Eco-Friendly Synthesis and Antimicrobial Activity of Silver Nanoparticles Using Dracocephalum moldavica Seed Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Seeds
2.2. Preparation of Aqueous D. Moldavica Seed Extract, and Synthesis of AgNPs
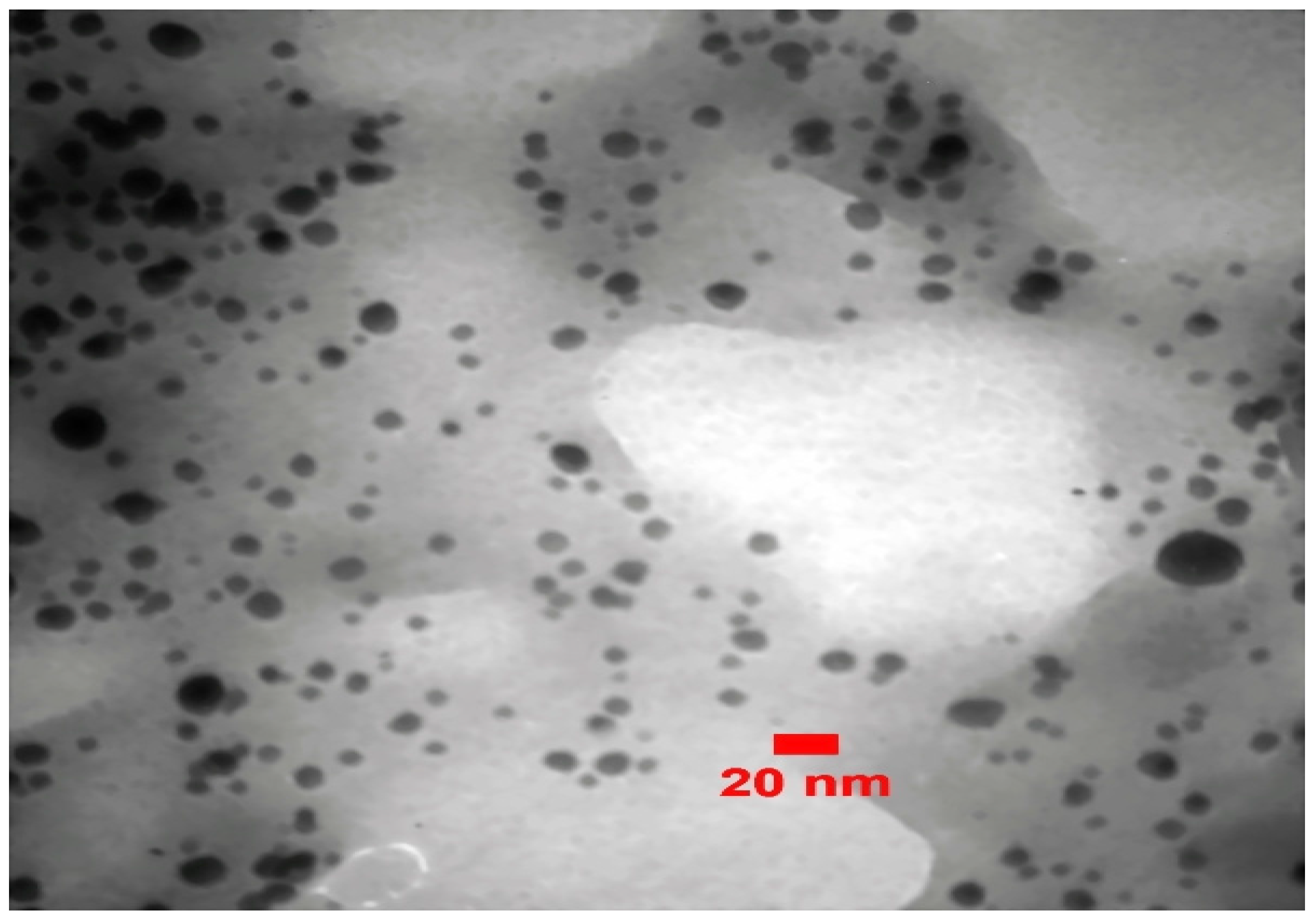
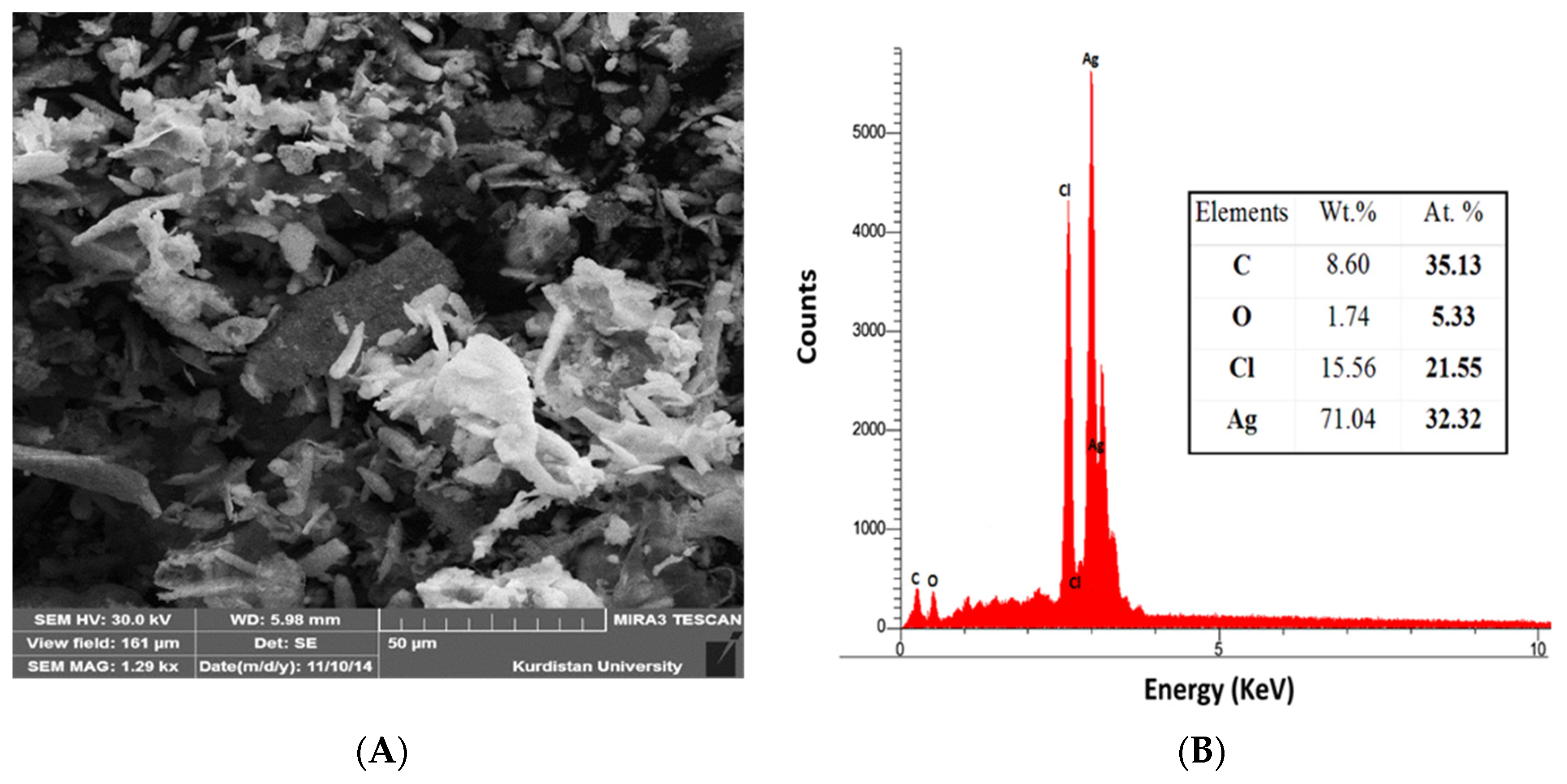
2.3. Characterization of Silver NPs
2.4. In-Vitro Antimicrobial Activity
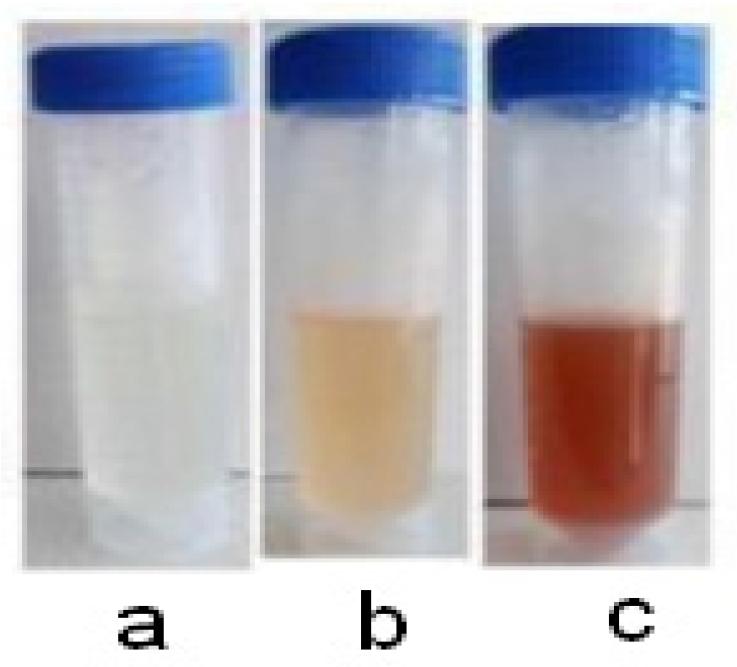
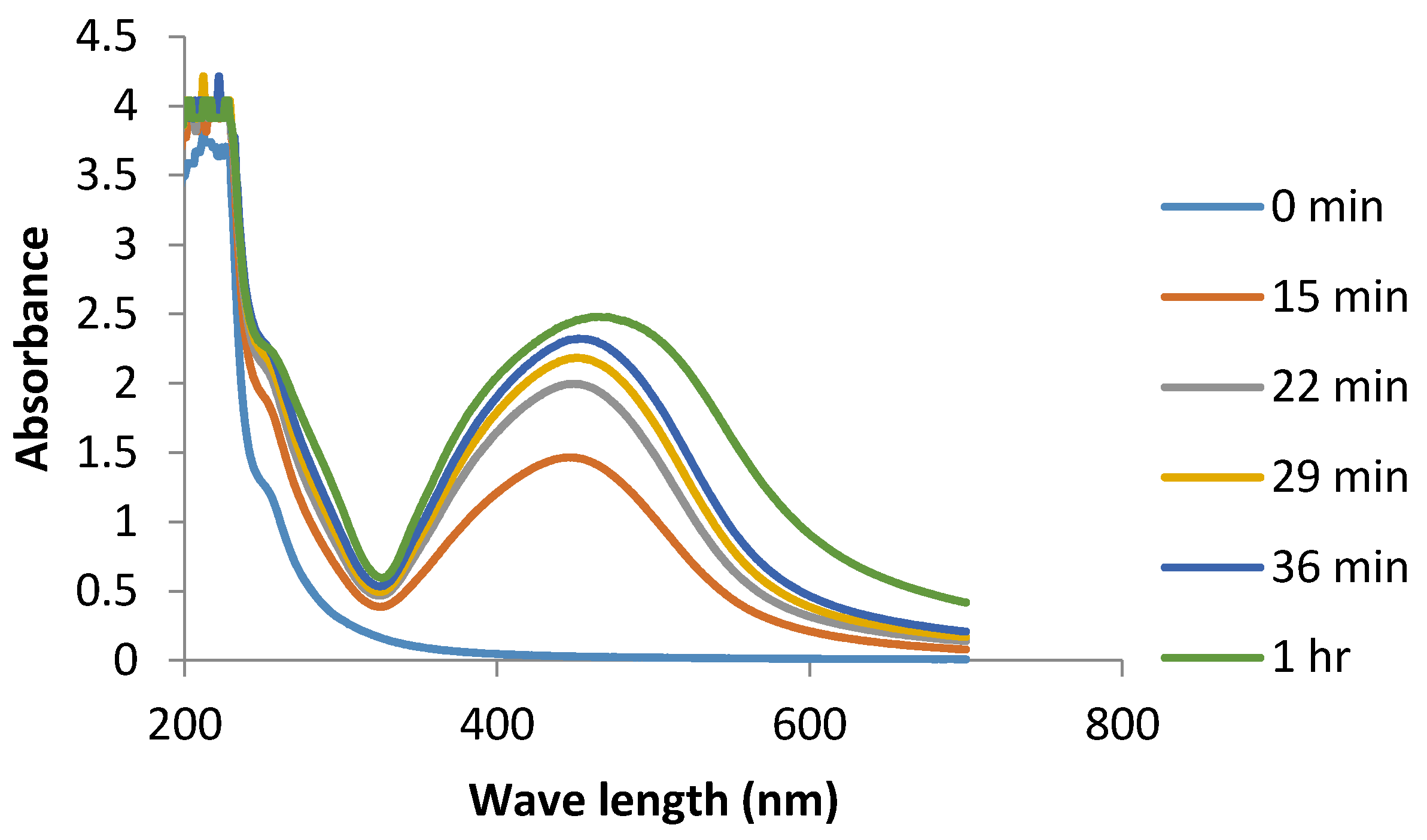
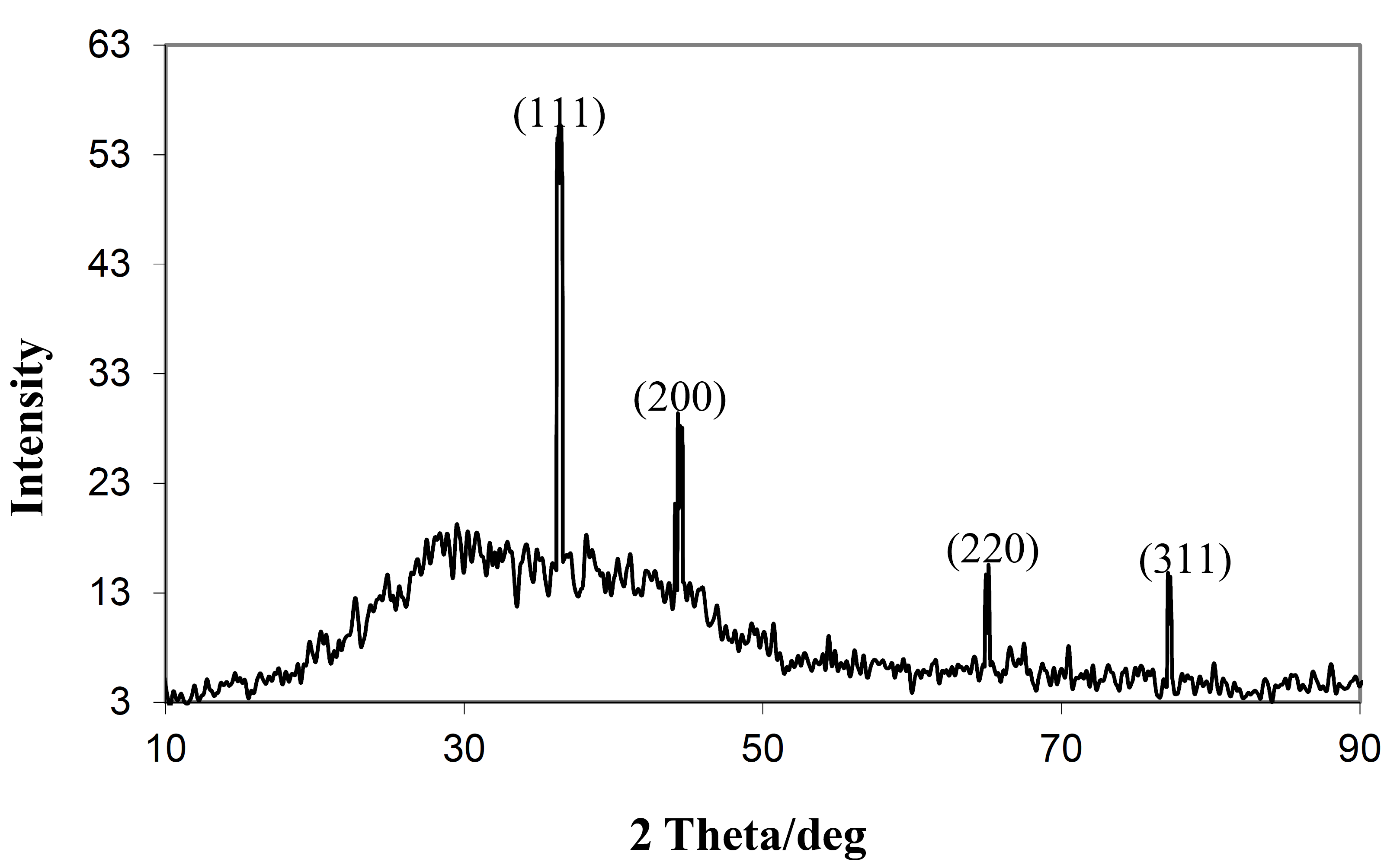
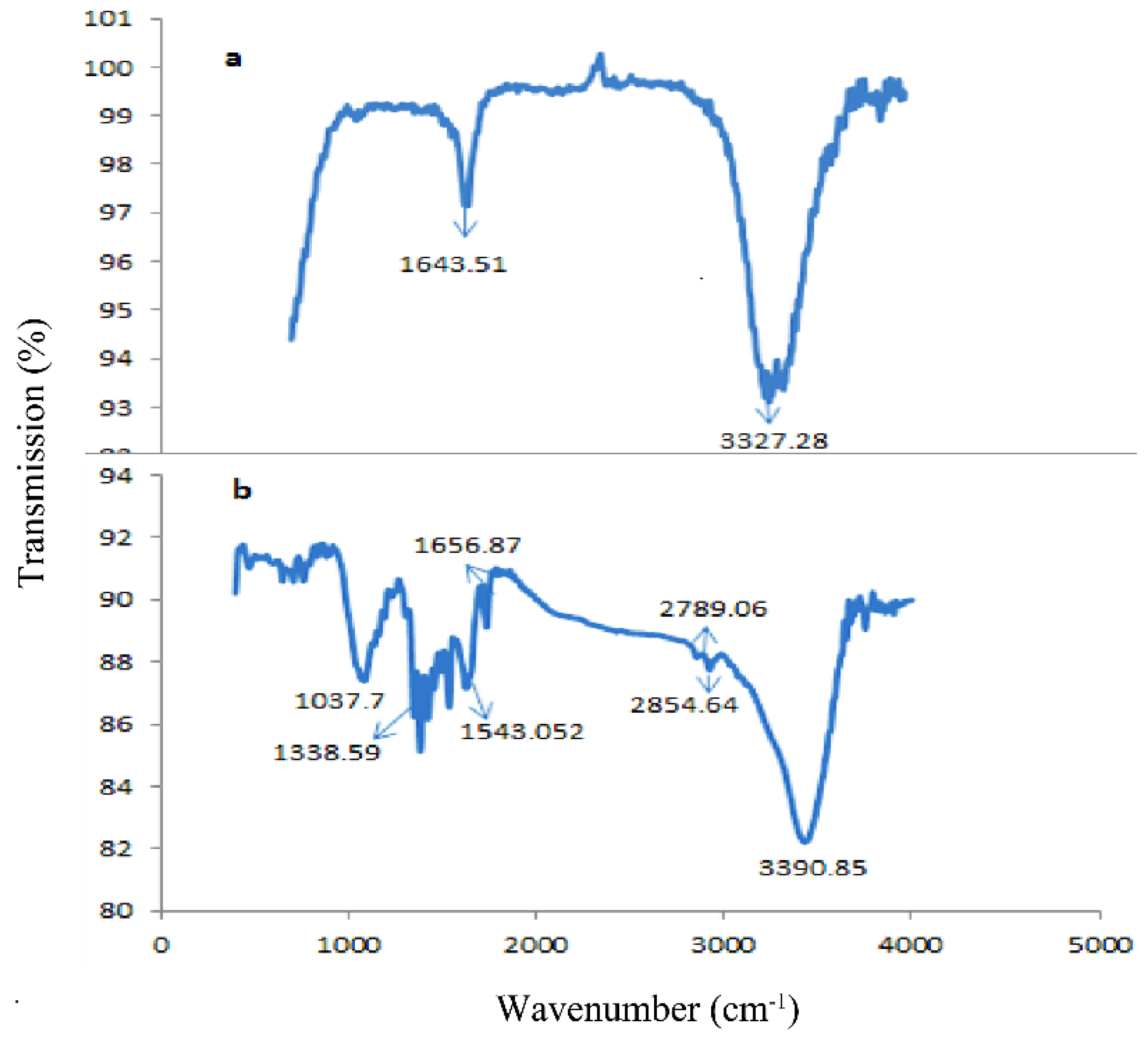
3. Results and Discussion
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Lin, C.A.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I.; et al. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. Sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Iram, F.; Iqbal, M.S.; Athar, M.M.; Saeed, M.Z.; Yasmeen, A.; Ahmad, R. Glucoxylan-mediated green synthesis of gold and silver nanoparticles and their phyto-toxicity study. Carbohydr. Polym. 2014, 104, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Nalini, S.; Prakash, N.U.; Madhankumar, D. One step green synthesis of silvernano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloid Surf. B Biointerfaces 2012, 94, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Nazeruddina, G.M.; Prasada, N.R.; Prasadd, S.R.; Shaikha, Y.I.; Waghmareb, S.R.; Adhyapak, P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind. Crops Prod. 2010, 60, 212–216. [Google Scholar]
- Joerger, R.; Klaus, T.; Granqvist, C.G. Biologically produced silvercarbon composite materials for optically functional thin-film coatings. Adv. Mater. 2000, 12, 407–409. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2003, 14, 95. [Google Scholar] [CrossRef]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Pawar, S.H. Green phytosynthesis of silver nanoparticles using aqueous extract of Manilkara zapota (L.) seeds and its inhibitory action against Candida species. Mater. Lett. 2014, 116, 367–369. [Google Scholar] [CrossRef]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Jayaseelana, C.; Ramkumarb, R.; Rahumana, A.A.; Perumalb, P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crops Prod. 2013, 45, 423–429. [Google Scholar] [CrossRef]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Jones, S.A.; Bowler, P.G.; Walker, M.; Parsons, D. Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair Regen. 2004, 12, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. Seed extract and its antibacterial activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatrophacurcas. Colloid Surface A 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Kora, A.; Arunachalam, J. Biosynthesis of silver nanoparticles by the seed extract of Strychnospotatorum: A natural phytocoagulant. IET Nanobiotechnol. 2013, 7, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Showmya, J.; Harini, K.; Pradeepa, M.; Thiyagarajan, M.; Manikandan, R.; Venkatachalam, P.; Geetha, N. Rapid green synthesis of silver nanoparticles using seed extract of Foenculum vulgare and screening of its antibacterial activity. Plant Cell Biotechnol. Mol. Biol. 2012, 13, 31–38. [Google Scholar]
- Mohammadinejad, R.; Pourseyedi, S.; Baghizadeh, A.; Ranjbar, S.; Mansoori, G.A. Synthesis of silver nanoparticles using Silybum marianum seed extract. Int. J. Nanosci. Nanotechnol. 2013, 9, 221–226. [Google Scholar]
- Venkateswarlu, S.; Kumar, B.N.; Prasad, C.H.; Venkateswarlu, P.; Jyothi, N.V.V. Bioinspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys. B Condens. Matter 2014, 449, 67–71. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Damien-Dorman, H.J.; Laakso, I.; Hiltunen, R. Chemical composition and antioxidative activity of Molavian balm (Dracocephalum moldavica L.) extracts. LWT Food Sci. Technol. 2007, 40, 1655–1663. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.; Varma, R. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Park, Y.; Hong, Y.N.; Weyers, A.Y.; Kim, S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kim, S.K. Biosynthesis of nanoparticles using marine algae: A review. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S.K., Chojnacka, K., Eds.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Marimuthu, V.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G.; et al. Efficacy of plant-mediated synthesized silver nanoparticles against hematophagous parasites. Parasitol. Res. 2013, 111, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Bhattacharya, J.; Mukherjee, M.; Ghosh, A.K.; Santra, C.R.; Dasgupta, A.K.; Karmakar, P. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614. [Google Scholar] [CrossRef]
- Chudasama, B.; Vala, A.K.; Andhariya, N.; Mehta, R.V.; Upadhyay, R.V. Highly bacterial resistant silver nanoparticles: Synthesis and antibacterial activities. J. Nanoparticle Res. 2010, 12, 1677–1685. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Bae, I.T.; Arey, B.W.; Exarhos, G.J. Facile stabilization of gold-silver alloy nanoparticles on cellulose nanocrystal. J. Phys. Chem. C 2008, 112, 4844–4848. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.; Lagashetty, D.A.; Rajasab, A.H.; Venkataraman, A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Raja, K.; Saravanakumar, A.; Vijayakumar, R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim. Acta Part A 2012, 97, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B.; Damle, C.; Ahmad, A.; Sastry, M.; Nanosci, J. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol. 2005, 5, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- El-Nour, K.M.A.; Eftaiha, A.A.; Al-Warthanb, A.; Ammar, R.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J. Biological evaluation of silver nanoparticles obtained from T. arjuna bark extract as both reducing and capping agent. J. Clust. Sci. 2014, 25, 1449–1462. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, D.; Zhang, G.; He, N.; Liu, H.; Huang, J.; Odoom-Wubah, T.; Li, Q. Investigation of active biomolecules involved in the nucleation and growth of gold nanoparticles by Artocarpus heterophyllus Lam leaf extract. J. Nanoparticle Res. 2013, 15, 1741–1751. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, D.; Ravishankar, B.; Sharanbasava, G.; Mahesh, D.B.; Harsoor, V.; Yalagatti, M.S.; Bhagawanraju, M.; Venkataraman, A. Anti-cancer studies of noble metal nanoparticles synthesized using different plant extracts. Cancer Nanotechnol. 2011, 2, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.G.; Chen, S.A.; Chen, H.M.; Wu, W.M.; Hung, C.F.; Yao, Y.D.; Tu, C.S.; Liang, Y.J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanotechnol. Biol. Med. 2012, 87, 8767–8775. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.M.; Chopade, B.A. Plasmid mediated silver resistance in Acinetobacter baumannii. Biometals 1994, 7, 749–756. [Google Scholar] [CrossRef]
- Van den Wildenberg, W. Roadmap Report on Nanoparticles; W&W. Espana sl.: Barcelona, Spain, 2005. [Google Scholar]
- Annamalai, J.; Nallamuthu, T. Green synthesis of silver nanoparticles: Characterization and determination of antibacterial potency. Appl. Nanosci. 2016, 6, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nanotechnol. 2010, 4, 6903–6913. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. Vitro 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. ACS Nanotechnol. 2007, 3, 1357–1364. [Google Scholar] [CrossRef]
- Yu, S.J.; Yin, Y.G.; Liu, J.F. Silver nanoparticles in the environment. Environ. Sci. 2013, 15, 78–92. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2010, 52, 662–668. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramfrez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Vertelov, G.K.; Krutyakov, Y.A.; Efremenkova, O.V.; Olenin, A.Y.; Lisichkin, G.V. A versatile synthesis of highly bactericidal Myramistin stabilized silver nanoparticles. Nanotechnology 2008, 19, 355707. [Google Scholar] [CrossRef] [PubMed]
- Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D.H.; Tessier, C.A.; Youngs, W.J. Silver (I) imidazole cyclophane gem-Dio complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005, 127, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
| Pathogens | D. moldavica Seed Extract | Synthesized Silver Nanoparticle | AgNO3 | |||
|---|---|---|---|---|---|---|
| Disk Diffusion Assay (mm·dia) | MIC (µg·mL−1) | Disk Diffusion Assay (mm·dia) | MIC (µg·mL−1) | Disk Diffusion Assay (mm·dia) | MIC (µg·mL−1) | |
| Staphylococcus aureus | - | - | 8.4 ± 0.76 | 29.6 ± 0.00 | 11.6 ± 0.88 | 16 ± 0.00 |
| Staphylococcus epidermidis | - | - | 11 ± 1.66 | 25 ± 0.00 | 9 ± 0.43 | 25 ± 0.00 |
| Bacillus subtilis | - | - | 10 ± 0.77 | 22 ± 0.00 | 12 ± 1.32 | 16 ± 0.00 |
| E. coli | - | - | 17.6 ± 0.66 | 11 ± 0.00 | 15.4 ± 1.13 | 9 ± 0.00 |
| Serratia marcescens | 2 ± 0.00 | - | 19.5 ± 1.76 | 8 ± 0.00 | 17.5 ± 1.38 | 6 ± 0.00 |
| Pseudomonas aeruginosa | - | - | 13.2 ± 1.87 | 12 ± 0.00 | 13.44 ± 1.12 | 12 ± 0.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi Pak, Z.; Abbaspour, H.; Karimi, N.; Fattahi, A. Eco-Friendly Synthesis and Antimicrobial Activity of Silver Nanoparticles Using Dracocephalum moldavica Seed Extract. Appl. Sci. 2016, 6, 69. https://doi.org/10.3390/app6030069
Haghighi Pak Z, Abbaspour H, Karimi N, Fattahi A. Eco-Friendly Synthesis and Antimicrobial Activity of Silver Nanoparticles Using Dracocephalum moldavica Seed Extract. Applied Sciences. 2016; 6(3):69. https://doi.org/10.3390/app6030069
Chicago/Turabian StyleHaghighi Pak, Zahra, Hossein Abbaspour, Naser Karimi, and Ali Fattahi. 2016. "Eco-Friendly Synthesis and Antimicrobial Activity of Silver Nanoparticles Using Dracocephalum moldavica Seed Extract" Applied Sciences 6, no. 3: 69. https://doi.org/10.3390/app6030069





