The Development of Piezo-Driven Tools for Cellular Piercing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mouse Oocytes
2.2. Preparation of Pipettes
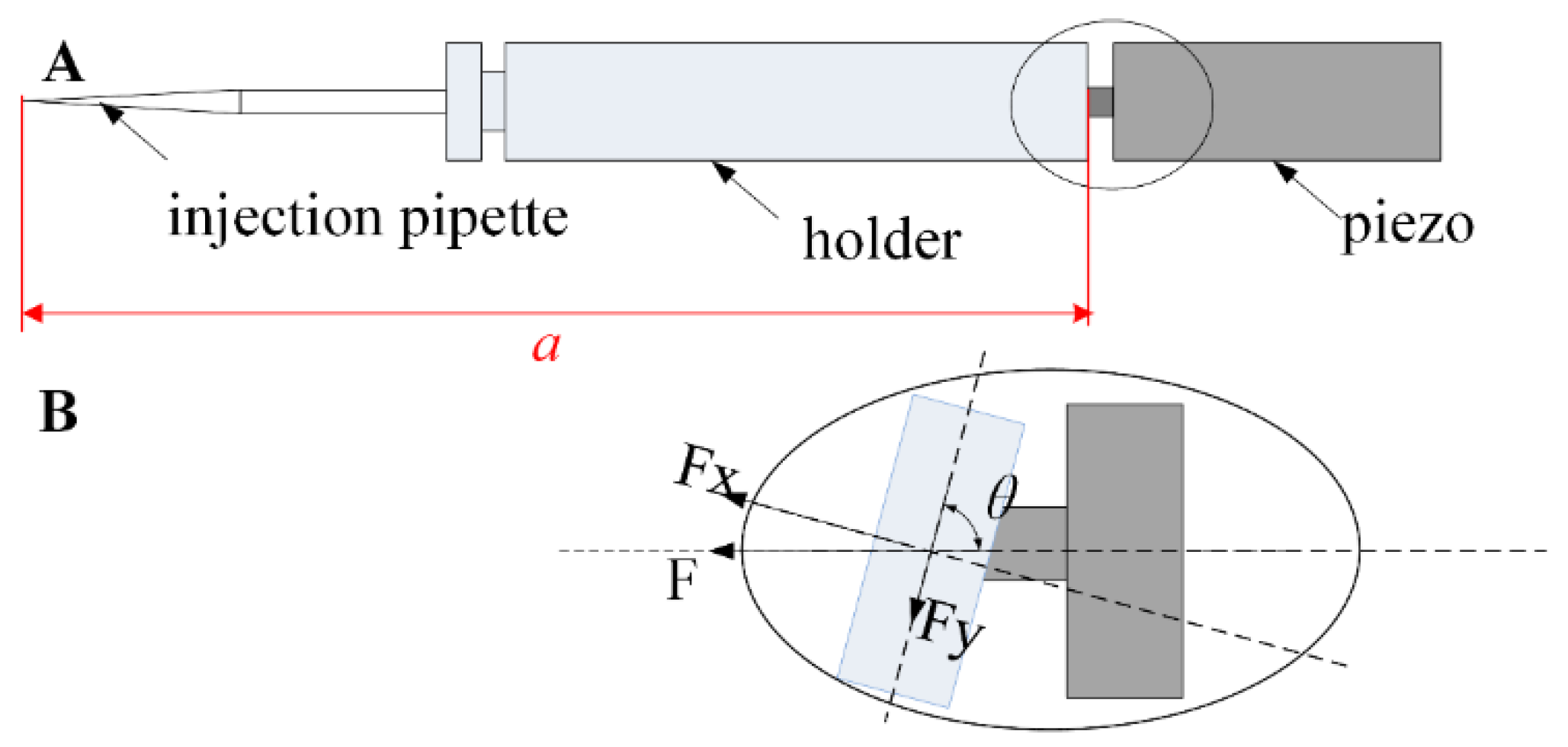
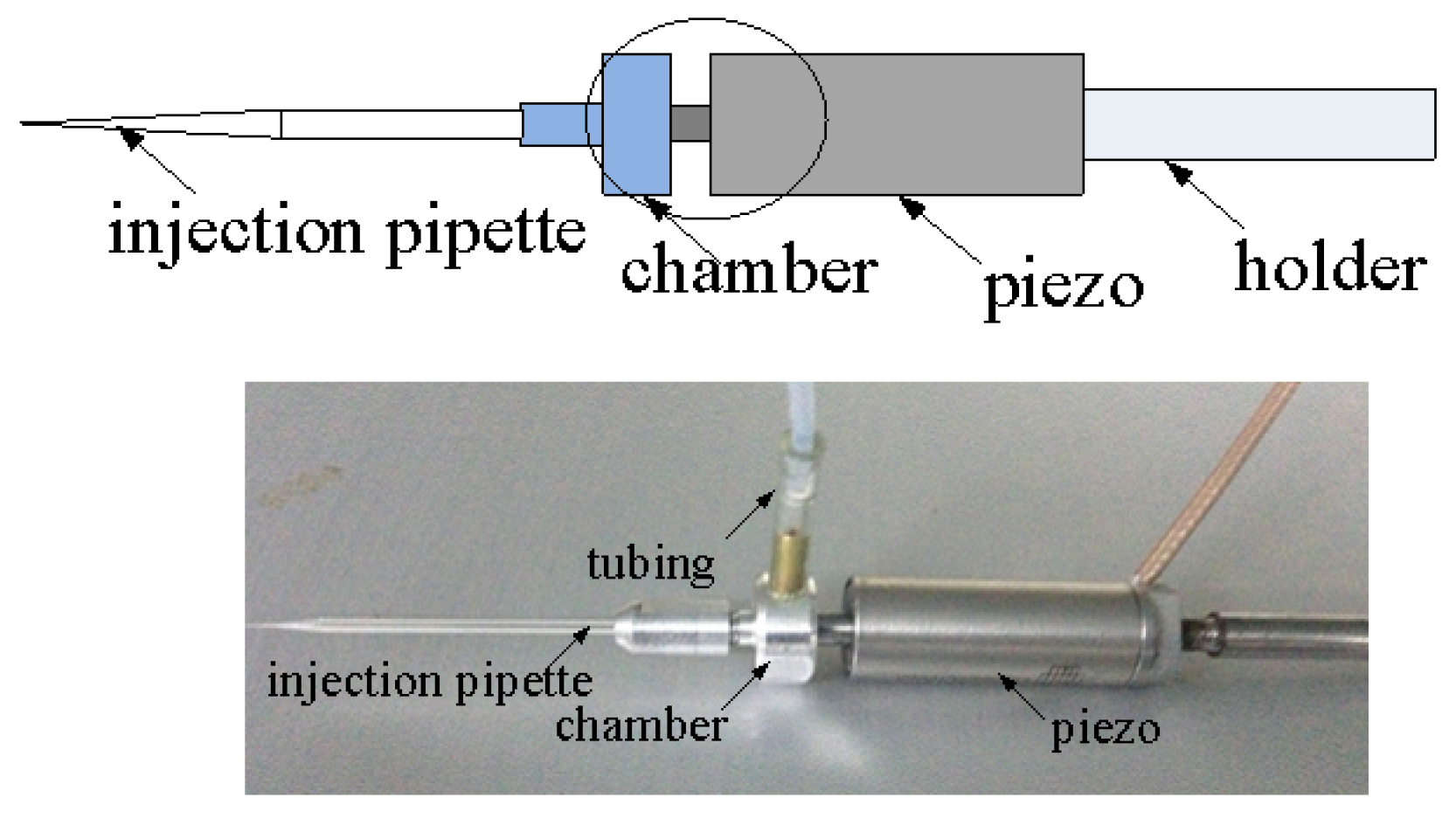
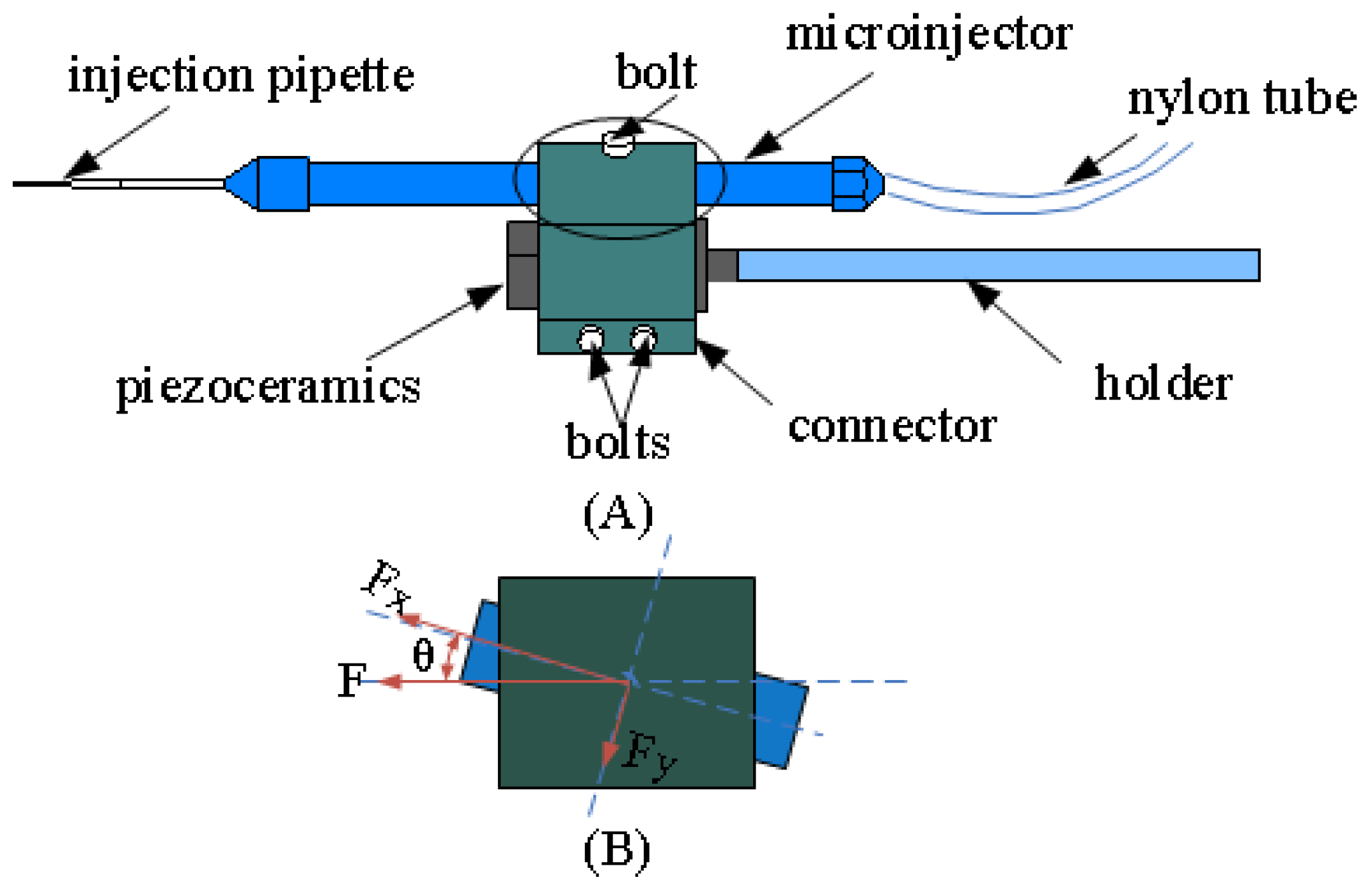
2.3. System Design
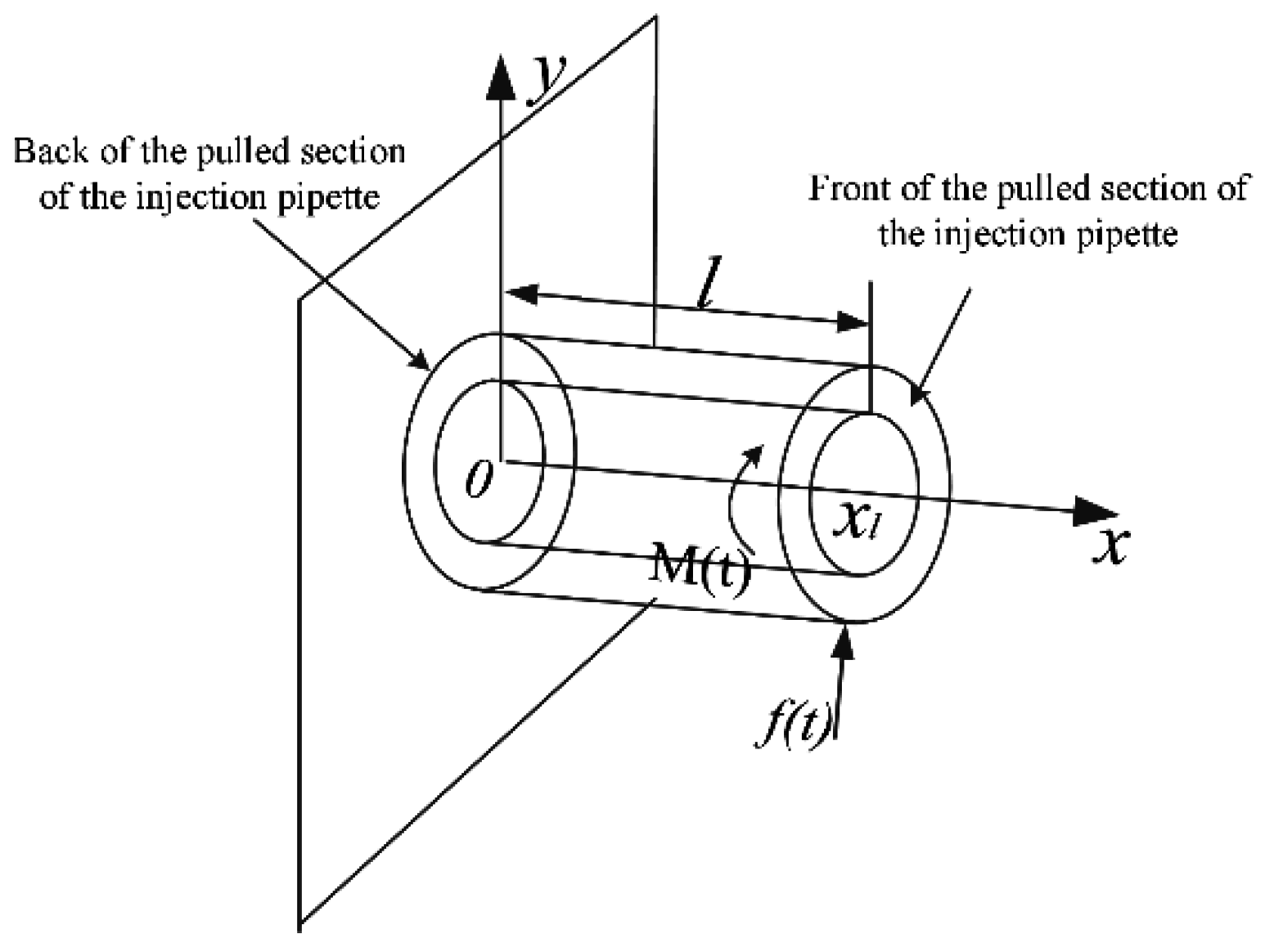
2.4. Design of the Piezo-Drill
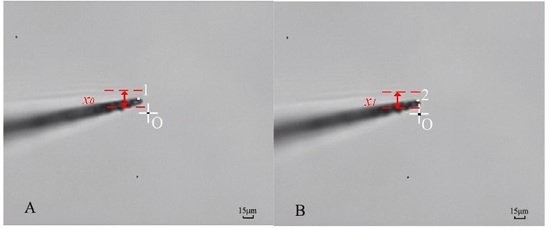
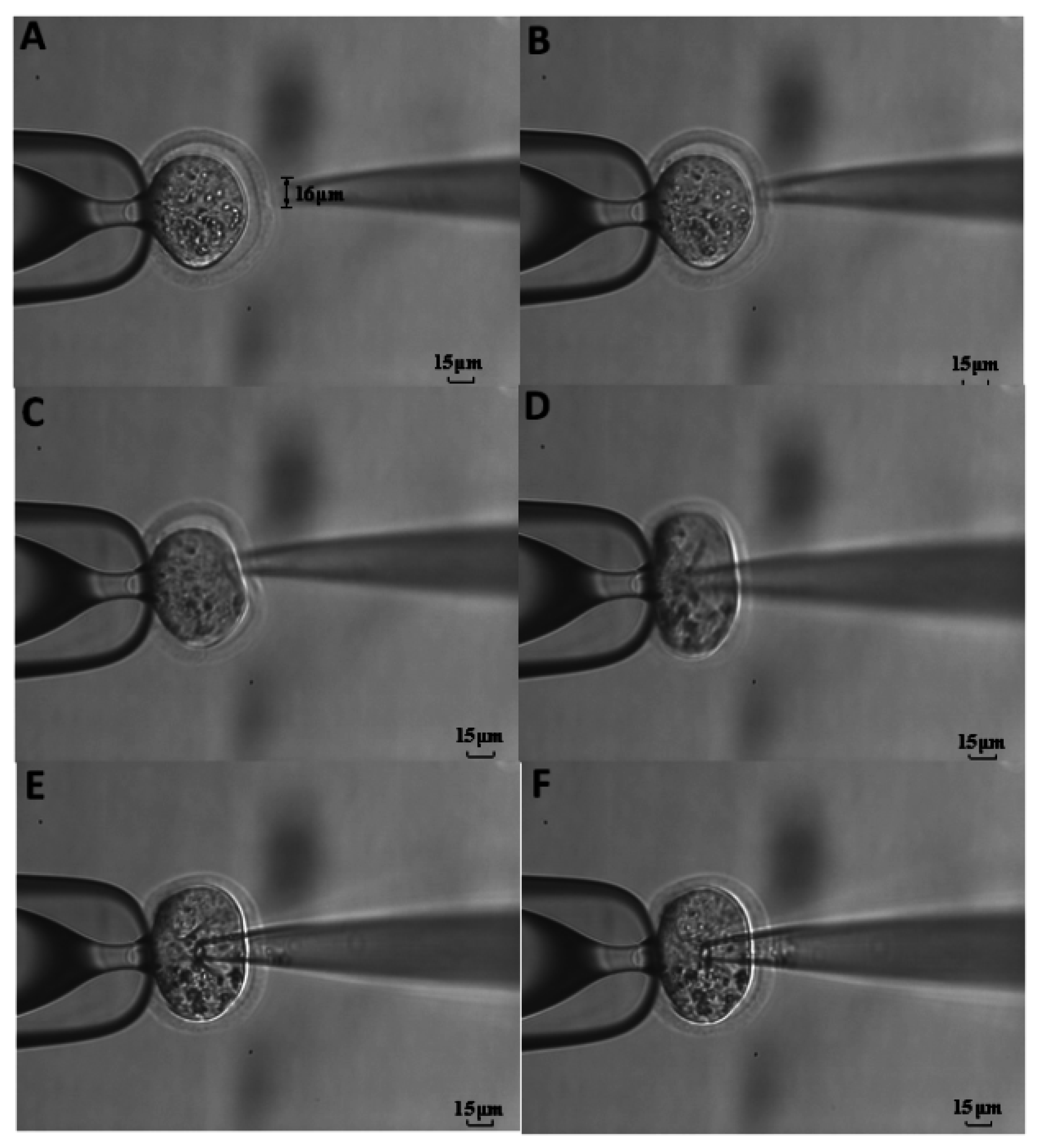
2.5. Procedure of Cellular Piercing
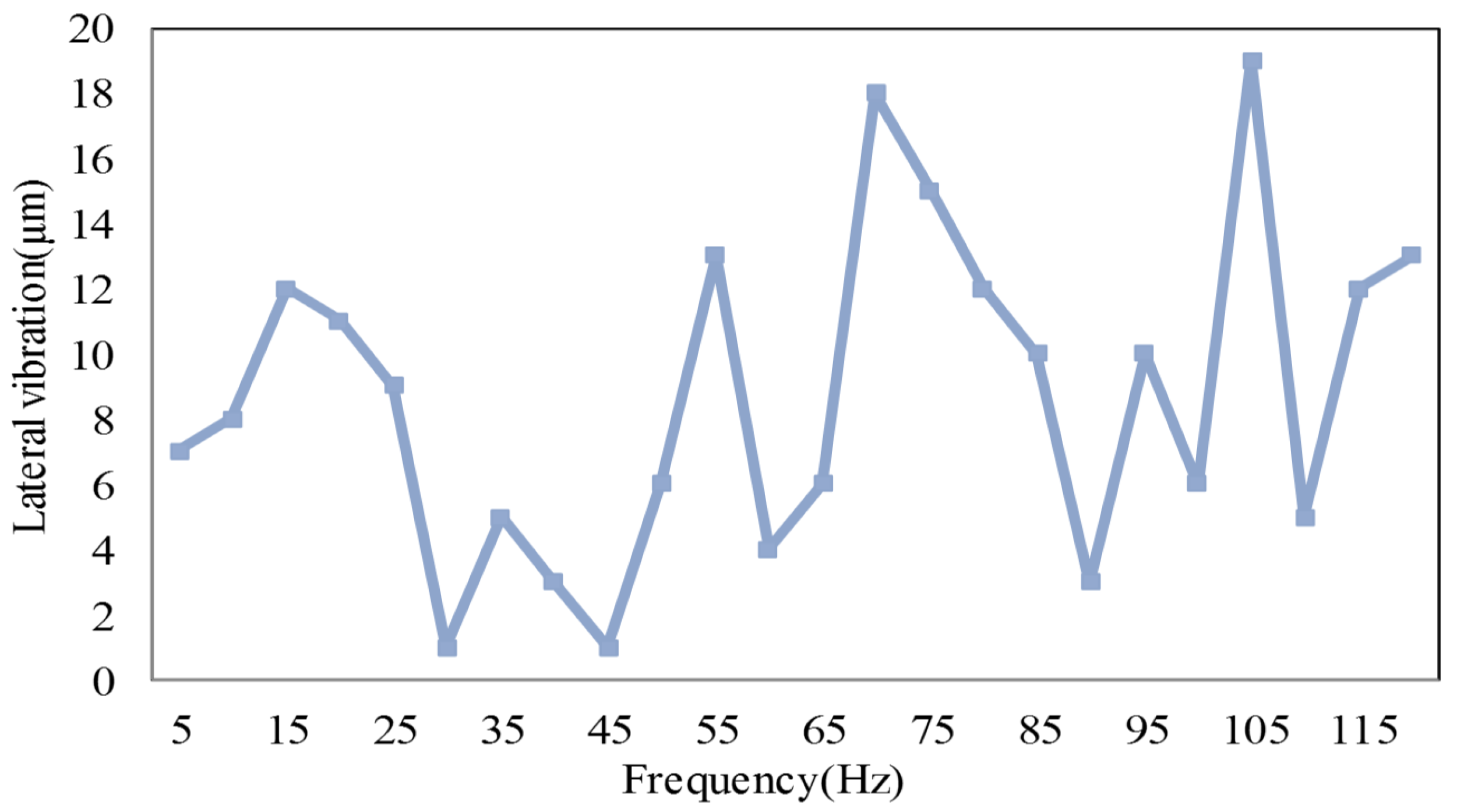
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palermo, G.; Joris, H.; Devroey, P.; van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Plachot, M.; Belaisch-Allart, J.; Mayenga, J.M.; Chouraqui, A.; Tesquier, L.; Serkine, A.M. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum. Reprod. 2002, 17, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Katayose, H.; Yazawa, H.; Kimura, Y.; Konnai, K.; Sato, A. The usefulness of a piezo-micromanipulator in intracytoplasmic sperm injection in humans. Hum. Reprod. 1999, 4, 448–453. [Google Scholar] [CrossRef]
- Katayose, H.; Yanagida, K.; Shinoki, T.; Kawahara, T.; Horiuchi, T.; Sato, A. Efficient injection of bull spermatozoa into oocytes using a Piezo-driven pipette. Theriogenology 1999, 52, 1215–1224. [Google Scholar] [CrossRef]
- Kimura, Y.; Yanagimachi, R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995, 52, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Wakayama, T. Development of normal mice from oocytes injected with freezes dried spermatozoa. Nat. Biotechnol. 1988, 16, 639–641. [Google Scholar]
- Dozortsev, D.; Wakaiama, T.; Ermilov, A.; Yanagimachi, R. Intracytoplasmic sperm injection in the rat. Zygote 1998, 6, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.B.; Getahun, M.N.; Wicher, D.; Hansson, B.S. Piezo controlled microinjection: An in vivo complement for in vitro sensory studies in insects. J. Neurosci. Methods 2011, 201, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, G.Y.; Lawitts, J.A.; Toner, M.; Biggers, J.D. Technical note: Mice produced by intracytoplasmic sperm injection using a modified conventional method. J. Anim. Sci. 2012, 90, 3739–3742. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Perry, A.C. Piezo-actuated mouse intracytoplasmic sperm injection (ICSI). Nat. Protoc. 2007, 2, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Markert, C.L. Fertilization of mammalian eggs by sperm injection. J. Exp. Zool. 1983, 228, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kawase, Y.; Iwata, T.; Watanabe, M.; Kamada, N.; Ueda, O.; Suzuki, H. Application of the piezo-micromanipulator for injection of embryonic stem cells into mouse blastocysts. J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 31–34. [Google Scholar]
- Ergenc, A.F.; Olgac, N. New technology for cellur piercing: Rotationally oscilating u-injector, description and validation tests. Biomed. Microdevices 2007, 9, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Ediz, K.; Olgac, N. Effect of the mercury column on the microdynamics of the piezo-driven pipettes. J. Biomech. Eng. 2005, 127, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Chen, Z.; Montgomery-Smith, S. Improved material point method for simulating the zona failure response in piezo-assisted intracytoplasmic sperm injection. Comput. Model. Eng. Sci. 2011, 73, 45. [Google Scholar] [CrossRef]
- Huang, H.; Hao, S.; Chen, H.; James, K. Piezoelectric driven non-toxic injector for automated cell manipulation. In Proceedings of the Medicine Meets Virtual Reality Conference (MMVR), Laško, Slovenia, 14–15 April 2011; pp. 231–235.
- Liu, J.; Lee, G.; Lawitts, J.; Toner, M.; Biggers, J.D. Mice produced by ICSI using a conventional injection pipette without Piezo. Biol. Reprod. 2011, 85, 731. [Google Scholar]
- Huang, H.; Mills, J.K.; Sun, D. A new piezo-driven ultrasonic cell microinjection system. In Proceedings of the 2010 IEEE International Conference on Information and Automation (ICIA), Harbin, China, 20–23 June 2010; pp. 18–23.
- Ediz, K.; Olgac, N. Microdynamics of the piezo-driven pipettes in ICSI. IEEE Trans. Biomed. Eng. 2004, 51, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.T.; Chan, V.; Dillard, D.A. Constitutive equation for elastic indentation of a thin-walled bio-mimetic microcapsule by an atomic force microscope tip. Colloids Surf. B Biointerfaces 2003, 27, 241–248. [Google Scholar] [CrossRef]


| Replicate | Number of Oocytes Prepared | Number of Oocytes Cleaved | Cleavage (%) |
|---|---|---|---|
| 1 | 13 | 10 | 76.9 |
| 2 | 21 | 17 | 81.0 |
| 3 | 19 | 16 | 84.2 |
| 4 | 25 | 20 | 80.0 |
| 5 | 27 | 22 | 81.5 |
| Total | 105 | 85 | 80.1 |
| Frequency (Hz) | 30 | 45 | ||
|---|---|---|---|---|
| Cleavage (%) | ||||
| Voltage (V) | ||||
| 30 (membrane) | 0 | 90 | ||
| 75 (zona pellucida) | 50 | 100 | ||
| Replicate | Number of Oocytes Prepared | Number of Oocytes Cleaved | Cleavage (%) |
|---|---|---|---|
| 1 | 13 | 12 | 92.3 |
| 2 | 21 | 19 | 90.5 |
| 3 | 19 | 18 | 94.7 |
| 4 | 25 | 22 | 88.0 |
| 5 | 27 | 25 | 92.6 |
| 6 | 12 | 11 | 91.7 |
| Total | 117 | 103 | 91.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, C.; Pan, P.; Chen, R. The Development of Piezo-Driven Tools for Cellular Piercing. Appl. Sci. 2016, 6, 314. https://doi.org/10.3390/app6110314
Ru C, Pan P, Chen R. The Development of Piezo-Driven Tools for Cellular Piercing. Applied Sciences. 2016; 6(11):314. https://doi.org/10.3390/app6110314
Chicago/Turabian StyleRu, Changhai, Peng Pan, and Ruihua Chen. 2016. "The Development of Piezo-Driven Tools for Cellular Piercing" Applied Sciences 6, no. 11: 314. https://doi.org/10.3390/app6110314