Evaluation of the Toxin-to-Protein Binding Rates during Hemodialysis Using Sorbent-Loaded Mixed-Matrix Membranes
Abstract
:1. Introduction
2. Materials and Methods
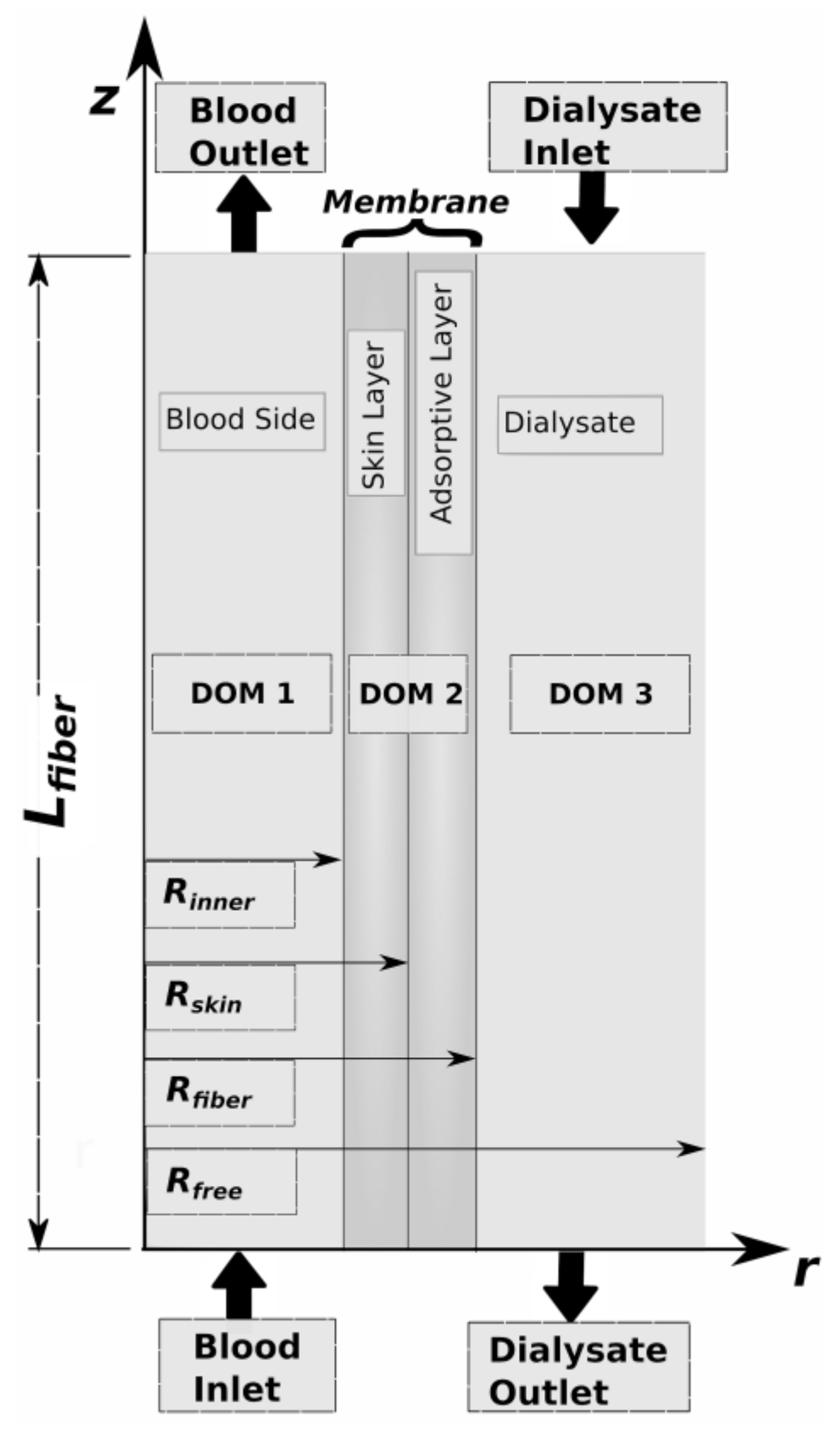
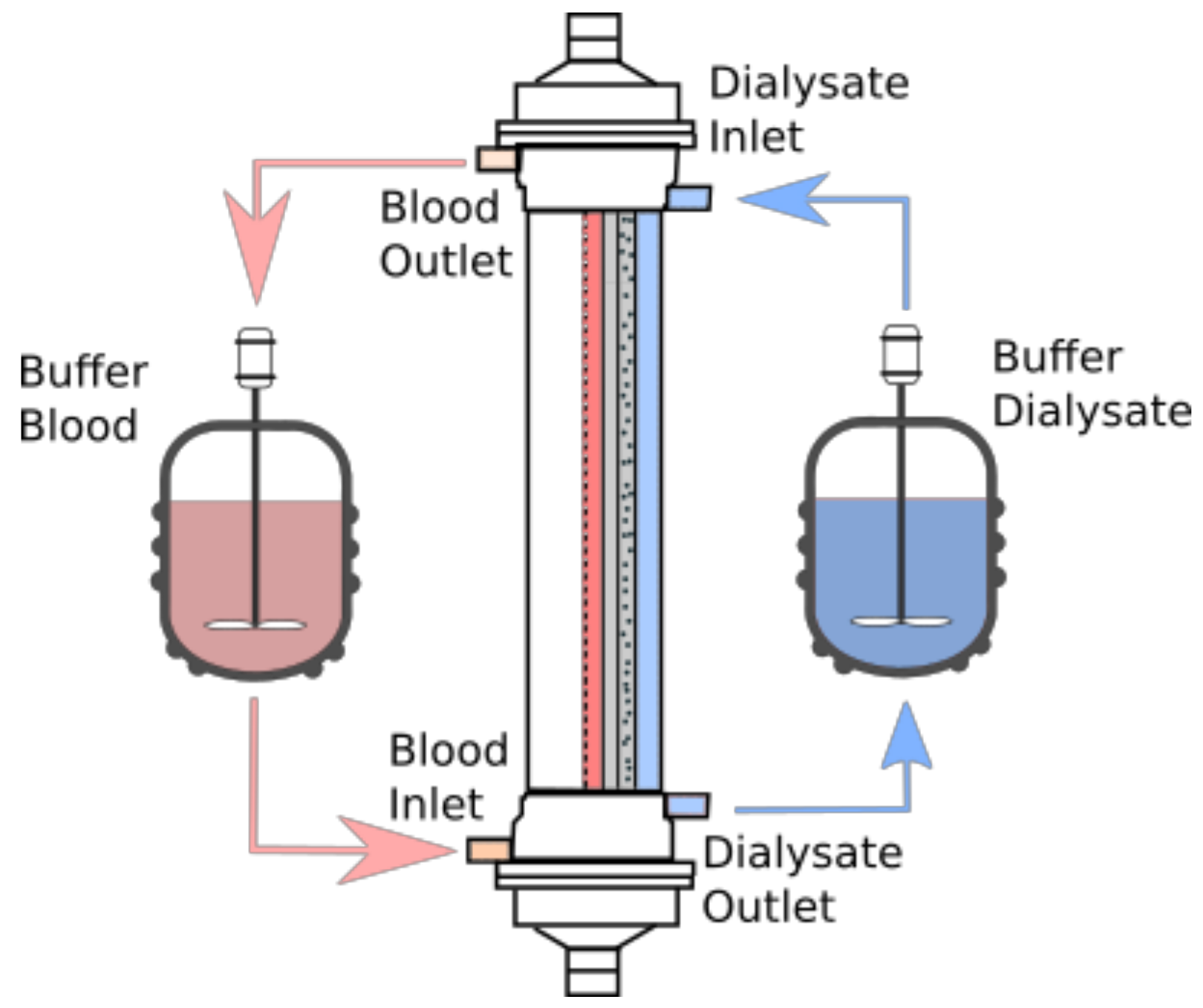
2.1. Model Description
2.2. Momentum Transport
2.3. Mass Transport
2.3.1. The Blood-Sided Domain (DOM1)
2.3.2. The Skin/Web Layer Domain (DOM2)
2.3.3. The Adsorptive Layer (DOM2)
- Film diffusion effects are negligible.
- Diffusion is fast compared to intra-particle adsorption; hence, local equilibrium conditions apply.
2.3.4. The Dialysate-Side Domain (DOM3)
2.4. Boundary Conditions for Mass Transport
2.4.1. The Blood Inlet
2.4.2. The Dialysate
2.4.3. The Blood and Dialysate Outlets
2.4.4. The Boundary Conditions at Layer Interfaces
2.5. Mass Transport
3. Results and Discussion
3.1. Effective Properties of the Membrane Layers
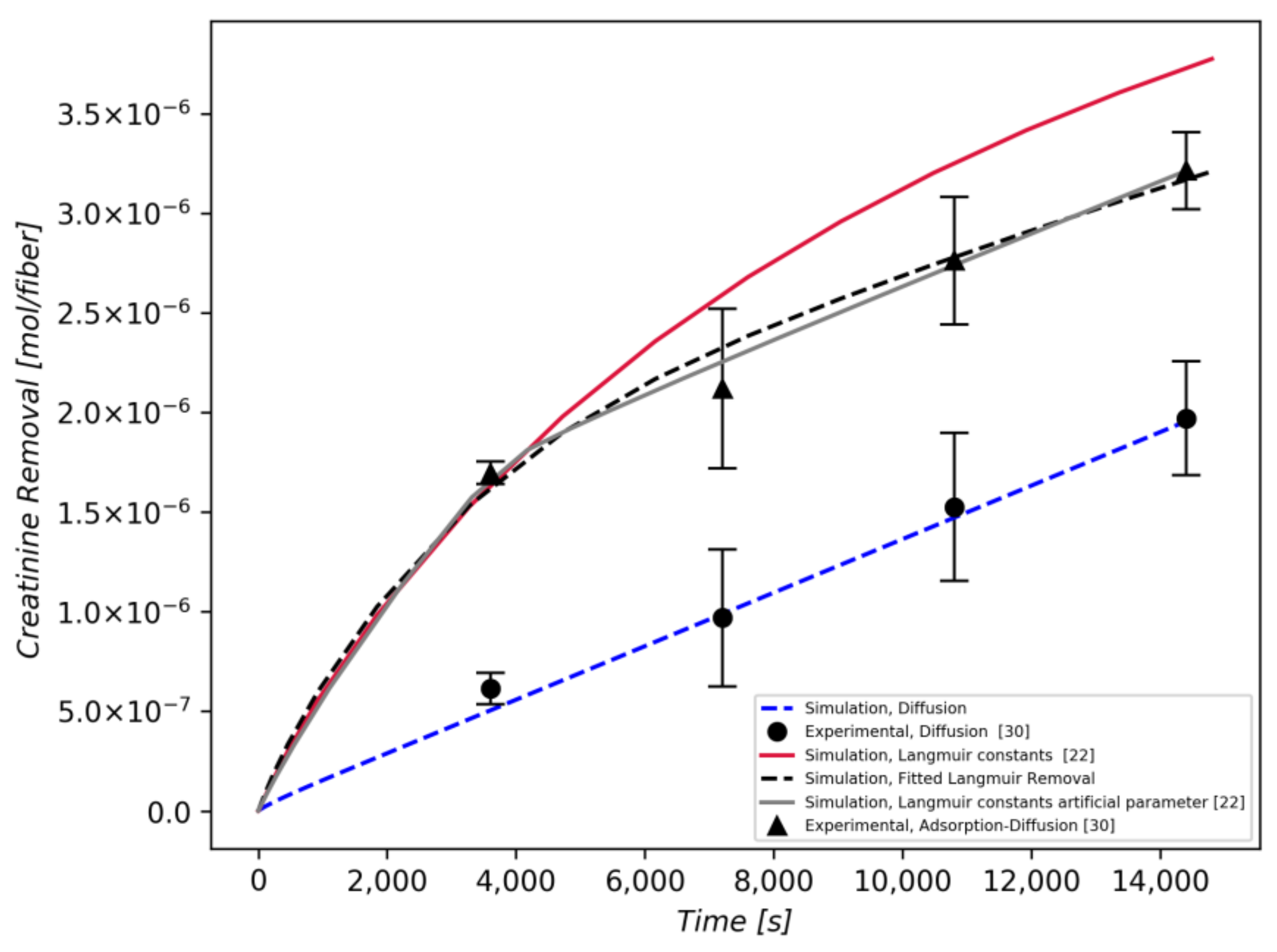
3.2. Creatinine Removal
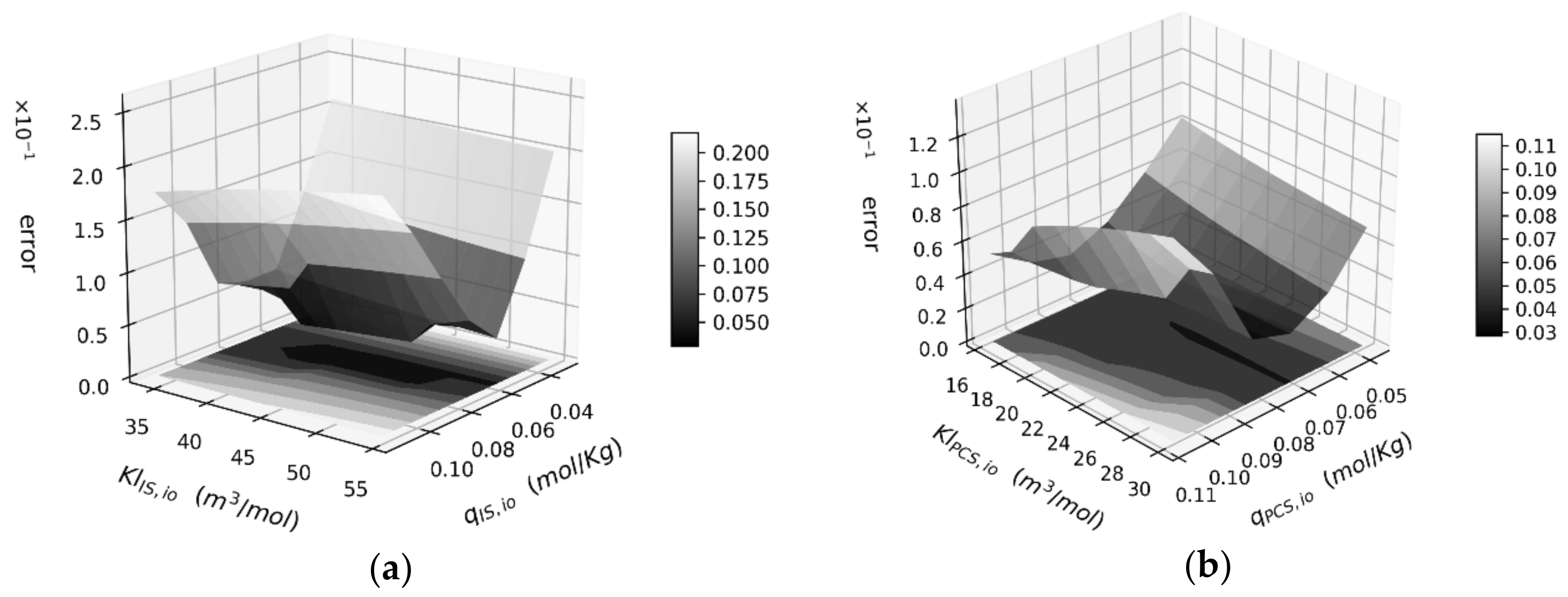
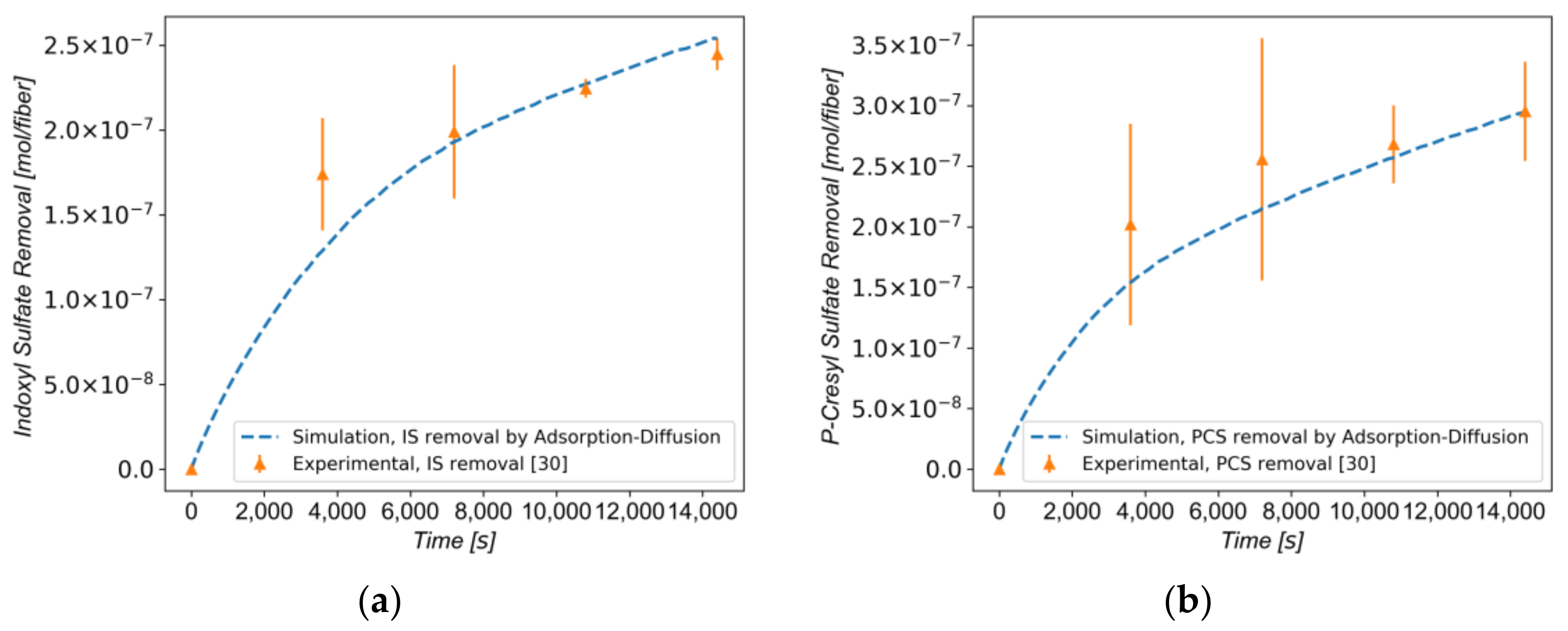
3.3. Protein-Bound Toxins Removal
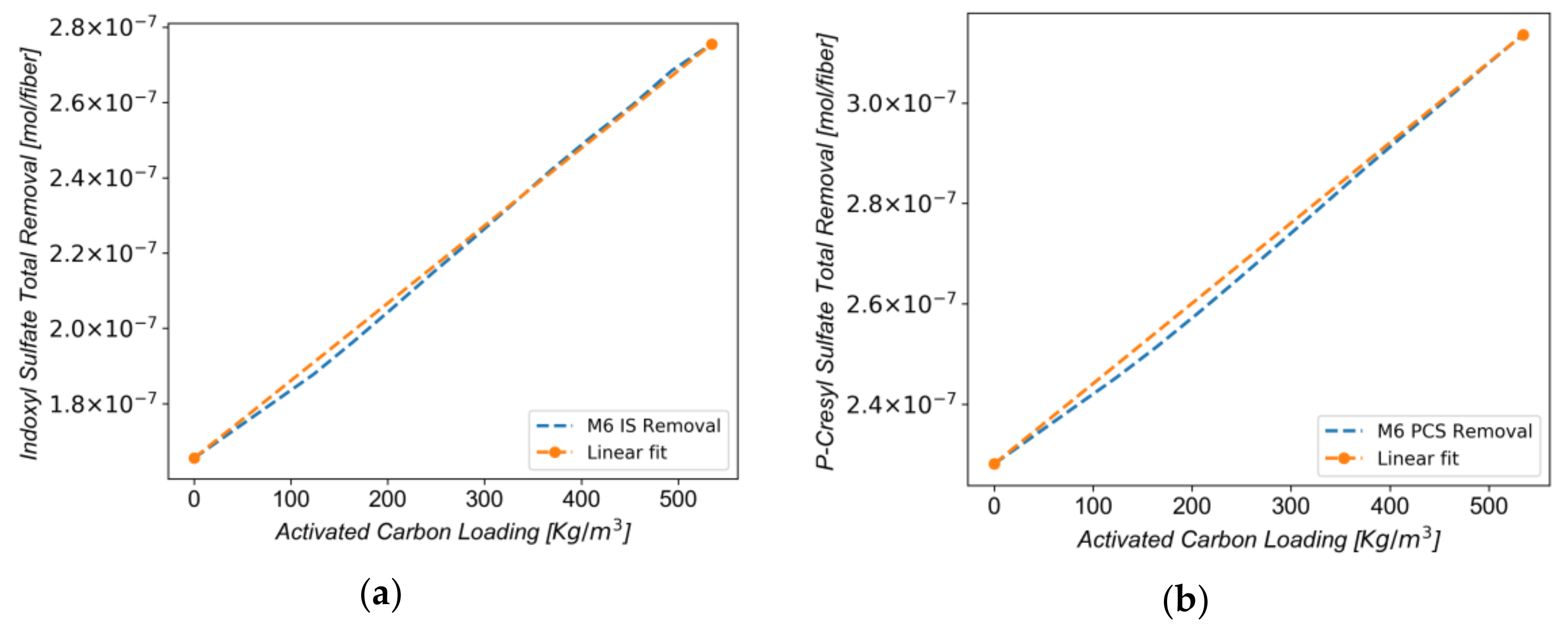
3.4. Effect of Carbon Loading on Toxin Removal
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tijink, M.; Kooman, J.; Wester, M.; Sun, J.; Saiful, S.; Joles, J.; Borneman, Z.; Wessling, M.; Stamatialis, D. Mixed matrix membranes: A new asset for blood purification therapies. Blood Purif. 2014, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tijink, M.S.L.; Wester, M.; Sun, J.; Saris, A.; Bolhuis-Versteeg, L.A.M.; Saiful, S.; Joles, J.A.; Borneman, Z.; Wessling, M.; Stamatialis, D.F. A novel approach for blood purification: Mixed-matrix membranes combining diffusion and adsorption in one step. Acta Biomater. 2012, 8, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Cristina Annesini, M.; Piemonte, V.; Turchetti, L. Simultaneous removal of albumin-bound toxins in liver support devices: Bilirubin and tryptophan adsorption on activated carbon. Chem. Eng. Trans. 2013, 32, 1069–1074. [Google Scholar]
- Watanabe, H.; Noguchi, T.; Miyamoto, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Miyamura, S.; Ishima, Y.; Otagiri, M.; Maruyama, T. Interaction between two sulfate-conjugated uremic toxins, p-cresyl sulfate and indoxyl sulfate, during binding with human serum albumin. Drug Metab. Dispos. 2012, 40, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Devine, E.; Krieter, D.H.; Rüth, M.; Jankovski, J.; Lemke, H.-D. Binding affinity and capacity for the uremic toxin indoxyl sulfate. Toxins 2014, 6, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Bergé-Lefranc, D.; Chaspoul, F.; Calaf, R.; Charpiot, P.; Brunet, P.; Gallice, P. Binding of p-cresylsulfate and p-cresol to human serum albumin studied by Microcalorimetry. J. Phys. Chem. B 2010, 114, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Abad, S.; Vega, A.; Quiroga, B.; Arroyo, D.; Panizo, N.; Reque, J.E.; López-Gómez, J.M. Protein-bound toxins: Added value in their removal with high convective volumes. Nefrología 2016, 36, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Stiller, S.; Mann, H.; Brunner, H. Backfiltration in hemodialysis with highly permeable membranes. In Highly Permeable Membranes; Karger Publishers: Basel, Switzerland, 1985; pp. 23–32. [Google Scholar]
- Wüpper, A.; Dellanna, F.; Baldamus, C.; Woermann, D. Local transport processes in high-flux hollow fiber dialyzers. J. Membr. Sci. 1997, 131, 181–193. [Google Scholar] [CrossRef]
- Katchalsky, A.; Curran, P.F. Nonequilibrium Thermodynamics in Biophysics; Harvard University Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Maheshwari, V.; Thijssen, S.; Tao, X.; Fuertinger, D.; Kappel, F.; Kotanko, P. A novel mathematical model of protein-bound uremic toxin kinetics during hemodialysis. Sci. Rep. 2017, 7, 10371. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Takadate, A.; Otagiri, M. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 1995, 18, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Boucif, N.; Nguyen, P.T.; Roizard, D.; Favre, E. Theoretical studies on carbon dioxide removal from a gas stream in hollow fiber membrane contactors. Desalination Water Treat. 2010, 14, 146–157. [Google Scholar] [CrossRef]
- Happel, J. Viscous flow relative to arrays of cylinders. AIChE J. 1959, 5, 174–177. [Google Scholar] [CrossRef]
- Chen, B.; Gao, Z.; Jin, W.; Zheng, S. Analytical mass transfer solution of longitudinal laminar flow of happel’s free surface model. Int. J. Heat Mass Transf. 2011, 54, 4000–4008. [Google Scholar] [CrossRef]
- Meijers, B.K.; Bammens, B.; Verbeke, K.; Evenepoel, P. A review of albumin binding in ckd. Am. J. Kidney Dis. 2008, 51, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.; Wade, D. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar] [PubMed]
- Takamura, N.; Maruyama, T.; Otagiri, M. Effects of uremic toxins and fatty acids on serum protein binding of furosemide: Possible mechanism of the binding defect in uremia. Clin. Chem. 1997, 43, 2274–2280. [Google Scholar] [PubMed]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ros production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Fournel, L.; Mocho, P.; Brown, R.; le Cloirec, P. Modeling breakthrough curves of volatile organic compounds on activated carbon fibers. Adsorption 2010, 16, 147–153. [Google Scholar] [CrossRef]
- Ochoa-Tapia, J.A.; Whitaker, S. Bulk and surface diffusion in porous media: An application of the surface-averaging theorem. Chem. Eng. Sci. 1993, 48, 2061–2082. [Google Scholar] [CrossRef]
- Tijink, M.S.L.; Wester, M.; Glorieux, G.; Gerritsen, K.G.F.; Sun, J.; Swart, P.C.; Borneman, Z.; Wessling, M.; Vanholder, R.; Joles, J.A.; et al. Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials 2013, 34, 7819–7828. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.A.; Rigatto, C.; Reslerova, M.; Ying, S.L.; Man, A.; Schattka, B.; Battrell, C.F.; Matthewson, J.; Mansfield, C. Toward point-of-care diagnostic metabolic fingerprinting: Quantification of plasma creatinine by infrared spectroscopy of microfluidic-preprocessed samples. Analyst 2009, 134, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, W.; Salpadoru, N.; Caro, C. Diffusion coefficients for protein molecules in blood serum. Atherosclerosis 1976, 25, 225–235. [Google Scholar] [CrossRef]
- Jackson, R. Transport in Porous Catalysts; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Bali, N.; Petsi, A.J.; Skouras, E.D.; Burganos, V.N. Three-dimensional reconstruction of bioactive membranes and pore-scale simulation of enzymatic reactions: The case of lactose hydrolysis. J. Membr. Sci. 2017, 524, 225–234. [Google Scholar] [CrossRef]
- Liao, Z.; Klein, E.; Poh, C.K.; Huang, Z.; Lu, J.; Hardy, P.A.; Gao, D. Measurement of hollow fiber membrane transport properties in hemodialyzers. J. Membr. Sci. 2005, 256, 176–183. [Google Scholar] [CrossRef]
- Islam, M.S.; Szpunar, J. Study of dialyzer membrane (Polyflux 210H) and effects of different parameters on dialysis performance. Open J. Nephrol. 2013, 3, 161–167. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, D.; van Geffen, E.; van Steenbergen, M.J.; Glorieux, G.; Vanholder, R.; Gerritsen, K.G.F.; Stamatialis, D. New low-flux mixed matrix membranes that offer superior removal of protein-bound toxins from human plasma. Sci. Rep. 2016, 6, 34429. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Hsu, S.-T.; Hu, S.-C. A one-compartment single-pore model for extracorporeal hemoperfusion. Comput. Biol. Med. 1989, 19, 83–94. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Syu, M.-J. Synthesis of creatinine-imprinted poly (β-cyclodextrin) for the specific binding of creatinine. Biomaterials 2005, 26, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Han, L. Implementing the nelder-mead simplex algorithm with adaptive parameters. Comput. Optim. Appl. 2012, 51, 259–277. [Google Scholar] [CrossRef]
- Schoning, L.-C.; Li, Y. Multivariable optimisation of a homogeneous charge microwave ignition system. In Proceedings of the 2013 19th International Conference on Automation and Computing (ICAC), London, UK, 13–14 September 2013; IEEE: Piscataway, NJ, USA; pp. 1–5. [Google Scholar]

| Layer | Thickness | ||
|---|---|---|---|
| Inner Layer | 21 μm | 1.6 × 10−19 m2 | 0.19 |
| Outer Layer | 47 μm | 1.6 × 10−12 m2 | 0.25 |
| Toxin | |||
|---|---|---|---|
| IS | 0.73 | 0.073 | 41 |
| PCS | 1.82 | 0.069 | 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiapis, C.S.; Skouras, E.D.; Pavlenko, D.; Stamatialis, D.; Burganos, V.N. Evaluation of the Toxin-to-Protein Binding Rates during Hemodialysis Using Sorbent-Loaded Mixed-Matrix Membranes. Appl. Sci. 2018, 8, 536. https://doi.org/10.3390/app8040536
Stiapis CS, Skouras ED, Pavlenko D, Stamatialis D, Burganos VN. Evaluation of the Toxin-to-Protein Binding Rates during Hemodialysis Using Sorbent-Loaded Mixed-Matrix Membranes. Applied Sciences. 2018; 8(4):536. https://doi.org/10.3390/app8040536
Chicago/Turabian StyleStiapis, Christos S., Eugene D. Skouras, Denys Pavlenko, Dimitrios Stamatialis, and Vasilis N. Burganos. 2018. "Evaluation of the Toxin-to-Protein Binding Rates during Hemodialysis Using Sorbent-Loaded Mixed-Matrix Membranes" Applied Sciences 8, no. 4: 536. https://doi.org/10.3390/app8040536






