Effect of Tillage Technology Systems for Seed Germination Rate in a Laboratory Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Locality and Field Experiment
- (i)
- Conventional tillage with ploughing (CT)—after the harvest of the preceding crop, ploughing to a depth of 0.20–0.24 m followed (medium deep) by the swivelling reversible Lemken plough. In the spring period, a sowing followed using the Accord seeding combination.
- (ii)
- Minimum tillage (MT)—the harvest of preceding crop was followed by the shallow tillage using a chisel-type soil loosener Kverneland to a depth of 0.10 m, after which a sowing followed in spring using the Accord seeding combination.
- (iii)
- Direct sowing (NT)—the soil surface was not treated after the harvest of the preceding crop and am Accord seeding combination was used for sowing. Pre-sowing soil preparation for seeding depth was made only in maize and sugar beet.
2.2. Methodology of Soil Sampling and Preparation of Soil Leachates
2.3. Methodology of Laboratory Experiments with Germination
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariotti, M.; Masoni, A.; Ercoli, L.; Arduini, I. Above- and below-ground competition between barley, wheat, lupin and vetch in a cereal and legume intercropping system. Grass Forage Sci. 2009, 64, 401–412. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 2012; 368p. [Google Scholar]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Qin, F.; Liu, S.; Yu, S. Effects of allelopathy and competition for water and nutrients on survival and growth of tree species in Eucalyptus urophylla plantations. For. Ecol. Manag. 2018, 424, 387–395. [Google Scholar] [CrossRef]
- Chon, S.U.; Nelson, C.J. Allelopathy in Compositae plants. A review. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Khaliq, A.; Al-Temimi, A.A.; Matloob, A. Integration of sunflower (Helianthus annuus) residues with a pre-plant herbicide enhances weed suppression in broad bean (Vicia faba). Planta Daninha 2011, 29, 849–859. [Google Scholar] [CrossRef][Green Version]
- TeKrony, D.M.; Egli, D.B.; Wickham, D.A. Corn seed vigour effect on no-tillage field performance. I. Field emergence. Crop Sci. 1989, 29, 1523–1528. [Google Scholar] [CrossRef]
- Wicks, G.A.; Crutchfield, D.A.; Burnside, O.C. Influence of wheat (Triticum aestivum) straw mulch and metolachlor on corn (Zea mays) growth and yield. Weed Sci. 1994, 42, 141–147. [Google Scholar] [CrossRef]
- Ngetich, K.F.; Diels, J.; Shisanya, C.A.; Mugwe, J.N.; Mucherumuna, M.; Mugendi, D.N. Effects of selected soil and water conservation techniques on runoff, sediment yield and maize productivity under sub-humid and semi-arid conditions in Kenya. Catena 2014, 121, 288–296. [Google Scholar] [CrossRef]
- Tuure, J.; Räsänen, M.; Hautala, M.; Pellikka, P.; Mäkelä, P.S.A.; Alakukku, L. Plant residue mulch increases measured and modelled soil moisture content in the effective root zone of maize in semi-arid Kenya. Soil Tillage Res. 2021, 209, 104945. [Google Scholar] [CrossRef]
- Cook, H.F.; Valdes, G.S.B.; Lee, H.C. Mulch effects on rainfall interception, soil physical characteristics and temperature under Zea mays L. Soil Tillage Res. 2006, 91, 227–235. [Google Scholar] [CrossRef]
- Alsaadawi, I.S. Allelopathic influence of decomposing wheat residues in agroecosystems. J. Crop Prod. 2001, 4, 185–196. [Google Scholar] [CrossRef]
- Morris, N.L.; Miller, P.C.H.; Orson, J.H.; Froud-Williams, R.J. The effect of wheat straw residue on the mergence and early growth of sugar beet (Beta vulgaris) and oilseed rape (Brassica napus). Eur. J. Agron. 2009, 30, 151–162. [Google Scholar] [CrossRef]
- Tisdall, J.M. Crop establishment a serious limitation to high productivity. Soil Tillage Res. 1996, 40, 1–2. [Google Scholar] [CrossRef]
- Gu, S.; Wu, S.; Guan, Y.; Zhai, C.; Zhang, Y.; Bello, A.; Guo, X.; Yang, W. Arbuscular mycorrhizal fungal community was affected by tillage practices rather than residue management in black soil of northeast China. Soil Tillage Res. 2020, 198, 104552. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Han, M.; Sun, L.; Gan, D.; Fu, L.; Zhu, B. Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biol. Biochem. 2020, 151, 108019. [Google Scholar] [CrossRef]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Zhao, Q.; Thompson, A.M.; Callister, S.J.; Tfaily, M.M.; Bell, S.L.; Hobbie, S.E.; Hofmockel, K.S. Dynamics of organic matter molecular composition under aerobic decomposition and their response to the nitrogen addition in grassland soils. Sci. Total Environ. 2022, 806, 150514. [Google Scholar] [CrossRef] [PubMed]
- Marendiak, D.; Kopčanová, L.; Leitgeb, S. Poľnohospodárska Mikrobiológia; Príroda: Bratislava, Slovakia, 1987; 444p. (In Slovak) [Google Scholar]
- Ocio, J.A.; Brookes, P.C.; Jenkinson, D.S. Field incorporation of straw and its effects on soil microbial biomass and soil inorganic N. Soil Biol. Biochem. 1991, 23, 171–176. [Google Scholar] [CrossRef]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Ning, X.; Wang, X.; Guan, Z.; Gu, Y.; Wu, C.; Hu, W. Effects of different patterns of maize-straw application on soil microorganisms, enzyme activities, and grain yield. Bioengineered 2021, 12, 3684–3698. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Tillage Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Tuure, J.; Korpela, A.; Hautala, M.; Hakojärvi, M.; Mikkola, H.; Räsänen, M.; Duplissy, J.; Pellikka, P.; Petäjä, T.; Kulmala, M.; et al. Comparison of surface foil materials and dew collectors location in an arid area: A one-year field experiment in Kenya. Agric. For. Meteorol. 2019, 15, 276–277. [Google Scholar] [CrossRef]
- Baeumer, K.; Bakermans, W.A.P. Zero-tillage. Adv. Agron. 1973, 25, 77–123. [Google Scholar]
- Chellappa, J.; Sagar, K.L.; Sekaran, U.; Kumar, S.; Sharma, P. Soil organic carbon, aggregate stability and biochemical activity under tilled and no-tilled agroecosystems. J. Agric. Food Res. 2021, 4, 100139. [Google Scholar] [CrossRef]
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Mañas, P.; De las Heras, J. Phytotoxicity test applied to sewage sludge using Lactuca sativa L. and Lepidium sativum L. seeds. Int. J. Environ. Sci. Technol. 2018, 15, 273–280. [Google Scholar] [CrossRef]
- Priac, A.; Badot, P.-M.; Crini, G. Treated wastewater phytotoxicity assessment using Lactuca sativa: Focus on germination and root elongation test parameters. Comptes Rendus Biol. 2017, 340, 188–194. [Google Scholar] [CrossRef]
- Chovancova, S.; Illek, F.; Winkler, J. The effect of three tillage treatments on weed infestation in maize monoculture. Pak. J. Bot. 2020, 52, 697–701. [Google Scholar] [CrossRef]
- Małecka-Jankowiak, I.; Blecharczyk, A.; Swedzrzynska, D.; Sawinska, Z.; Piechota, T. The effect of long-term tillage systems on some soil properties and yield of pea (Pisum sativum L.). Acta Sci. Pol. Agric. 2016, 15, 37–50. [Google Scholar]
- Mancinelli, R.; Muleo, R.; Marinari, S.; Radicetti, E. How Soil Ecological Intensification by Means of Cover Crops Affects Nitrogen Use Effciency in Pepper Cultivation. Agriculture 2019, 9, 145. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Malhi, S.S.; Vera, C.L.; Zhang, Y.; Wang, J. Effects of rainfall harvesting and mulching technologies on water use efficiency and crop yield in the semi-arid Loess Plateau, China. Agric. Water Manag. 2009, 96, 374–382. [Google Scholar] [CrossRef]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems—A meta-analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, L.; Wang, J.; Ma, J.; Zheng, N.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: An in-situ uptake test using 13C, 15N labelling, and 13C-PLFA analysis. Soil Biol. Biochem. 2018, 125, 319–327. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Upadhyay, N. Effects of organophosphate pesticides on siderophore producing soils microorganisms. Biocatal. Agric. Biotechnol. 2019, 21, 101359. [Google Scholar] [CrossRef]
- Chovancova, S.; Neugschwandtner, R.; Ebrahimi, E.; Kaul, H. Effects of aqueous above-ground biomass extracts of cover crops on germination and seedlings of maize. Die Bodenkultur. Austrian J. Agric. Res. 2015, 66, 17–23. [Google Scholar]
- Marcinkevičiene, A.; Kriauciuniene, Z.; Bogužas, V.; Velička, R. Allelopathic effects of cover crops on spring barley germination and establishment. J. Food Agric. Environ. 2013, 1, 684–688. [Google Scholar]
- Gniazdowska, A.; Oracz, K.; Bogatek, R. Phytotoxic effects of sunflower (Helianthus annus L.) leaf extracts on germinating mustard (Sinapis alba L.) seeds. Allelopath. J. 2007, 19, 215–226. [Google Scholar]
- Oracz, K.; Bailly, C.; Gniazdowska, A.; Côme, D.; Corbineau, F.; Bogatek, R. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J. Chem. Ecol. 2007, 33, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Kupidłowska, E.; Gniazdowska, A.; Stepien, J.; Corbineau, F.; Vinel, D.; Skoczowski, A.; Janeczko, A.; Bogatek, R. Impact of sunflower (Helianthus annuus L.) extracts upon reserve mobilization and energy metabolism in germinating mustard (Sinapis alba L.) seeds. J. Chem. Ecol. 2006, 32, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, S.; Neudert, L.; Winkler, J. The effect of three soil tillage treatments on weed infestation in forage maize. Acta Agrobot. 2019, 72, 1756. [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M.; Bronowicka-Mielniczuk, U.; Łukasz, J. Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage Systém. Agriculture 2020, 10, 208. [Google Scholar] [CrossRef]
- Winkler, J.; Trojan, V.; Hrubešová, V. Effects of the tillage technology and the forecrop on weeds in stands of winter wheat. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 477–483. [Google Scholar] [CrossRef]

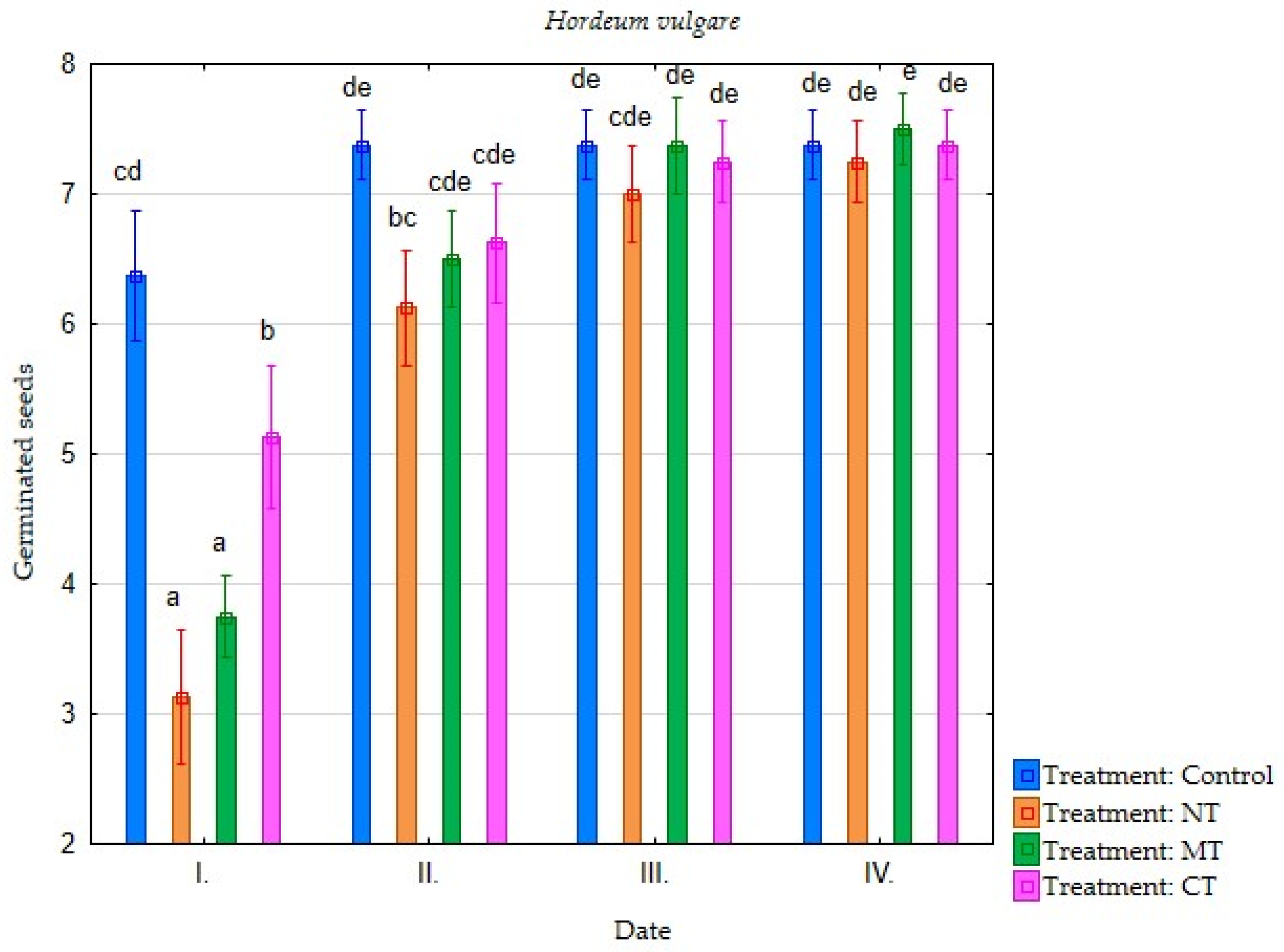
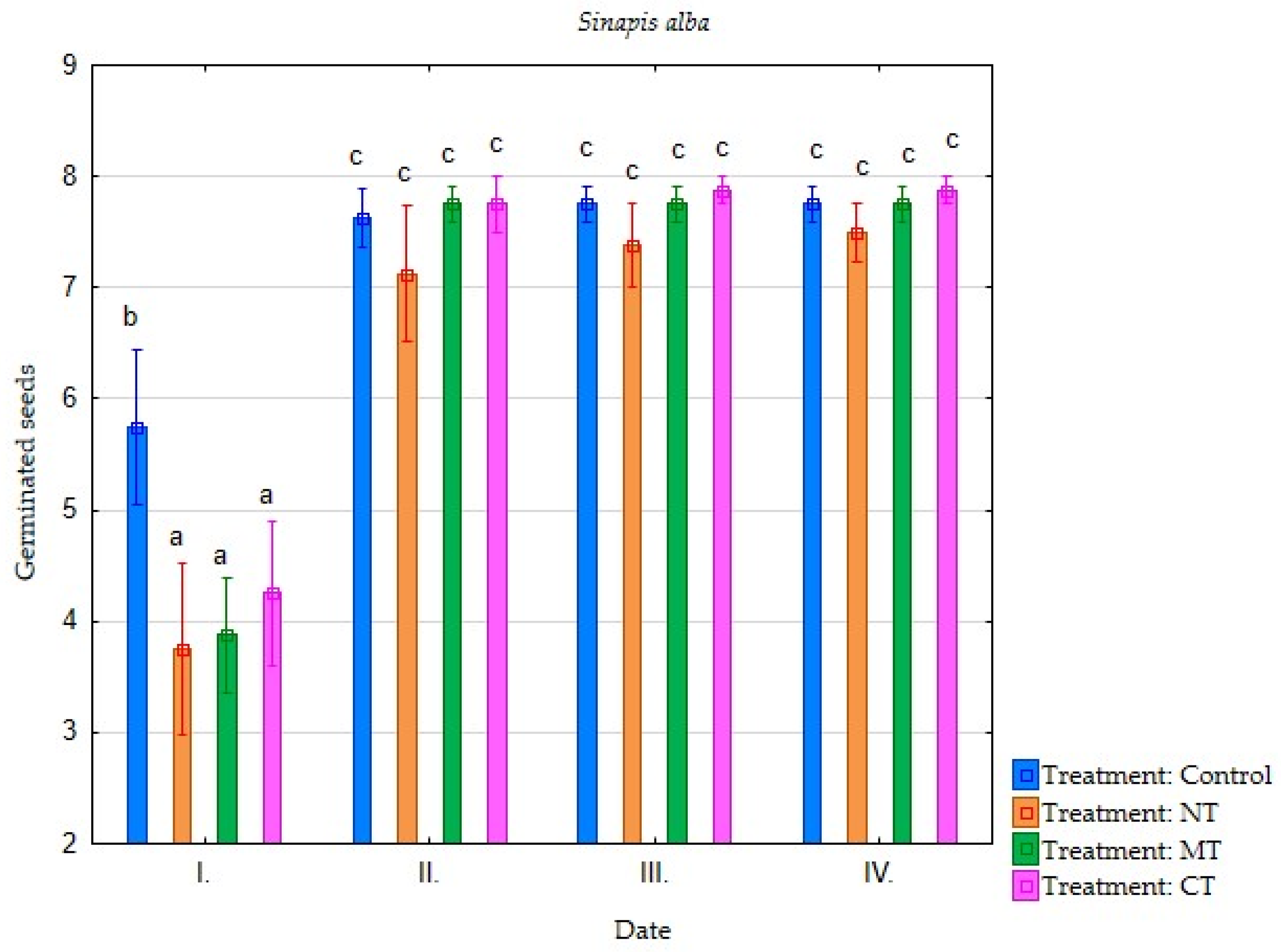
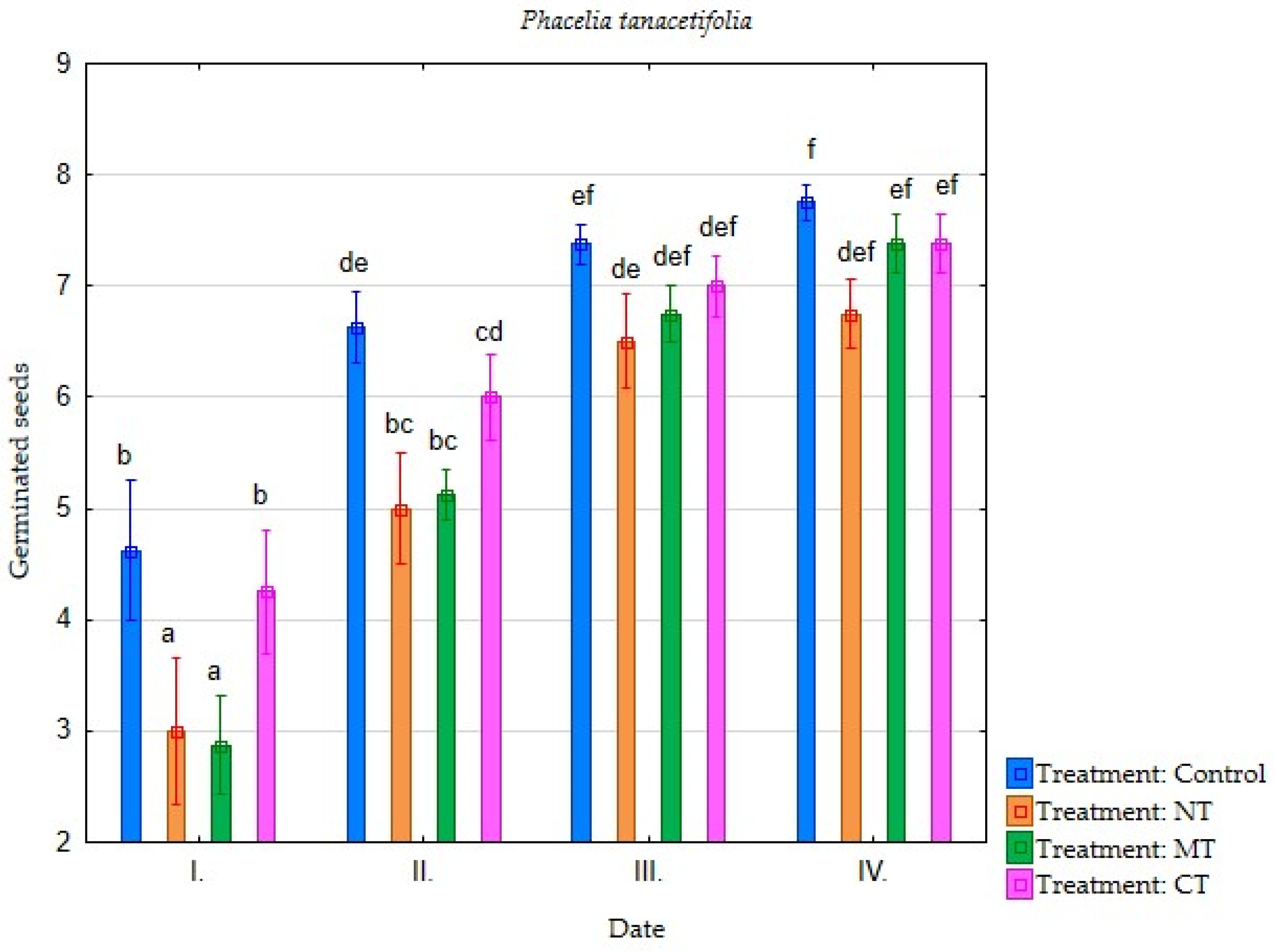
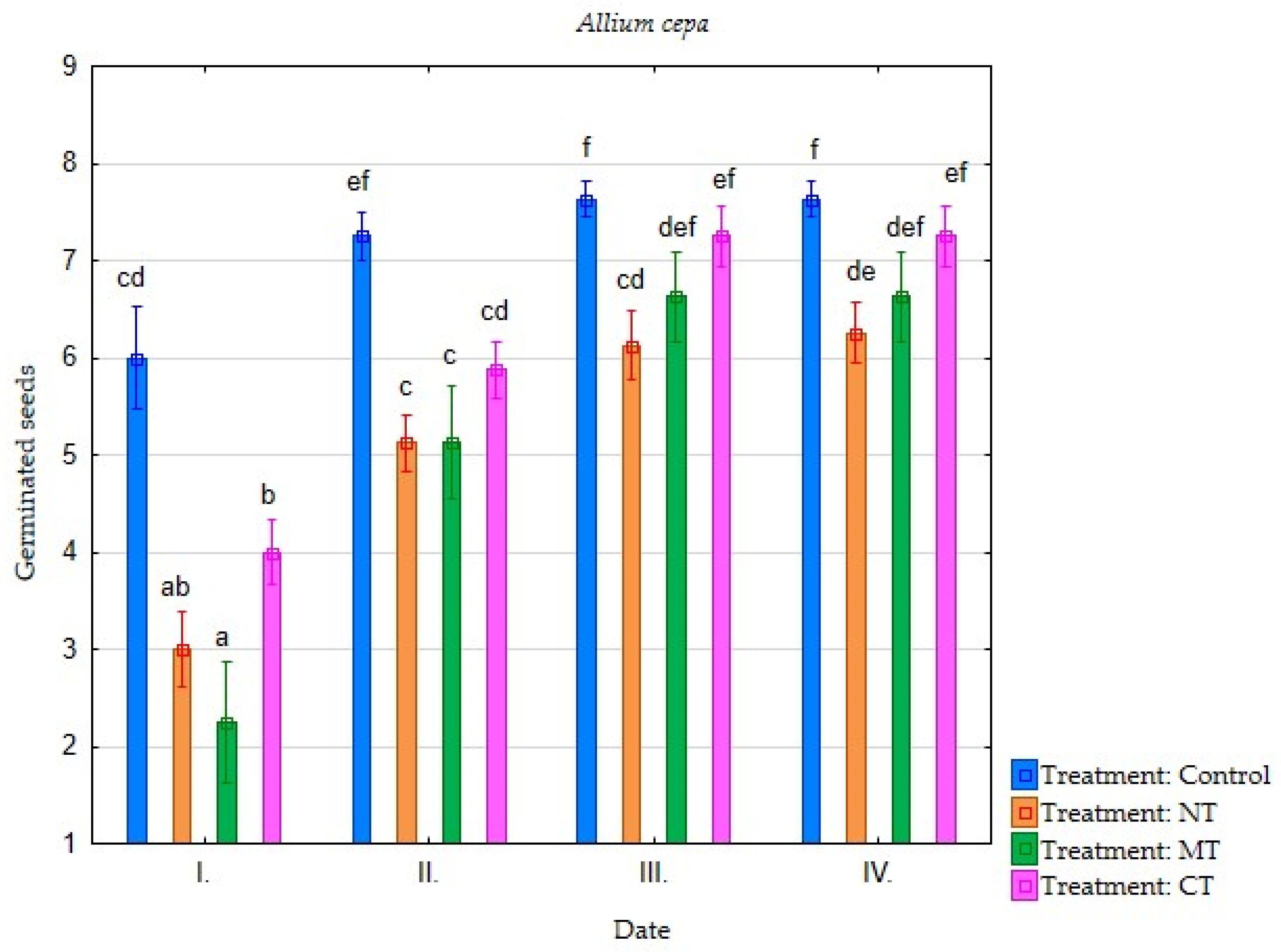
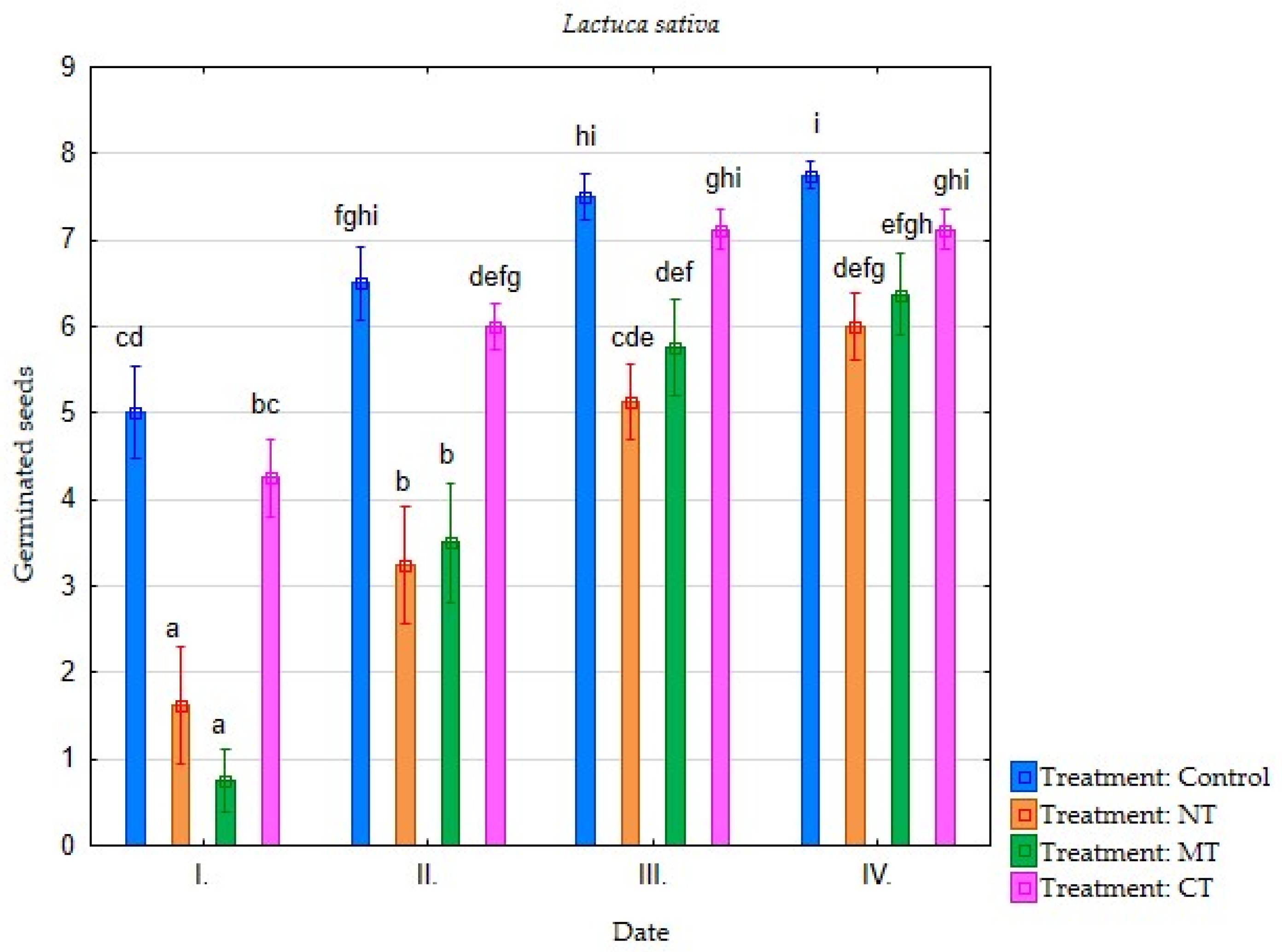
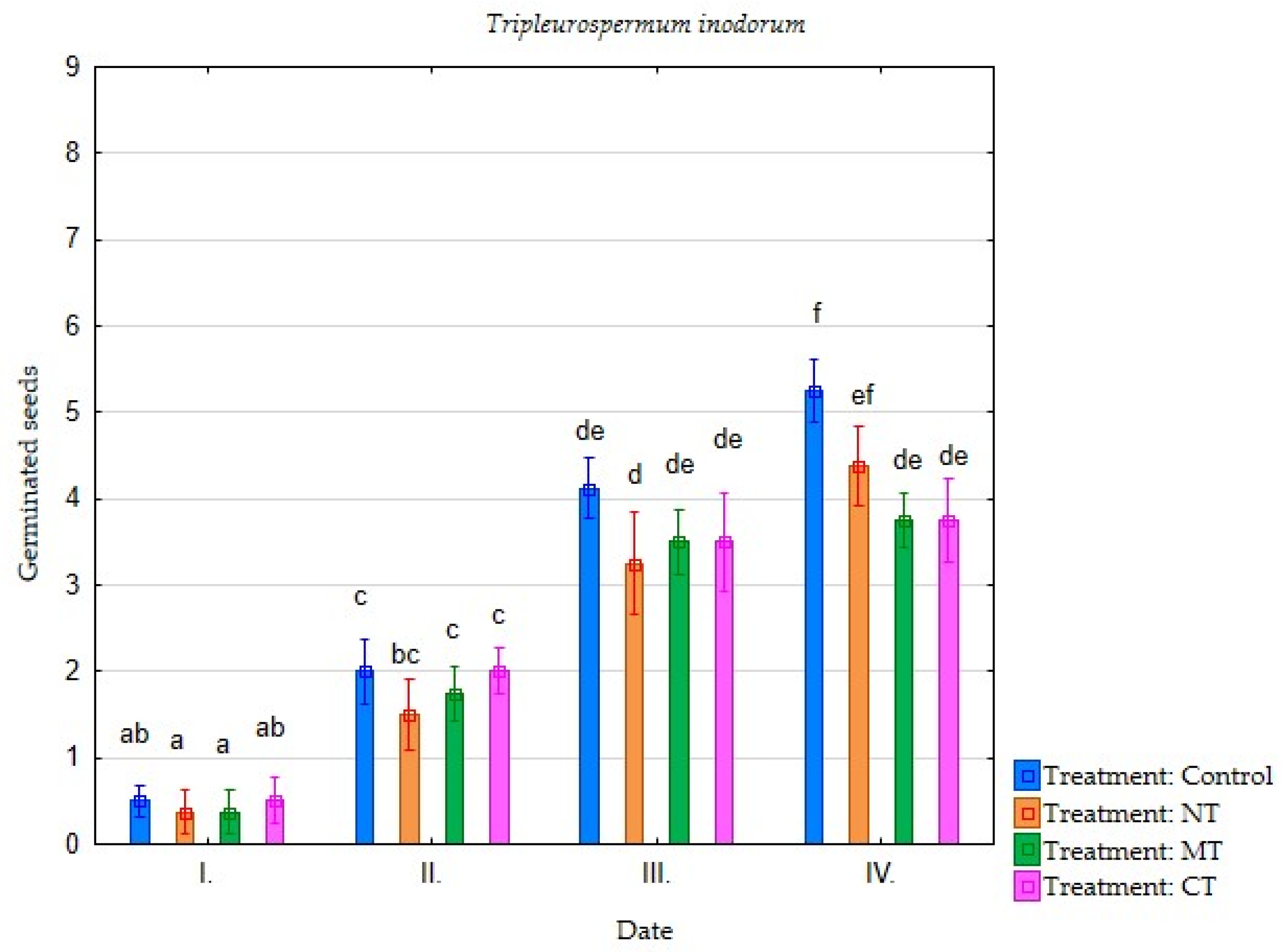
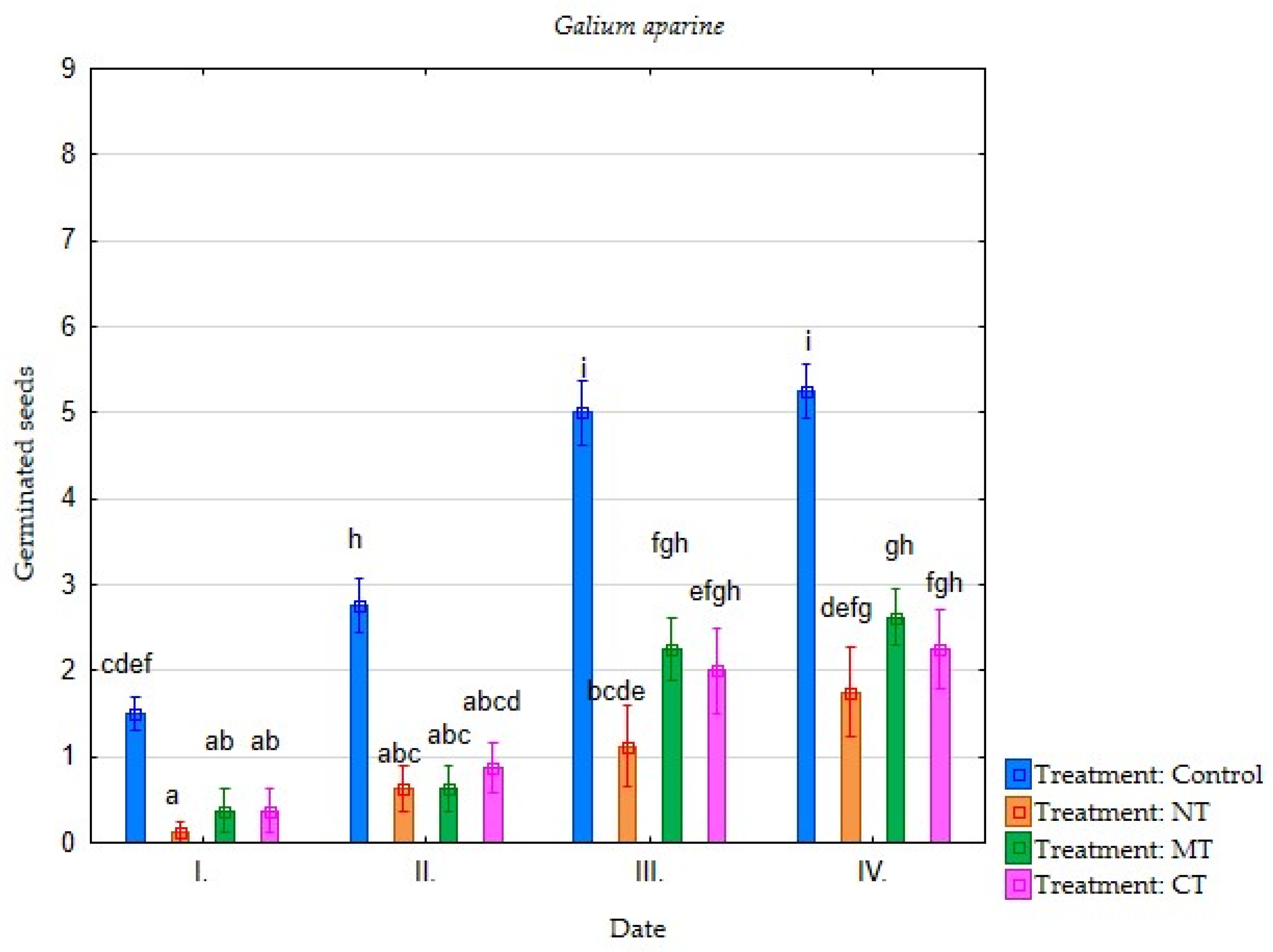
| Irrigation with Soil Leachate | Terms of Evaluation (Date) | Average Germination of Selected Plant Species (Number of Germinated Seeds) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Triticum aestivum | Hordeum vulgare | Sinapis alba | Phacelia tanacetifolia | Allium cepa | Lactuca sativa | Tripleurospermum inodorum | Galium aparine | ||
| Minimum tillage (MT) | I. | 4.25 | 3.75 | 3.88 | 2.88 | 2.25 | 0.75 | 0.38 | 0.38 |
| II. | 7.25 | 6.50 | 7.75 | 5.13 | 5.13 | 3.50 | 1.75 | 0.63 | |
| III. | 7.38 | 7.38 | 7.75 | 6.75 | 6.63 | 5.75 | 3.50 | 2.25 | |
| IV. | 7.38 | 7.50 | 7.75 | 7.38 | 6.63 | 6.38 | 3.75 | 2.63 | |
| Direct sowing (NT) | I. | 2.88 | 3.13 | 3.75 | 3.00 | 3.00 | 1.63 | 0.38 | 0.13 |
| II. | 5.88 | 6.13 | 7.13 | 5.00 | 5.13 | 3.25 | 1.50 | 0.63 | |
| III. | 7.13 | 7.00 | 7.38 | 6.50 | 6.13 | 5.13 | 3.25 | 1.13 | |
| IV. | 7.25 | 7.25 | 7.50 | 6.75 | 6.25 | 6.00 | 4.38 | 1.75 | |
| Conventional tillage CT) | I. | 5.38 | 5.13 | 4.25 | 4.25 | 4.00 | 4.25 | 0.50 | 0.38 |
| II. | 7.38 | 6.63 | 7.75 | 6.00 | 5.88 | 6.00 | 2.00 | 0.88 | |
| III. | 7.63 | 7.25 | 7.88 | 7.00 | 7.25 | 7.13 | 3.50 | 2.00 | |
| IV. | 7.63 | 7.38 | 7.88 | 7.38 | 7.25 | 7.13 | 3.75 | 2.25 | |
| Water (Control) | I. | 6.13 | 6.38 | 5.75 | 4.63 | 6.00 | 5.00 | 0.50 | 1.50 |
| II. | 7.75 | 7.38 | 7.63 | 6.63 | 7.25 | 6.50 | 2.00 | 2.75 | |
| III. | 7.88 | 7.38 | 7.75 | 7.38 | 7.63 | 7.50 | 4.13 | 5.00 | |
| IV. | 7.88 | 7.38 | 7.75 | 7.75 | 7.63 | 7.75 | 5.25 | 5.25 | |
| Source of Variance | Triticum aestivum | Hordeum vulgare | Sinapis alba | Phacelia tanacetifolia | Allium cepa | Lactuca sativa | Tripleurospermum inodorum | Galium aparine |
|---|---|---|---|---|---|---|---|---|
| Treatment | *** | *** | NS | *** | *** | *** | NS | *** |
| Time of evaluation | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatment × time of evaluation | * | * | NS | NS | NS | NS | NS | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, J.; Kopta, T.; Ferby, V.; Neudert, L.; Vaverková, M.D. Effect of Tillage Technology Systems for Seed Germination Rate in a Laboratory Tests. Environments 2022, 9, 13. https://doi.org/10.3390/environments9020013
Winkler J, Kopta T, Ferby V, Neudert L, Vaverková MD. Effect of Tillage Technology Systems for Seed Germination Rate in a Laboratory Tests. Environments. 2022; 9(2):13. https://doi.org/10.3390/environments9020013
Chicago/Turabian StyleWinkler, Jan, Tomáš Kopta, Vojtěch Ferby, Lubomír Neudert, and Magdalena Daria Vaverková. 2022. "Effect of Tillage Technology Systems for Seed Germination Rate in a Laboratory Tests" Environments 9, no. 2: 13. https://doi.org/10.3390/environments9020013
APA StyleWinkler, J., Kopta, T., Ferby, V., Neudert, L., & Vaverková, M. D. (2022). Effect of Tillage Technology Systems for Seed Germination Rate in a Laboratory Tests. Environments, 9(2), 13. https://doi.org/10.3390/environments9020013








