Assessment of the Lowland Bog Biomass for Ex Situ Remediation of Petroleum-Contaminated Soils
Abstract
1. Introduction
2. Materials and Methods
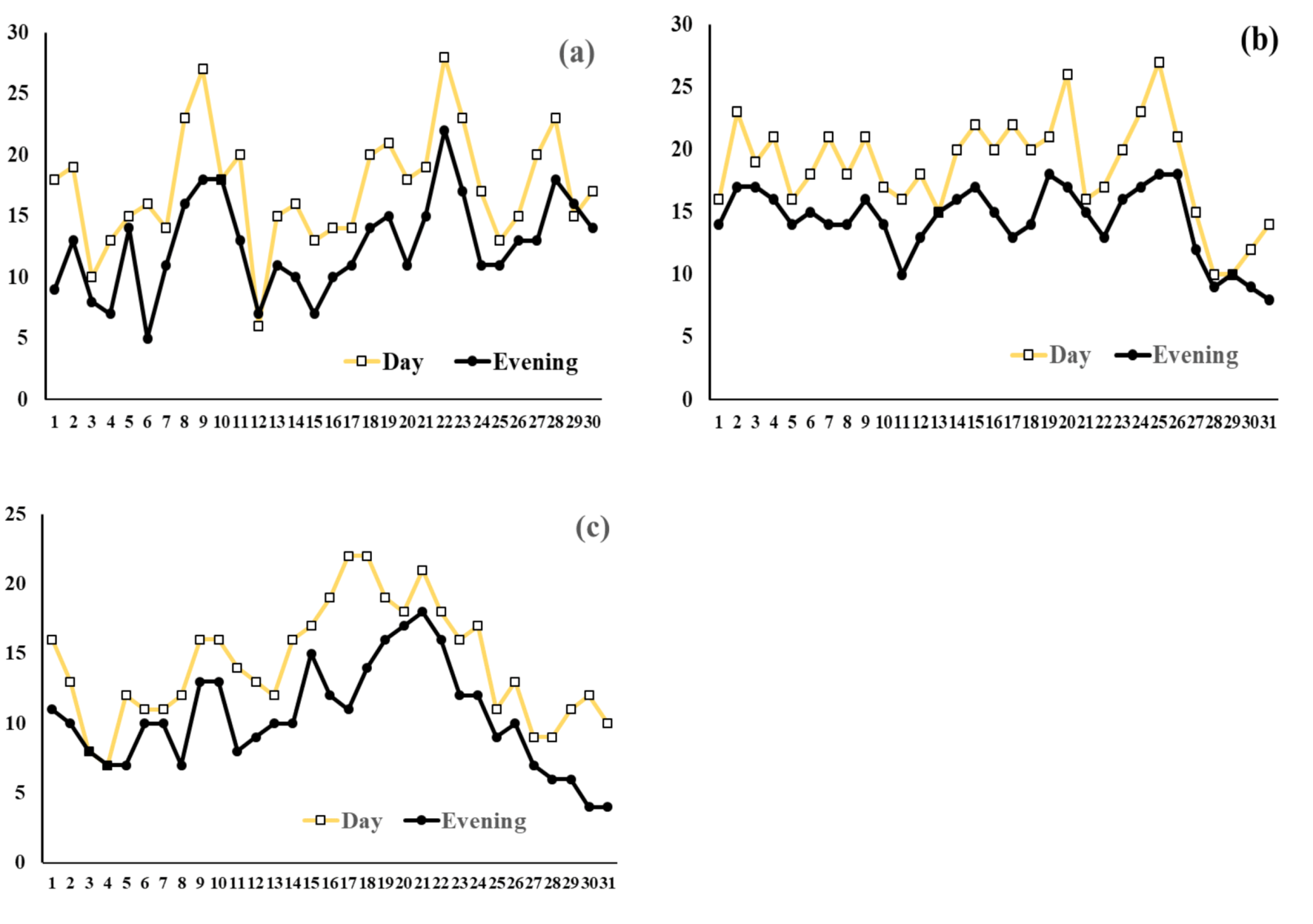
2.1. Soil Types and Ex Situ Remediation Conditions
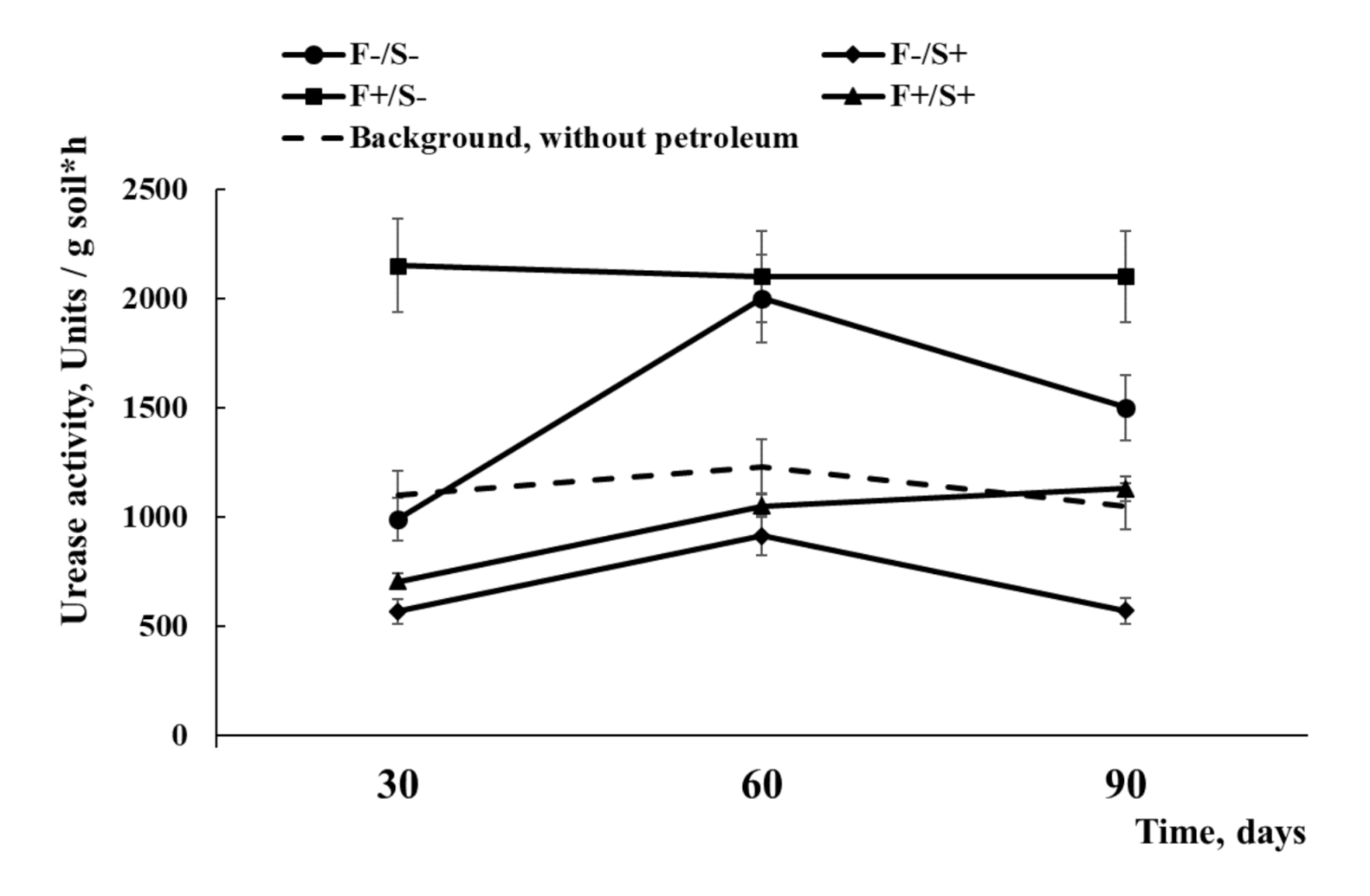
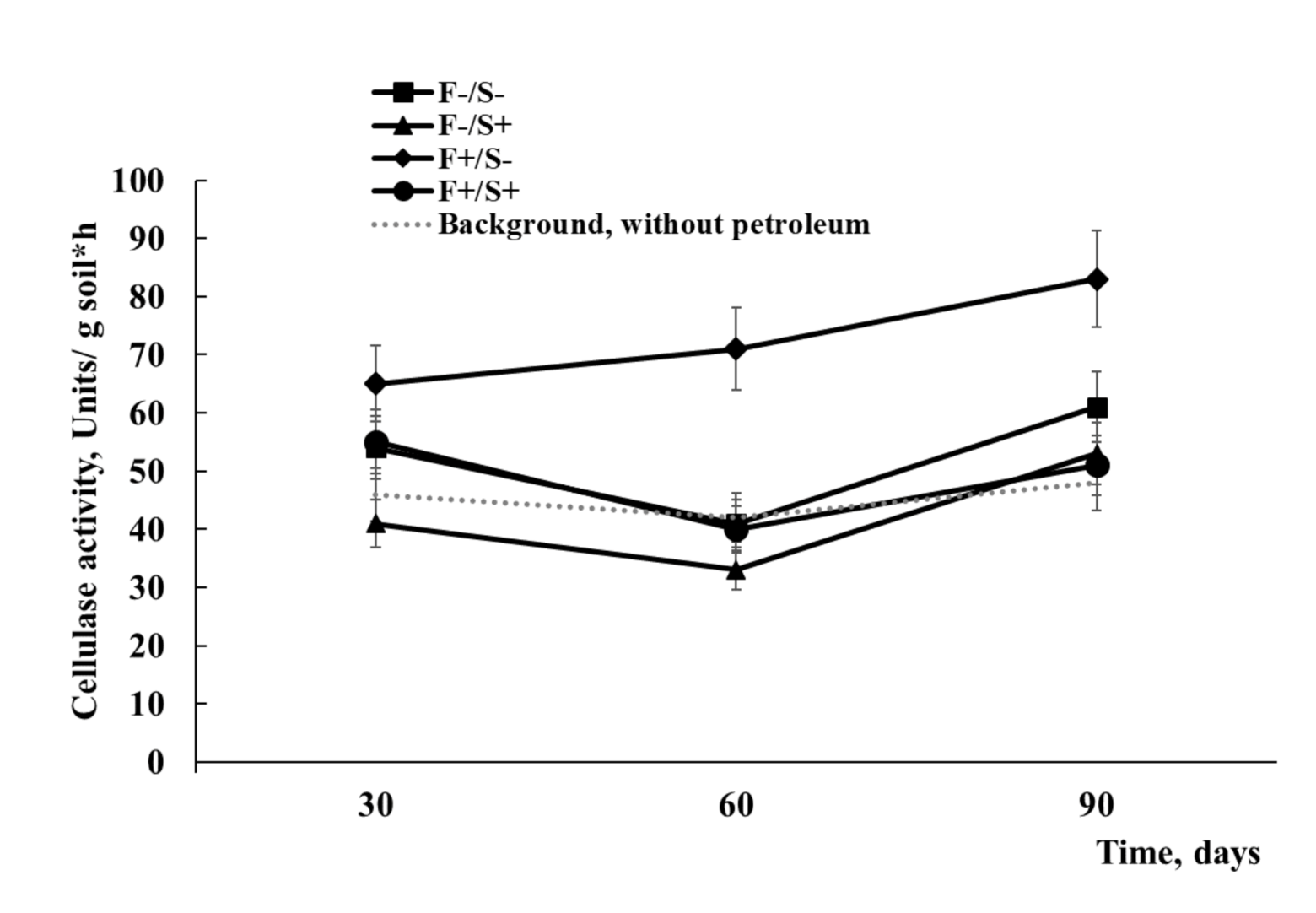
2.2. Soil Biochemical Activity Analysis
2.3. Quantification of the Content of Microscopic Fungi
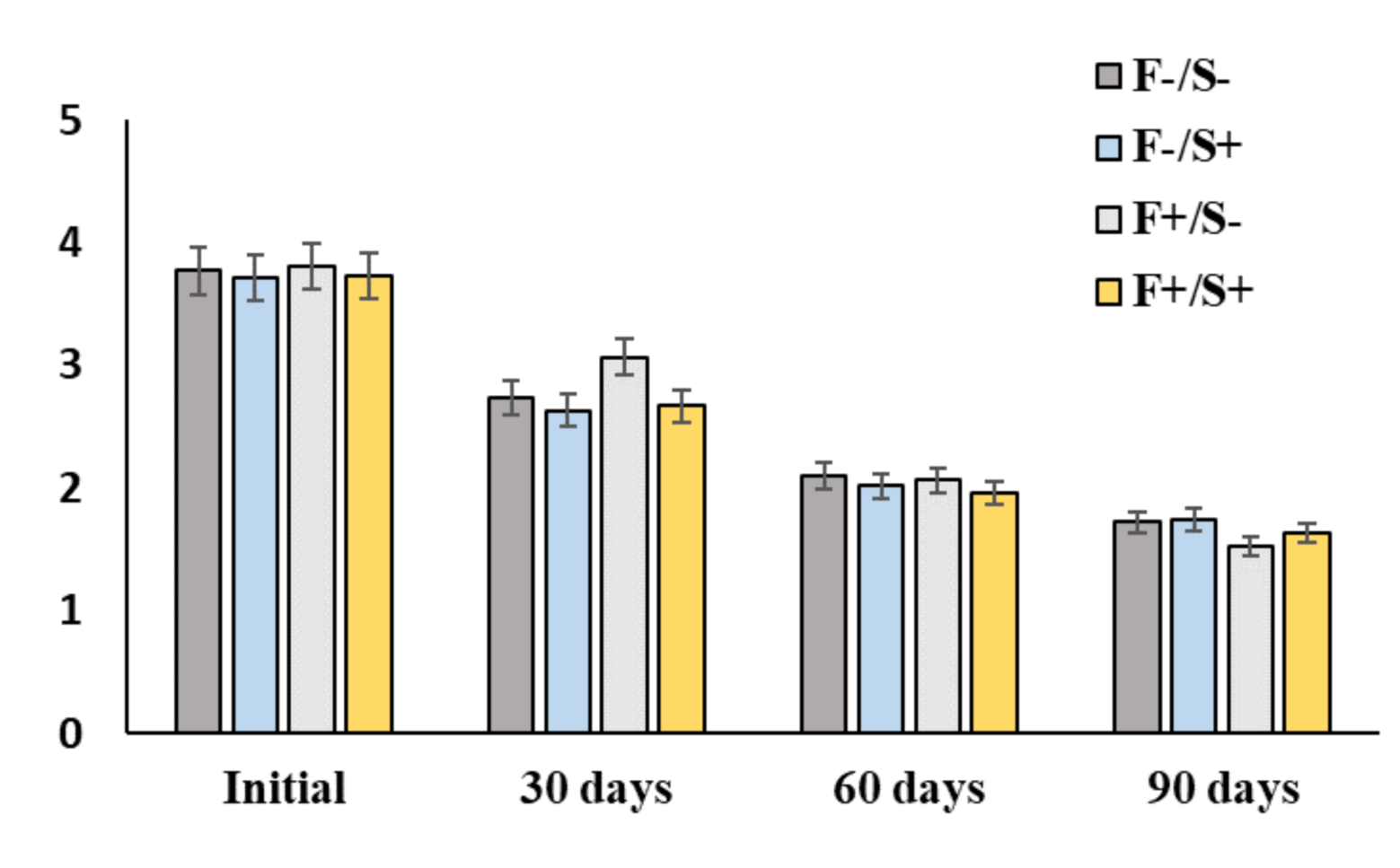
2.4. Assessment of Residual TPH Content
3. Results and Discussion
3.1. Dynamics of TPH Decomposition
3.2. Enzymatic and Microbial Diagnostics of Recultivated Bog Soil
Funding
Conflicts of Interest
References
- Barenboim, G.; Ertsev, G.; Taskaev, A.I.; Ulyashev, A.I.; Shubin, Y.P. Environmental Monitoring in the Accident Area. Experience of Elimination of Oil Spills in the Usinsk District of the Komi Republic; Syktyvkar: Komimeliovodkhozproject, Russia, 2000; pp. 83–146. (In Russian) [Google Scholar]
- Shoba, S.A.; Yakovlev, A.S.; Rybalsky, N.G. (Eds.) Ecological Standardization and Regulation of Environmental and Soils Quality and Land Management; NIA-Priroda: Moscow, Russian, 2013; 373p. (In Russian) [Google Scholar]
- Si-Zhong, Y.; Hui-Jun, J.; Zhi, W.; Rui-Xia, H.; Yan-Jun, J.; Xiu-Mei, L.; Shao-Peng, Y. Bioremediation of Oil Spills in Cold Environments: A Review. Pedosphere 2009, 19, 371–381. [Google Scholar]
- Chaudhary, D.K.; Kim, J. New insights into bioremediation strategies for oil-contaminated soil in cold environments. Int. Biodeterior. Biodegrad. 2019, 142, 58–72. [Google Scholar] [CrossRef]
- Schaefer, M.; Petersen, S.O.; Filser, J. Effects of Lumbricus terrestris, Allolobophora chlorotica and Eisenia fetida on microbial community dynamics in oil-contaminated soil. Soil Biol. Biochem. 2005, 37, 2065–2076. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, D.; Huang, R.; Huang, G.; Yuan, Y.; Fan, H. Effects of bacterial-feeding nematodes on soil microbial activity and the microbial community in oil-contaminated soil. J. Environ. Manag. 2019, 234, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Fodelianakis, S.; Antoniou, E.; Mapelli, F.; Magagnini, M.; Nikolopoulou, M.; Marasco, R.; Barbato, M.; Tsiola, A.; Tsikopoulou, I.; Giaccaglia, L.; et al. Allochthonous bioaugmentation in ex situ treatment of crude oil-polluted sediments in the presence of an effective degrading indigenous microbiome. J. Hazard. Mat. 2015, 287, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodet. Biodegr. 2018, 26, 57–68. [Google Scholar] [CrossRef]
- Lin, T.-C.; Pan, P.-T.; Cheng, S.-S. Ex situ bioremediation of oil-contaminated soil. J. Haz. Mat. 2010, 176, 27–34. [Google Scholar] [CrossRef]
- Filatov, D.A.; Gulaya, E.V.; Svarovskaya, L.I.; Altunina, L.K. Biochemical Oxidation of High Viscosity Oil by Indigenous Soil Microflora. Pet. Chem. 2013, 53, 59–64. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Gojgic-Cvijovic, G.; Milic, J.; Ilic, M.; Miletic, S.; Šolevic, T.; Vrvic, M.M. Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil)—A field experiment. Chemosphere 2011, 83, 34–40. [Google Scholar] [CrossRef]
- Nikolopoulou, M.; Pasadakis, N.; Norf, H.; Kalogerakis, N. Enhanced ex situ bioremediation of crude oil contaminated beach sand by supplementation with nutrients and rhamnolipids. Mar. Pollut. Bull. 2013, 77, 37–44. [Google Scholar] [CrossRef]
- Utobo, E.B.; Tewari, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2015, 13, 147–169. [Google Scholar]
- Taskaev, A.I. (Ed.) Virgin Forests of Komi: The UNESCO World Cultural and Natural Heritage Site; Publishing Centre Design, Information, Cartography: Moscow, Russia, 2006; 288p. (In Russian) [Google Scholar]
- World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; 106p.
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil. Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Pancholy, S.K.; Rice, E.L. Soil enzymes in relation to old field succession: Amylase, cellulase, invertase, dehydrogenase, and urease. Soil Sci. Soc. Am. J. 1973, 37, 47–50. [Google Scholar] [CrossRef]
- McCleary, B.V.; McGeough, P. A comparison of polysaccharide substrates and reducing sugar methods for the measurement of endo-1,4-β-xylanase. Appl. Biochem. Biotech. 2015, 177, 1152–1163. [Google Scholar] [CrossRef]
- Polyanskaya, L.M.; Pinchuk, I.P.; Stepanov, A.L. Comparative Analysis of the Luminescence Microscopy and Cascade Filtration Methods for Estimating Bacterial Abundance and Biomass in the Soil: Role of Soil Suspension Dilution Eurasian Soil Science. Eurasian Soil Sci. 2017, 50, 1173–1176. [Google Scholar] [CrossRef]
- Tarabukin, D.V.; Torlopov, M.A.; Shchemelinina, T.N.; Anchugova, E.M.; Shergina, N.N.; Istomina, E.I.; Belyy, V.A. Biosorbents based on esterified starch carrying immobilized oil-degrading microorganisms. J. Biotech. 2017, 260, 31–37. [Google Scholar] [CrossRef]
- Lin, X.; Yang, B.; Shen, J.; Du, N. Biodegradation of Crude Oil by an Arctic Psychrotrophic Bacterium Pseudoalteromomas sp. P29. Curr. Microbiol. 2009, 59, 341–345. [Google Scholar] [CrossRef]
- Vergeynst, L.; Kjeldsen, K.U.; Lassen, P.; Rysgaard, S. Bacterial community succession and degradation patterns of hydrocarbons in seawater at low temperature. J. Haz. Mat. 2018, 353, 127–134. [Google Scholar] [CrossRef]
- Shintani, M.; Sugiyama, K.; Sakurai, T.; Yamada, K.; Kimbara, K. Biodegradation of A-fuel oil in soil samples with bacterial mixtures of Rhodococcus and Gordonia strains under low temperature conditions. J. Biosci. Bioeng. 2019, 127, 197–200. [Google Scholar] [CrossRef]
- Daane, L.L.; Harjono, I.; Zylstra, G.J.; Häggblom, M.M. Isolation and characterization of polycyclic aromatic hydrocarbon-degrading bacteria associated with the rhizosphere of salt marsh plants. Appl. Environ. Microbiol. 2001, 67, 2683–2691. [Google Scholar] [CrossRef]
- Escalante-Espinosa, E.; Gallegos-Martínez, M.E.; Favela-Torres, E.; Gutiérrez-Rojas, M. Improvement of the hydrocarbon phytoremediation rate by Cyperus laxus Lam. inoculated with a microbial consortium in a model system. Chemosphere 2005, 59, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Pan, P.T.; Young, C.C.; Chang, J.S.; Chang, T.C.; Cheng, S.S. Evaluation of the optimal strategy for ex situ bioremediation of diesel oil-contaminated soil. Environ. Sci. Pollut. Res. 2011, 18, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Biria, D. The synergetic effect of starch and alpha amylase on the biodegradation of n-alkanes. Chemosphere 2016, 152, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-K.; Jho, E.H.; Choi, H.S.; Kang, G. Role of hemoglobin in hemoglobin-based remediation of the crude oil-contaminated soil. Sci. Total Environ. 2018, 627, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Al-Kindi, S. Effect of disturbance by oil pollution on the diversity and activity of bacterial communities in biological soil crusts from the Sultanate of Oman. Appl. Soil Ecol. 2017, 110, 88–96. [Google Scholar] [CrossRef]
- Ansari, N.; Hassanshahian, M.; Ravan, H. Study the Microbial Communities’ Changes in Desert and Farmland Soil After Crude Oil Pollution. Int. J. Environ. Res. 2018, 12, 391–398. [Google Scholar] [CrossRef]
- Melekhina, E.N.; Markarova, M.Y.; Shchemelinina, T.N.; Anchugova, E.M.; Kanev, V.A. Secondary successions of biota in oil-polluted peat soil upon different biological remediation methods. Eurasian Soil Sci. 2015, 48, 643–653. [Google Scholar] [CrossRef]
- Petrov, A.M.; Versioning, A.A.; Karimullin, L.K.; Akaikin, D.V.; Tarasov, O.Y. Dynamics of Ecological and Biological Characteristics of Soddy-Podzolic Soils under Long-Term Oil Pollution. Eurasian Soil Sci. 2016, 49, 784–791. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Tatosyan, M.L.; Aznaur’yan, D.K. Change in Enzymatic Activity of Common Chernozem Polluted with Crude Oil and Its Products in Model Experiments. Russ. Agric. Sci. 2007, 33, 318–320. [Google Scholar] [CrossRef]
- Kiss, S.; Drăgan-Bulard, M.; Paşca, D. Enzymology of the Recurvation of Technogenic Soils. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1989; Volume 42, pp. 229–278. [Google Scholar]
- Zhang, X.; Liu, Z.; Luc, N.T.; Liang, X.; Liu, X. Dynamics of the biological properties of soil and the nutrient release of Amorpha fruticosa L. litter in soil polluted by crude oil. Environ. Sci. Pollut. Res. 2015, 22, 16749–16757. [Google Scholar] [CrossRef]
- Ooshima, H.; Sakata, M.; Harano, Y. Enhancement of enzymatic hydrolysis of cellulose by surfactant. Biotechnol. Bioeng. 1986, 11, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zeng, M.; Hu, Q.; Cai, C.; Lin, X.; Qiu, X.; Yang, D. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour. Technol. 2018, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Liu, H.-F.; Tian, Z.-L.; Zhu, L.-H.; Wu, Y.-H.; Tang, H.-Q. Diesel pollution biodegradation: Synergetic effect of Mycobacterium and Filamentous Fungi. Biomed. Environ. Sci. 2008, 21, 181–187. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Ahmad, R.; Jonathan, S.G. Synergistic action of rhizospheric fungi with Megathyrsus maximus root speeds up hydrocarbon degradation kinetics in oil polluted soil. Chemosphere 2017, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.-T.; Tsang, D.C.W.; Dong, C.-D.; Chen, C.-W.; Luo, S.-G.; Kao, C.-M. Enhanced bioremediation of TCE-contaminated groundwater using gamma poly-glutamic acid as the primary substrate. J. Clean. Prod. 2018, 178, 108–118. [Google Scholar] [CrossRef]
- Agamuthu, P.; Tan, Y.S.; Fauziah, S.H. Bioremediation of hydrocarbon contaminated soil using selected organic wastes. Procedia Environ. Sci. 2013, 18, 694–702. [Google Scholar] [CrossRef]
- Mathavan, G.N.; Viraraghavan, T. Use of peat in the treatment of oily waters. Water Air Soil Pollut. 1989, 45, 17–26. [Google Scholar]
- Galiulin, R.V.; Bashkin, V.N.; Galiulina, R.A. Degradation of Petroleum Hydrocarbons in Soil under the Action of Peat Compost. Solid Fuel Chem. 2012, 46, 328–329. [Google Scholar] [CrossRef]
- Cojocaru, C.; Macoveanu, M.; Cretescu, I. Peat-based sorbents for the removal of oil spills from water surface: Application of artificial neural network modeling. Coll. Surf. A Phys. Eng. Asp. 2011, 384, 675–684. [Google Scholar] [CrossRef]


| Options | Fertilizer (Ammonium Nitrogen—10%; Total Phosphates—25%; Potassium in Terms of K2O—25%), 0.5 g per 100 g Soil Sample (F) | Nonionic Surfactant (Laurylglucoside), 10 mg per 100 g Soil Sample (S) |
|---|---|---|
| F−/S− | − | − |
| F−/S+ | − | + |
| F+/S− | + | − |
| F+/S+ | + | + |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabukin, D.V. Assessment of the Lowland Bog Biomass for Ex Situ Remediation of Petroleum-Contaminated Soils. Environments 2020, 7, 86. https://doi.org/10.3390/environments7100086
Tarabukin DV. Assessment of the Lowland Bog Biomass for Ex Situ Remediation of Petroleum-Contaminated Soils. Environments. 2020; 7(10):86. https://doi.org/10.3390/environments7100086
Chicago/Turabian StyleTarabukin, Dmitriy V. 2020. "Assessment of the Lowland Bog Biomass for Ex Situ Remediation of Petroleum-Contaminated Soils" Environments 7, no. 10: 86. https://doi.org/10.3390/environments7100086
APA StyleTarabukin, D. V. (2020). Assessment of the Lowland Bog Biomass for Ex Situ Remediation of Petroleum-Contaminated Soils. Environments, 7(10), 86. https://doi.org/10.3390/environments7100086





