, 159, 930–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef] [PubMed]
- Ondimu, S.; Murase, H. Water stress detection in Sunagoke moss (Rhacomitrium canescens) using combined thermal infrared and visible light imaging techniques. Biosyst. Eng. 2008, 100, 4–13. [Google Scholar] [CrossRef]
- Vermeulen, K.; Aerts, J.M.; Dekock, J.; Bleyaert, P.; Berckmans, D.; Steppe, K. Automated leaf temperature monitoring of glasshouse tomato plants by using a leaf energy balance model. Comput. Electron. Agric. 2012, 87, 19–31. [Google Scholar] [CrossRef]
- Behmann, J.; Steinrucken, J.; Plumer, L. Detection of early plant stress responses in hyperspectral images. ISPRS J. Photogramm. Remote Sens. 2014, 93, 98–111. [Google Scholar] [CrossRef]
- Musse, M.; Leport, L.; Cambert, M.; Debrandt, W.; Sorin, C.; Bouchereau, A.; Mariette, F. A mobile NMR lab for leaf phenotyping in the field. Plant Methods 2017, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Leaf Rank | Treatment | WC (%) 42 DAS | WC (%) 57 DAS | RWC (%) 42 DAS | RWC (%) 57 DAS |
|---|---|---|---|---|---|
| 4 | Control | 96.5 ± 0.4 a,1 | 96.7 ± 0.8 a,b,1 | 96.2 ± 1.8 a,1 | 95.2 ± 2.8 a,1 |
| Stress | 96.0 ± 0.3 a,1 | 96.6 ± 0.1 a,1 | 92.3 ± 4.3 a c,1 | 95.4 ± 2.4 a,1 | |
| Stress-Ti | 96.0 ± 0.4 a,1 | 96.1 ± 0.2 b,1 | 94.5 ± 3.4 a c,1 | 96.0 ± 2.1 a,1 | |
| 8 | Control | 93.4 ± 0.6 b,1 | 95.4 ± 1.1 a,b,c,2 | 84.4 ± 5.1 b,1 | 93.2 ± 1.2 a,b,2 |
| Stress | 93.6 ± 1.0 b,1 | 93.9 ± 0.9 c,1 | 87.4 ± 6.5 b,c,1 | 88.8 ± 3.5 b,1 | |
| Stress-Ti | 93.3 ± 0.6 b,1 | 94.4 ± 0.4 c,2 | 91.6 ± 3.8 a,b,c,1 | 91.1 ± 4.5 a,b,1 |
| Leaf Rank | Treatment | FW per Leaf Area 42 DAS | FW per Leaf Area 57 DAS | DW per Leaf Area 42 DAS | DW per Leaf Area 57 DAS |
|---|---|---|---|---|---|
| 4 | Control | 72.8 ± 5.6 a,1 | 76.3 ± 2.1 a,1 | 2.55 ± 0.15 a,1 | 2.55 ± 0.45 a,1 |
| Stress | 72.6 ± 2.5 a,1 | 77.1 ± 0.2 a,2 | 2.41 ± 0.15 a,1 | 2.61 ± 0.09 a,2 | |
| Stress-Ti | 76.2 ± 5.3 a,1 | 80.7 ± 7.4 a,1 | 2.88 ± 0.43 a,1 | 3.12 ± 0.20 b,2 | |
| 8 | Control | 36.6 ± 0.6 b,1 | 49.6 ± 6.4 b,2 | 2.65 ± 0.12 a,1 | 2.53 ± 0.22 a,1 |
| Stress | 40.4 ± 3.6 b,1 | 45.7 ± 4.3 b,1 | 2.60 ± 0.20 a,1 | 2.77 ± 0.16 a,1 | |
| Stress-Ti | 40.5 ± 3.6 b,1 | 52.0 ± 4.7 b,2 | 2.42 ± 0.14 a,1 | 2.92 ± 0.06 b,2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorin, C.; Mariette, F.; Musse, M.; Leport, L.; Cruz, F.; Yvin, J.-C. Leaf Development Monitoring and Early Detection of Water Deficiency by Low Field Nuclear Magnetic Resonance Relaxation in Nicotiana tabacum Plants. Appl. Sci. 2018, 8, 943. https://doi.org/10.3390/app8060943
Sorin C, Mariette F, Musse M, Leport L, Cruz F, Yvin J-C. Leaf Development Monitoring and Early Detection of Water Deficiency by Low Field Nuclear Magnetic Resonance Relaxation in Nicotiana tabacum Plants. Applied Sciences. 2018; 8(6):943. https://doi.org/10.3390/app8060943
Chicago/Turabian StyleSorin, Clément, François Mariette, Maja Musse, Laurent Leport, Florence Cruz, and Jean-Claude Yvin. 2018. "Leaf Development Monitoring and Early Detection of Water Deficiency by Low Field Nuclear Magnetic Resonance Relaxation in Nicotiana tabacum Plants" Applied Sciences 8, no. 6: 943. https://doi.org/10.3390/app8060943






