Environmental Pollution by Lost Fishing Tackle: A Systematic Assessment in Lake Eixendorf
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Collection and Treatment of Items
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2016, 92, 572–607. [Google Scholar] [CrossRef]
- Häder, D.-P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Verhougstraete, M.P.; Martin, S.L.; Kendall, A.D.; Hyndman, D.W.; Rose, J.B. Linking fecal bacteria in rivers to landscape, geochemical, and hydrologic factors and sources at the basin scale. Proc. Natl. Acad. Sci. USA 2015, 112, 10419–10424. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental effects of the Deepwater Horizon oil spill: A review. Mar. Pollut. Bull. 2016, 110, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Snyder, S.A. Critical assessment of the ubiquitous occurrence and fate of the insect repellent N,N-diethyl-m-toluamide in water. Environ. Int. 2016, 96, 98–117. [Google Scholar] [CrossRef]
- Wheate, N.J. A review of environmental contamination and potential health impacts on aquatic life from the active chemicals in sunscreen formulations. Aust. J. Chem. 2022, 75, 241–248. [Google Scholar] [CrossRef]
- Lewin, W.-C.; Arlinghaus, R.; Mehner, T. Documented and Potential Biological Impacts of Recreational Fishing: Insights for Management and Conservation. Rev. Fish. Sci. 2006, 14, 305–367. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Lorenzen, K.; Johnson, B.M.; Cooke, S.J.; Cowx, I.G. Management of freshwater fisheries, addressing habitat, people and fishes. In Freshwater Fisheries Ecology; Craig, J.F., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 557–579. [Google Scholar] [CrossRef]
- Smith, E.R.C.; Bennion, H.; Sayer, C.D.; Aldridge, D.C.; Owen, M. Recreational angling as a pathway for invasive non-native species spread: Awareness of biosecurity and the risk of long distance movement into Great Britain. Biol. Invasions 2020, 22, 1135–1159. [Google Scholar] [CrossRef]
- Roberts, A.; Munday, M.; Roche, N.; Brown, A.; Armstrong, M.; Hargreaves, J.; Pilgrim-Morrison, S.; Williamson, K.; Hyder, K. Assessing the contribution of recreational sea angling to the English economy. Mar. Policy 2017, 83, 146–152. [Google Scholar] [CrossRef]
- Bell, D.V.; Odin, N.; Torres, E. Accumulation of angling litter at game and coarse fisheries in South Wales, UK. Biol. Conserv. 1985, 34, 369–379. [Google Scholar] [CrossRef]
- Forbes, I.J. The quantity of lead shot, nylon fishing line and other litter discarded at a coarse fishing lake. Biol. Conserv. 1986, 38, 21–34. [Google Scholar] [CrossRef]
- O’Toole, A.C.; Hanson, K.C.; Cooke, S.J. The Effect of Shoreline Recreational Angling Activities on Aquatic and Riparian Habitat within an Urban Environment: Implications for Conservation and Management. Environ. Manag. 2009, 44, 324–334. [Google Scholar] [CrossRef]
- Macfayden, G.; Huntington, T.; Cappell, R. Abandoned, Lost or Otherwise Discarded Fishing Gear; UNEP Regional Seas Reports and Studies, No 185; FAO Fisheries and Aquaculture Technical Paper, No 523; UNEP: Nairobi, Kenya; FAO: Rome, Italy, 2009; p. 115. [Google Scholar]
- Wilcox, C.; Heathcote, G.; Goldberg, J.; Gunn, R.; Peel, D.; Hardesty, B.D. Understanding the sources and effects of abandoned, lost, and discarded fishing gear on marine turtles in northern Australia. Conserv. Biol. 2015, 29, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.; Łopata, I.; Osial, M. The ghost nets phenomena from the chemical perspective. Pure Appl. Chem. 2021, 93, 479–496. [Google Scholar] [CrossRef]
- Knott, J.; Nagel, C.; Geist, J. Wasted effort or promising approach—Does it make sense to build an engineered spawning ground for rheophilic fish in reservoir cascades? Ecol. Eng. 2021, 173, 106434. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Niesar, M. Nutrient digestibility of angling baits for carp, Cyprinus carpio, with implications for groundbait formulation and eutrophication control. Fish. Manag. Ecol. 2005, 12, 91–97. [Google Scholar] [CrossRef]
- Hofman, R.J. The changing focus of marine mammal conservation. Trends Ecol. Evol. 1995, 10, 462–465. [Google Scholar] [CrossRef]
- Laist, D.W. Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine Debris. Springer Series on Environmental Management; Coe, J.M., Rogers, D.B., Eds.; Springer: New York, NY, USA, 1997; pp. 99–139. [Google Scholar]
- Yoshikawa, T.; Asoh, K. Entanglement of monofilament fishing lines and coral death. Biol. Conserv. 2004, 117, 557–560. [Google Scholar] [CrossRef]
- Barbosa-Filho, M.L.; Seminara, C.I.; Tavares, D.C.; Siciliano, S.; Hauser-Davis, R.A.; da Silva Mourão, J. Artisanal fisher perceptions on ghost nets in a tropical South Atlantic marine biodiversity hotspot: Challenges to traditional fishing culture and implications for conservation strategies. Ocean Coast. Manag. 2020, 192, 105189. [Google Scholar] [CrossRef]
- Link, J.; Segal, B.; Casarini, L.M. Abandoned, lost or otherwise discarded fishing gear in Brazil: A review. Perspect. Ecol. Conserv. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Borkowski, R. Lead poisoning and intestinal perforations in a snapping turtle (Chelydra serpentina) due to fishing gear ingestion. J. Zoo Wildl. Med. 1997, 28, 109–113. Available online: http://www.jstor.org/stable/20079497 (accessed on 24 August 2022).
- Fäth, J.; Feiner, M.; Beggel, S.; Geist, J.; Göttlein, A. Leaching behavior and ecotoxicological effects of different game shot materials in freshwater. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 24. [Google Scholar] [CrossRef]
- Thomas, V.G. Chemical compositional standards for non-lead hunting ammunition and fishing weights. Ambio 2019, 48, 1072–1078. [Google Scholar] [CrossRef]
- Arnemo, J.M.; Andersen, O.; Stokke, S.; Thomas, V.G.; Krone, O.; Pain, D.J.; Mateo, R. Health and Environmental Risks from Lead-based Ammunition: Science Versus Socio-Politics. EcoHealth 2016, 13, 618–622. [Google Scholar] [CrossRef]
- Fisher, I.J.; Pain, D.J.; Thomas, V.G. A review of lead poisoning from ammunition sources in terrestrial birds. Biol. Conserv. 2006, 131, 421–432. [Google Scholar] [CrossRef]
- Sciteuhammer, A.M.; Norris, S.L. The ecotoxicology of lead shot and lead fishing weights. Ecotoxicology 1996, 5, 279–295. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- McGrath, S.P.; Reichelt-Brushett, A.J.; Butcher, P.A.; Cairns, S.C. Absorption of metals in mulloway (Argyrosomus japonicus) after ingesting nickel-plated carbon-steel hooks. Mar. Environ. Res. 2014, 99, 188–197. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, 9–11 September 2008; University of Washington Tacoma: Tacoma, WA, USA, 2009; p. 528. [Google Scholar]
- Collignon, A.; Hecq, J.-H.; Galgani, F.; Collard, F.; Goffart, A. Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica). Mar. Pollut. Bull. 2014, 79, 293–298. [Google Scholar] [CrossRef]
- Michael, P.J. Fish and Wildlife Issues Related to the Use of Lead Fishing Gear; Washington Department of Fish and Wildlife Program: Washington, DC, USA, 2006; p. 33.
- Copeland, C.; Baker, E.; Koehn, J.D.; Morris, S.G.; Cowx, I.G. Motivations of recreational fishers involved in fish habitat management. Fish. Manag. Ecol. 2017, 24, 82–92. [Google Scholar] [CrossRef]



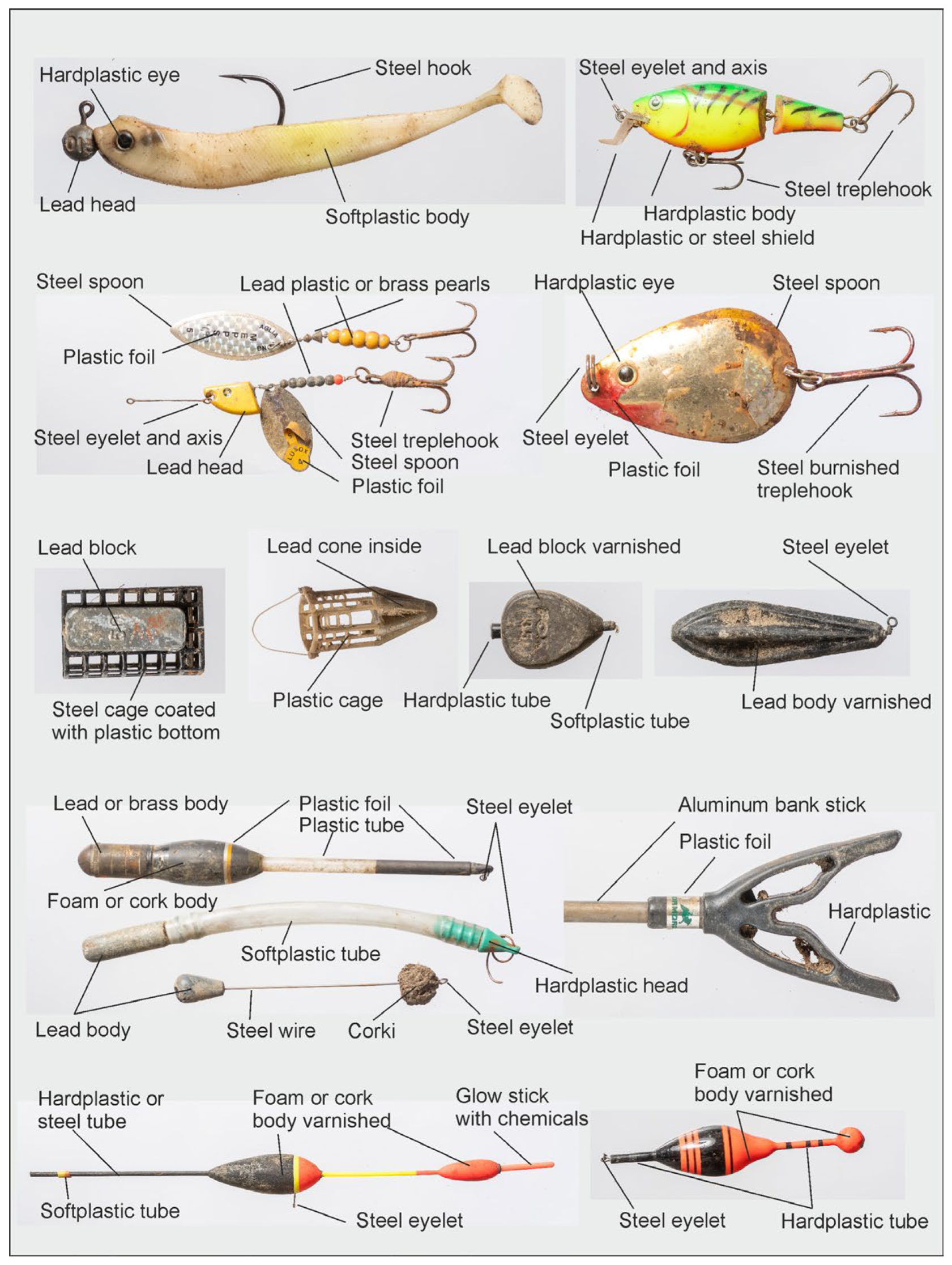
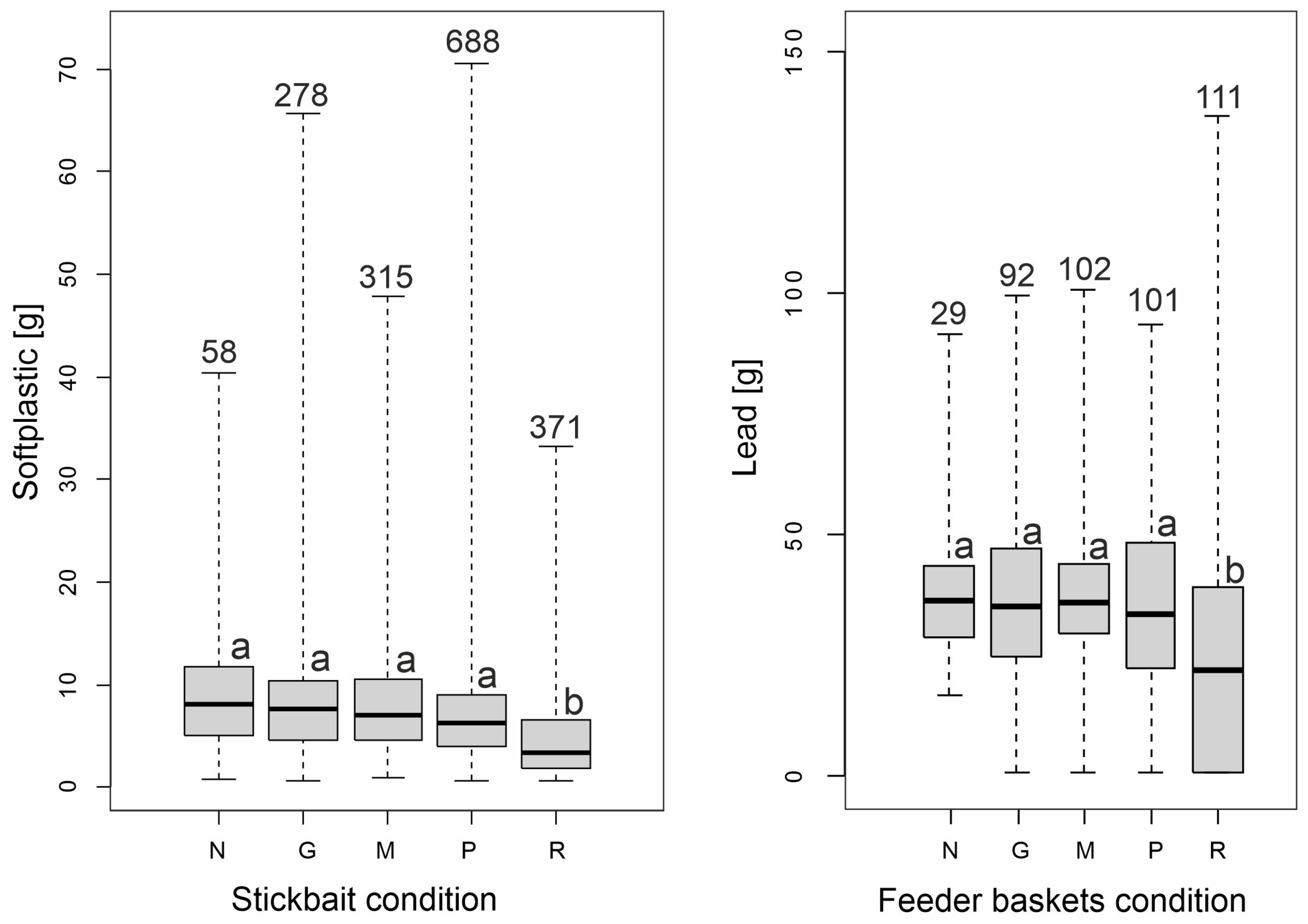

| Overall | N | G | M | P | R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | n | (g) | n | (g) | n | (g) | n | (g) | n | (g) | n | ||
| Active | Shads and twister | 26,868 | 1690 | 835 | 58 | 4695 | 278 | 5548 | 315 | 11,204 | 668 | 4621 | 371 |
| Jigheads | 2820 | 247 | 105 | 11 | 484 | 42 | 617 | 52 | 1170 | 106 | 444 | 36 | |
| Spoons | 2679 | 116 | 142 | 7 | 474 | 24 | 681 | 27 | 1072 | 47 | 311 | 12 | |
| Spinner | 1059 | 85 | 194 | 12 | 157 | 10 | 204 | 13 | 501 | 48 | 4 | 2 | |
| Lures and jerkbait | 681 | 30 | 85 | 2 | 158 | 7 | 158 | 7 | 273 | 13 | 6 | 1 | |
| Trolling devices | 551 | 40 | 53 | 3 | 208 | 15 | 170 | 14 | 107 | 7 | 12 | 1 | |
| Flies | 126 | 13 | 0 | 0 | 2 | 1 | 0 | 0 | 79 | 9 | 45 | 3 | |
| Passive | Feeder baskets | 15,717 | 435 | 1196 | 29 | 4234 | 92 | 4210 | 102 | 3912 | 101 | 2763 | 111 |
| Sinkers | 12,109 | 413 | 659 | 45 | 5219 | 148 | 2327 | 69 | 3728 | 147 | 176 | 3 | |
| Boilie bombs | 296 | 6 | 0 | 0 | 129 | 2 | 163 | 3 | 5 | 1 | 0 | 0 | |
| Antitangle boom | 287 | 213 | 0 | 0 | 99 | 73 | 47 | 31 | 125 | 89 | 13 | 17 | |
| Floats | 226 | 26 | 47 | 1 | 67 | 8 | 1 | 1 | 93 | 10 | 18 | 6 | |
| Hooks | 175 | 344 | 18 | 35 | 32 | 63 | 47 | 92 | 43 | 85 | 35 | 69 | |
| N.A. | Cast connectors | 481 | 163 | 28 | 94 | 70 | 238 | 202 | 684 | 134 | 453 | 47 | 159 |
| Not classified | 475 | 158 | 147 | 1 | 115 | 6 | 78 | 123 | 111 | 13 | 23 | 15 | |
| Weight (g) | |||||
|---|---|---|---|---|---|
| n | Overall | Mean/Item | Min | Max | |
| Steel | 4567 | 6387 | 1.4 | 0.1 | 55.6 |
| Lead | 2409 | 44,998 | 18.7 | 0.2 | 183.6 |
| Soft plastic | 1879 | 11,368 | 6.1 | 0.1 | 70.0 |
| Hard plastic | 1085 | 1642 | 1.5 | 0.1 | 65.9 |
| Copper | 13 | 47 | 3.6 | 0.2 | 10.4 |
| Balsawood | 12 | 26 | 2.1 | 0.2 | 4.8 |
| Chemicals | 11 | 25 | 2.2 | 1.0 | 4.2 |
| Brass | 11 | 22 | 2.0 | 0.2 | 6.6 |
| Aluminum | 8 | 307 | 38.3 | 4.0 | 140.0 |
| Cork | 7 | 9 | 1.3 | 1.0 | 3.0 |
| Carbon fiber/Fiberglass | 5 | 21 | 4.3 | 0.1 | 13.5 |
| Stone | 1 | 26 | - | - | - |
| Length (m) | Weight (g) | Diameter (mm) | |
|---|---|---|---|
| Monofilament line | 3138 | 104 | 0.10–1.00 |
| Braided line | 1724 | 336 | 0.08–1.20 |
| Steel leader | 334 | 309 | 0.08–0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pander, J.; Dobler, A.H.; Hoos, P.; Geist, J. Environmental Pollution by Lost Fishing Tackle: A Systematic Assessment in Lake Eixendorf. Environments 2022, 9, 144. https://doi.org/10.3390/environments9110144
Pander J, Dobler AH, Hoos P, Geist J. Environmental Pollution by Lost Fishing Tackle: A Systematic Assessment in Lake Eixendorf. Environments. 2022; 9(11):144. https://doi.org/10.3390/environments9110144
Chicago/Turabian StylePander, Joachim, Andreas H. Dobler, Philipp Hoos, and Juergen Geist. 2022. "Environmental Pollution by Lost Fishing Tackle: A Systematic Assessment in Lake Eixendorf" Environments 9, no. 11: 144. https://doi.org/10.3390/environments9110144
APA StylePander, J., Dobler, A. H., Hoos, P., & Geist, J. (2022). Environmental Pollution by Lost Fishing Tackle: A Systematic Assessment in Lake Eixendorf. Environments, 9(11), 144. https://doi.org/10.3390/environments9110144






