Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composites Preparation
2.2. Composite Characterization
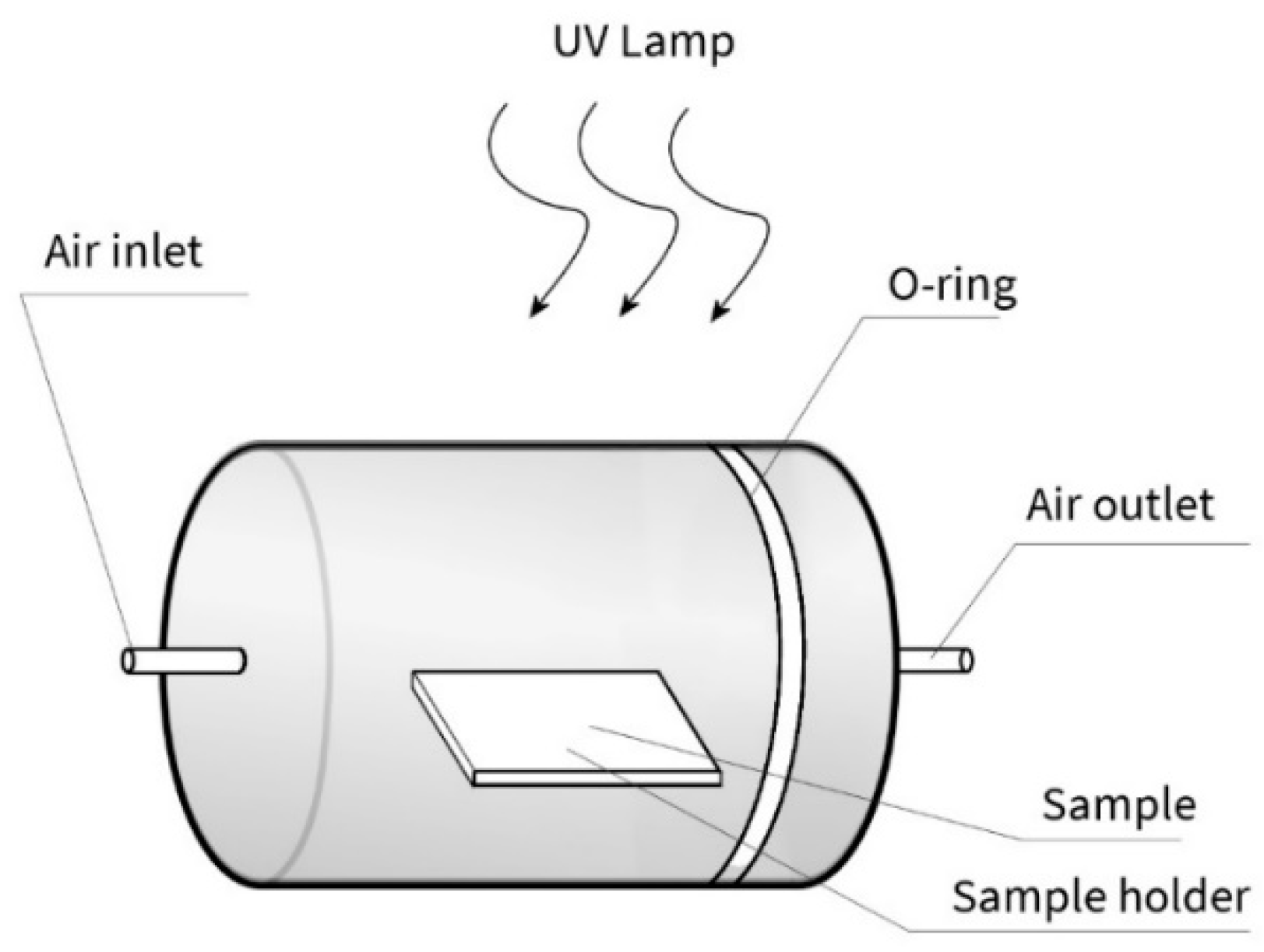
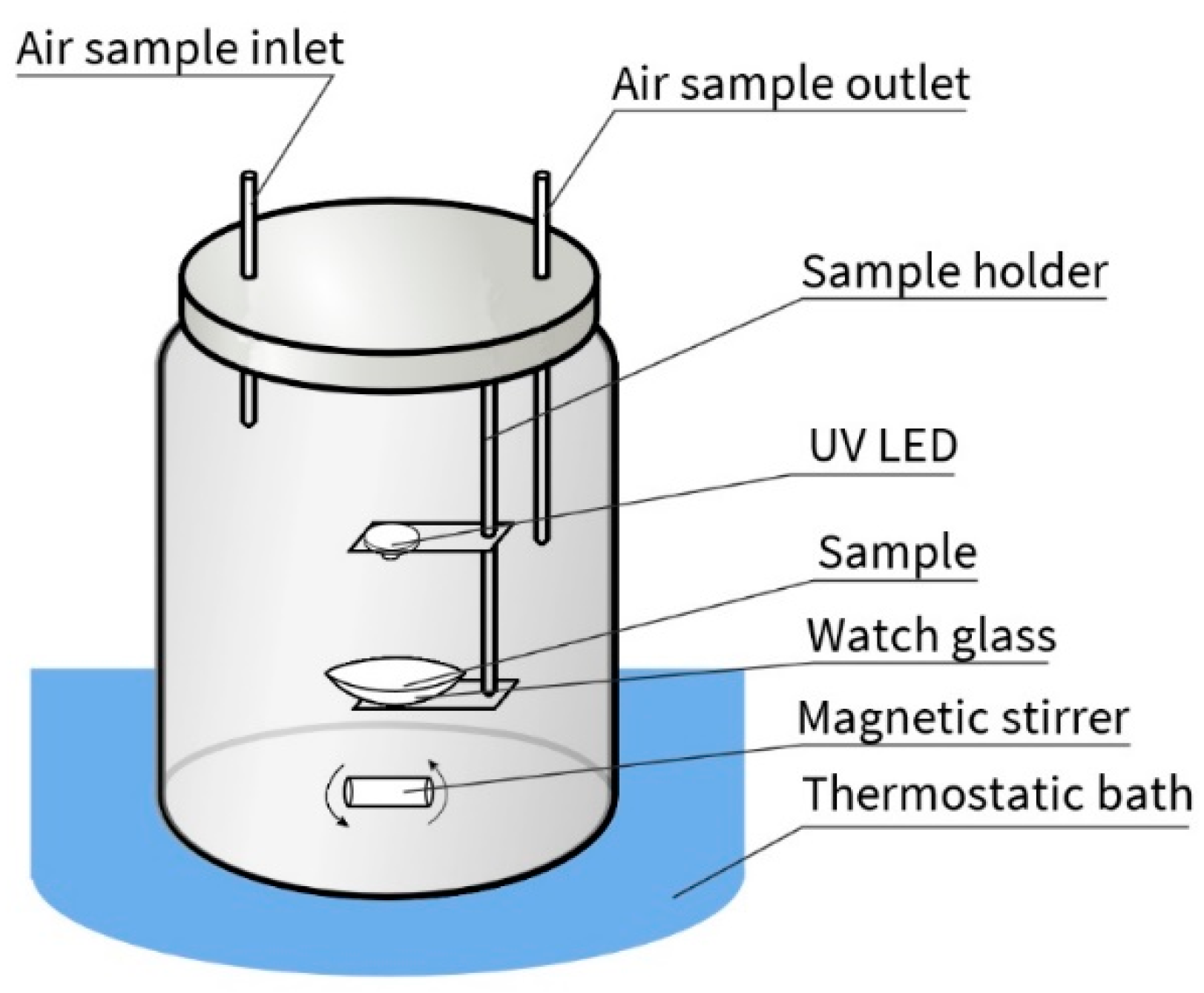
2.3. Photocatalytic Activity
3. Results
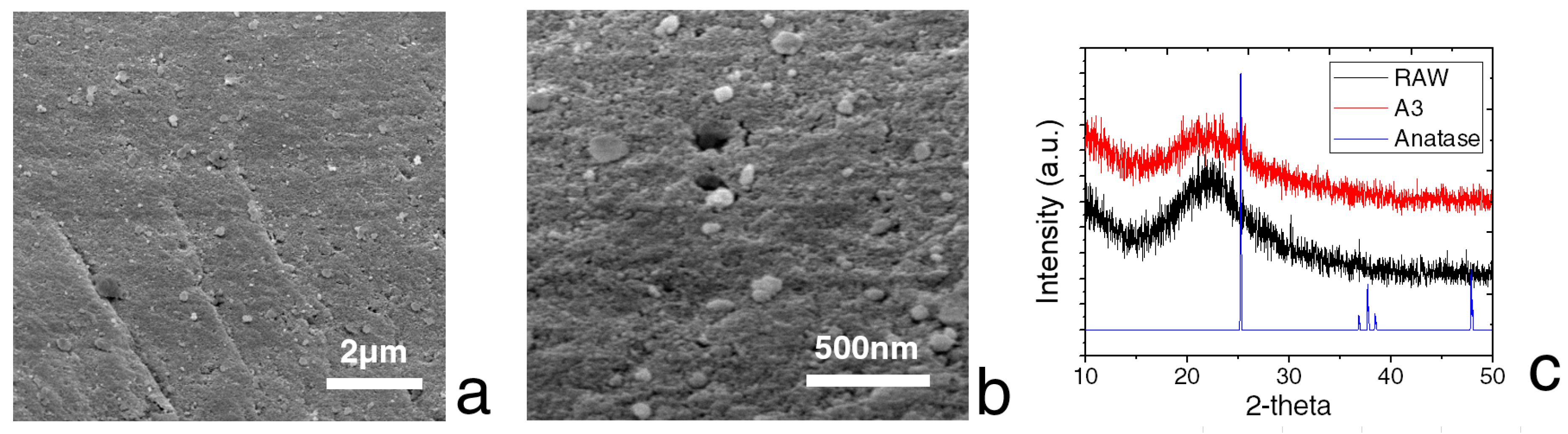
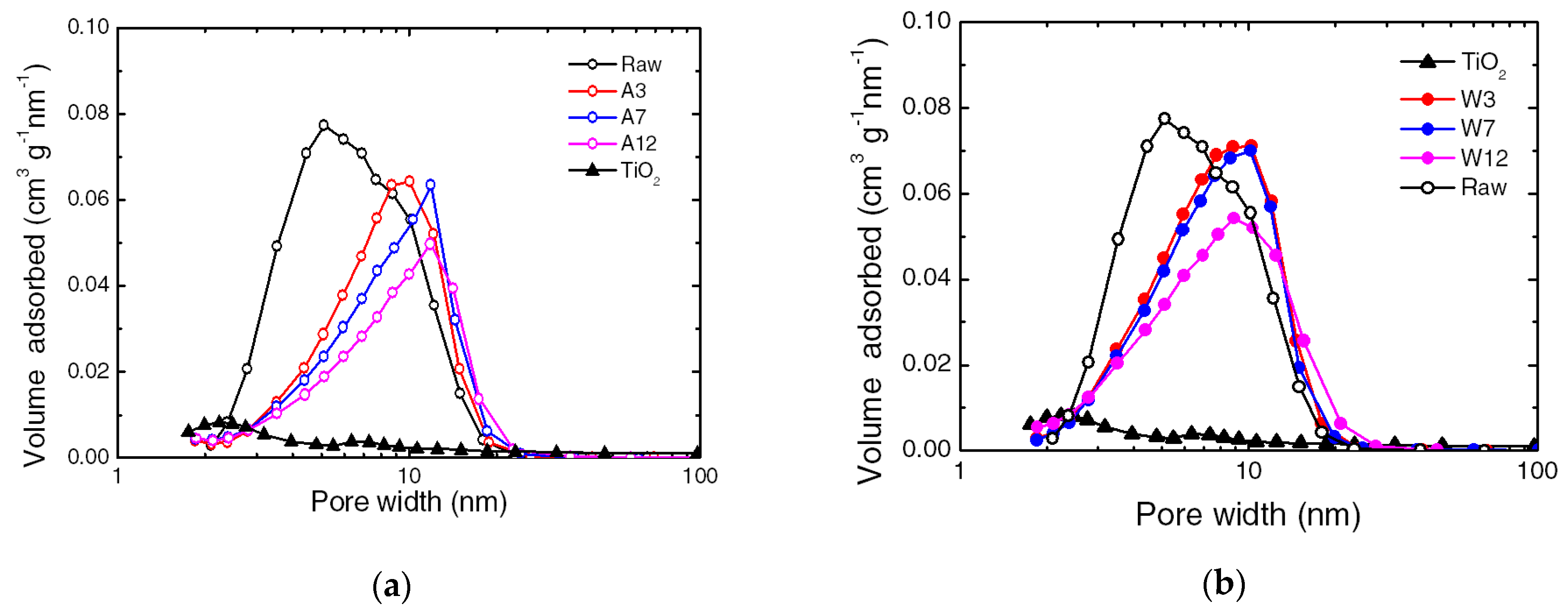
3.1. Characterization of the Composite
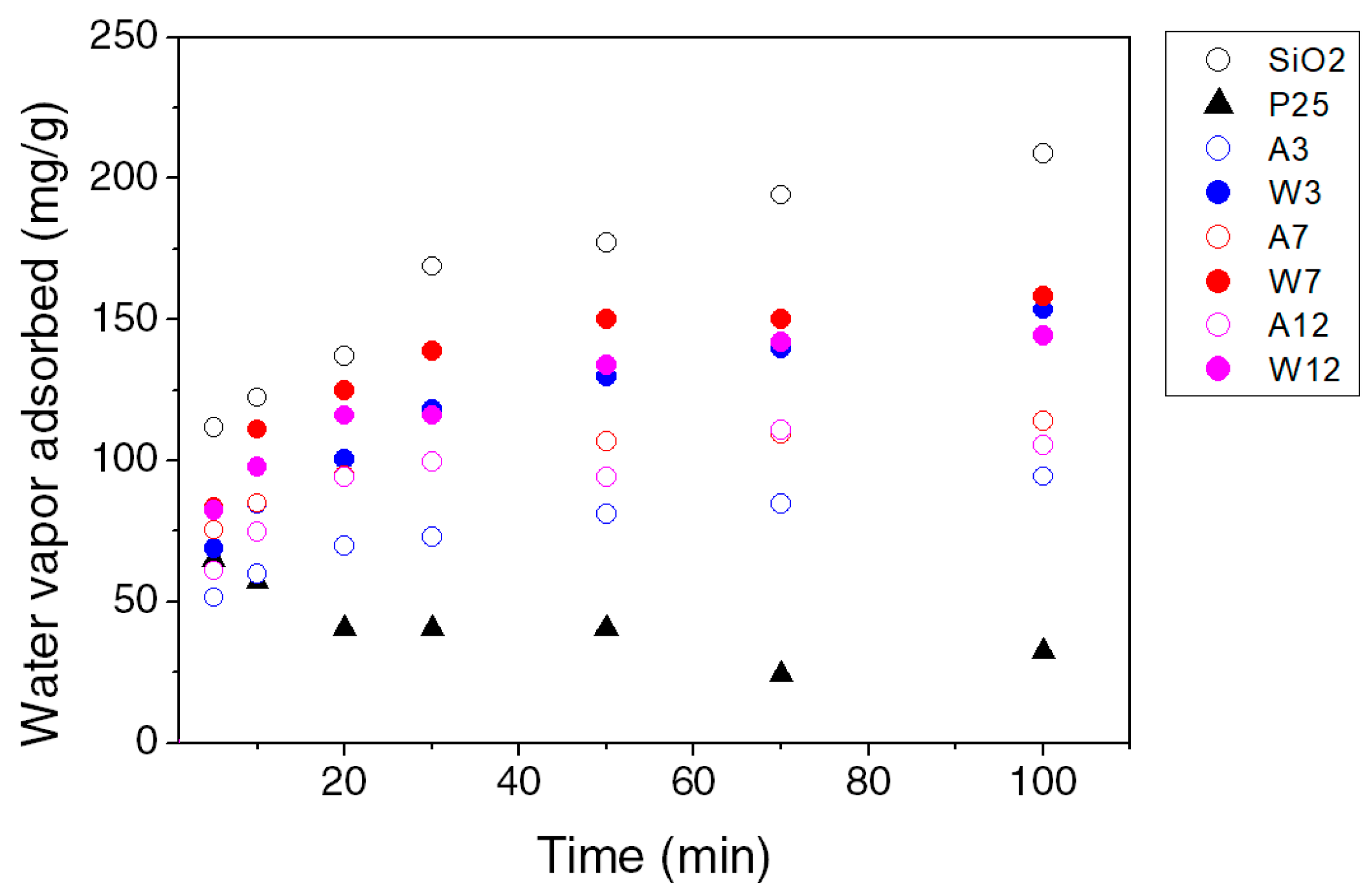
3.2. Photocatalytic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- R.S.C. TiO2: Manufacture of Titanium Dioxide. RSC Adv. Chem. Sci. 2016, 5, 2. [Google Scholar]
- Gázquez, M.J.; Bolívar, J.P.; Garcia-Tenorio, R.; Vaca, F. A Review of the Production Cycle of Titanium Dioxide Pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Hakki, A.; Yang, L.; Wang, F.; Macphee, D.E. The Effect of Interfacial Chemical Bonding in TiO2-SiO2 Composites on Their Photocatalytic NOx Abatement Performance. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bao, N.; Wang, X.; Hu, X.; Miao, X.; Chaker, M.; Ma, D. Advanced Fabrication of Chemically Bonded Graphene/TiO2 Continuous Fibers with Enhanced Broadband Photocatalytic Properties and Involved Mechanisms Exploration. Sci. Rep. 2016, 6, 38066. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, R. Mechanisms of Enhanced Activity of Model TiO2/Carbon and TiO2/Metal Nanocomposite Photocatalysts; Department of Applied Physics Chalmers, University of Technology: Göteborg, Sweden, 2013; ISBN 9789173858717. [Google Scholar]
- Pierpaoli, M.; Lewkowicz, A.; Ficek, M.; Ruello, M.L.; Bogdanowicz, R. Preparation and characterization of TiO2/carbon nanowall composite on a transparent substrate. Photonics Lett. Pol. 2018, 10, 54–56. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved Photocatalytic Activity and Characterization of Mixed TiO2/SiO2 and TiO2/Al2O3 Materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Dutoit, D.C.M.; Schneider, M.; Baiker, A. Titania-Silica Mixed Oxides: I. Influence of Sol-Gel and Drying Conditions on Structural Properties. J. Catal. 1995, 153, 165–176. [Google Scholar] [CrossRef]
- Xianzhi, F.U.; Clark, L.A.; Yang, Q.; Anderson, M.A.; Fu, X.; Clark, L.A.; Yang, Q.; Anderson, M.A. Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996, 30, 647–653. [Google Scholar] [CrossRef]
- Aguado, J.; Vangrieken, R.; López-Muñoz, M.; Marugan, J.; López-Muñoz, M.-J.; Marugan, J.; van Grieken, R.; López-Muñoz, M.-J.; Marugán, J. A comprehensive study of the synthesis, characterization and activity of TiO2 and mixed TiO2/SiO2 photocatalysts. Appl. Catal. A Gen. 2006, 312, 202–212. [Google Scholar] [CrossRef]
- Kochkar, H.; Figueras, F. Synthesis of Hydrophobic TiO2–SiO2Mixed Oxides for the Epoxidation of Cyclohexene. J. Catal. 1997, 171, 420–430. [Google Scholar] [CrossRef]
- Li, X.; He, J. Synthesis of Raspberry-Like SiO–TiO2 Nanoparticles toward Antireflective and Self-Cleaning Coatings. ACS Appl. Mater. Interfaces 2013, 5, 5282–5290. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L.; Ji, G.; Gan, S.; Zou, H.; Xu, X. Uniform TiO2–SiO2 hollow nanospheres: Synthesis, characterization and enhanced adsorption–photodegradation of azo dyes and phenol. Appl. Surf. Sci. 2014, 305, 562–574. [Google Scholar] [CrossRef]
- Dong, W.; Sun, Y.; Lee, C.W.; Hua, W.; Lu, X.; Shi, Y.; Zhang, S.; Chen, J.; Zhao, D. Controllable and Repeatable Synthesis of Thermally Stable Anatase Nanocrystal−Silica Composites with Highly Ordered Hexagonal Mesostructures. J. Am. Chem. Soc. 2007, 129, 13894–13904. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, S.J. Synthesis and Characterization of Nano titania Particles Embedded in Mesoporous Silica with Both High Photocatalytic Activity and Adsorption Capability. J. Phys. Chem. B 2005, 109, 12309–12315. [Google Scholar] [CrossRef] [PubMed]
- Beyers, E.; Biermans, E.; Ribbens, S.; De Witte, K.; Mertens, M.; Meynen, V.; Bals, S.; Van Tendeloo, G.; Vansant, E.F.; Cool, P. Combined TiO2/SiO2 mesoporous photocatalysts with location and phase controllable TiO2 nanoparticles. Appl. Catal. B Environ. 2009, 88, 515–524. [Google Scholar] [CrossRef]
- van Grieken, R.; Aguado, J.; López-Muñoz, M.J.; Marugán, J. Synthesis of size-controlled silica-supported TiO2 photocatalysts. J. Photochem. Photobiol. A Chem. 2002, 148, 315–322. [Google Scholar] [CrossRef]
- Itoh, M.; Hattori, H.; Tanabe, K. The acidic properties of TiO2-SiO2 and its catalytic activities for the amination of phenol, the hydration of ethylene and the isomerization of butene. J. Catal. 1974, 35, 225–231. [Google Scholar] [CrossRef]
- Nilchi, A.; Janitabar-Darzi, S.; Mahjoub, A.R.; Rasouli-Garmarodi, S. New TiO2/SiO2 nanocomposites—Phase transformations and photocatalytic studies. Colloids Surf. Physicochem. Eng. Asp. 2010, 361, 25–30. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Yang, S. Fabrication, characterization, and photocatalytic performance of TiO2 hybridized with SiO2. Russ. J. Appl. Chem. 2016, 89, 2050–2060. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Chan, K.-Y. Synthesis of titania–silica mixed oxide mesoporous materials, characterization and photocatalytic properties. Appl. Catal. A Gen. 2005, 284, 193–198. [Google Scholar] [CrossRef]
- Xie, C.; Xu, Z.; Yang, Q.; Xue, B.; Du, Y.; Zhang, J. Enhanced photocatalytic activity of titania–silica mixed oxide prepared via basic hydrolyzation. Mater. Sci. Eng. B 2004, 112, 34–41. [Google Scholar] [CrossRef]
- Bellardita, M.; Addamo, M.; Di Paola, A.; Marcì, G.; Palmisano, L.; Cassar, L.; Borsa, M. Photocatalytic activity of TiO2/SiO2 systems. J. Hazard. Mater. 2010, 174, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Belhekar, A.A.; Awate, S.V.; Anand, R. Photocatalytic activity of titania modified mesoporous silica for pollution control. Catal. Commun. 2002, 3, 453–458. [Google Scholar] [CrossRef]
- Leboda, R.; Gun’ko, V.; Marciniak, M.; Malygin, A.; Malkin, A.; Grzegorczyk, W.; Trznadel, B.; Pakhlov, E.; Voronin, E. Structure of Chemical Vapor Deposition Titania/Silica Gel. J. Colloid Interface Sci. 1999, 218, 23–39. [Google Scholar] [CrossRef]
- Li, Z.; Hou, B.; Xu, Y.; Wu, D.; Sun, Y. Hydrothermal synthesis, characterization, and photocatalytic performance of silica-modified titanium dioxide nanoparticles. J. Colloid Interface Sci. 2005, 288, 149–154. [Google Scholar] [CrossRef]
- Anpo, M.; Nakaya, H.; Kodama, S.; Kubokawa, Y.; Domen, K.; Onishi, T. Photocatalysis over binary metal oxides. Enhancement of the photocatalytic activity of titanium dioxide in titanium-silicon oxides. J. Phys. Chem. 1986, 90, 1633–1636. [Google Scholar] [CrossRef]
- He, C.; Tian, B.; Zhang, J. Thermally stable SiO2-doped mesoporous anatase TiO2 with large surface area and excellent photocatalytic activity. J. Colloid Interface Sci. 2010, 344, 382–389. [Google Scholar] [CrossRef]
- Inumaru, K.; Kasahara, T.; Yasui, M.; Yamanaka, S. Direct nanocomposite of crystalline TiO2 particles and mesoporous silica as a molecular selective and highly active photocatalyst. Chem. Commun. 2005, 16, 2131–2133. [Google Scholar] [CrossRef]
- Paušová, Š.; Krýsa, J.; Jirkovský, J.; Prevot, V.; Mailhot, G. Preparation of TiO2-SiO2 composite photocatalysts for environmental applications. J. Chem. Technol. Biotechnol. 2014, 89, 1129–1135. [Google Scholar] [CrossRef]
- Smitha, V.S.; Manjumol, K.A.; Baiju, K.V.; Ghosh, S.; Perumal, P.; Warrier, K.G.K. Sol–gel route to synthesize titania-silica nano precursors for photoactive particulates and coatings. J. Sol-Gel Sci. Technol. 2010, 54, 203–211. [Google Scholar] [CrossRef]
- Alaoui, O.T.; Nguyen, Q.T.; Rhlalou, T. Preparation and characterization of a new TiO2/SiO2 composite catalyst for photocatalytic degradation of indigo carmin. Environ. Chem. Lett. 2009, 7, 175–181. [Google Scholar] [CrossRef]
- Hirano, M.; Ota, K.; Inagaki, M.; Iwata, H. Hydrothermal Synthesis of TiO2/SiO2 Composite Nanoparticles and Their Photocatalytic Performances. J. Ceram. Soc. Jpn. 2004, 112, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Montes, M.; Getton, F.P.; Vong, M.S.W.; Sermon, P.A. Titania on silica. A comparison of sol-gel routes and traditional methods. J. Sol-Gel Sci. Technol. 1997, 8, 131–137. [Google Scholar] [CrossRef]
- Hirano, M.; Ota, K.; Iwata, H. Direct Formation of Anatase (TiO2)/Silica (SiO2) Composite Nanoparticles with High Phase Stability of 1300 °C from Acidic Solution by Hydrolysis under Hydrothermal Condition. Chem. Mater. 2004, 16, 3725–3732. [Google Scholar] [CrossRef]
- Hirano, M.; Ota, K. Preparation of photoactive anatase-type TiO2/silica gel by direct loading anatase-type TiO2 nanoparticles in acidic aqueous solutions by thermal hydrolysis. J. Mater. Sci. 2004, 39, 1841–1844. [Google Scholar] [CrossRef]
- Grubb, G.F.; Bakshi, B.R. Life Cycle of Titanium Dioxide Nanoparticle Production. J. Ind. Ecol. 2011, 15, 81–95. [Google Scholar] [CrossRef]
- Middlemas, S.; Fang, Z.Z.; Fan, P. Life cycle assessment comparison of emerging and traditional Titanium dioxide manufacturing processes. J. Clean. Prod. 2015, 89, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Babaizadeh, H.; Hassan, M. Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr. Build. Mater. 2013, 40, 314–321. [Google Scholar] [CrossRef]
- Giosuè, C.; Pierpaoli, M.; Mobili, A.; Ruello, M.L.; Tittarelli, F. Influence of binders and lightweight aggregates on the properties of cementitious mortars: From traditional requirements to indoor air quality improvement. Materials (Basel) 2017, 10, 978. [Google Scholar] [CrossRef]
- Bondarenko, V.; Ruello, M.L.; Bondarenko, A. Ageing of Photocatalytic Materials: Investigation, Assessment and Possible Solving. Chem. Eng. Trans. 2016, 47, 133–138. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Giosuè, C.; Ruello, M.L.; Fava, G. Appraisal of a hybrid air cleaning process. Environ. Sci. Pollut. Res. 2017, 24, 12638–12645. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, M.; Ruello, M.; Fava, G. Enhanced Adsorption of Organic Compounds over an Activated Carbon Cloth by an External-Applied Electric Field. Environments 2017, 4, 33. [Google Scholar] [CrossRef]
- Machida, M.; Norimoto, K.; Watanabe, T.; Hashimoto, K.; Fujishima, A. Effect of SiO2 addition in super-hydrophilic property of TiO2 photocatalyst. J. Mater. Sci. 1999, 34, 2569–2574. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.M.; Dupont, O.; Grange, P. TiO2–SiO2 mixed oxide modified with H2SO4: I. Characterization of the microstructure of metal oxide and sulfate. Appl. Catal. A Gen. 2001, 208, 393–401. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Hattori, H.; Ono, Y. New Solid Acids and Bases: Their Catalytic Properties; Kodansha: Bunkyō, Tokyo, Japan, 1989; Volume 51, ISBN 9780080887555. [Google Scholar]
- Pierpaoli, M.; Ruello, M.L. Indoor Air Quality: A Bibliometric Study. Sustainability 2018, 2018 10, 3830. [Google Scholar] [CrossRef]
- Tittarelli, F.; Giosuè, C.; Mobili, A.; Ruello, M.L. Influence of binders and aggregates on VOCs adsorption and moisture buffering activity of mortars for indoor applications. Cem. Concr. Compos. 2015, 57, 75–83. [Google Scholar] [CrossRef]
- Fava, G.; Naik, T.; Pierpaoli, M.; Fava, G.; Naik, T.R.; Pierpaoli, M. Compressive Strength and Leaching Behavior of Mortars with Biomass Ash. Recycling 2018, 3, 46. [Google Scholar] [CrossRef]



| Author | Year | Synthesis Type | TiO2 Precursor | SiO2 Precursor | Objective | Ref. |
|---|---|---|---|---|---|---|
| Anderson et al. | 1997 | sol-gel | TTIP | TEOS | Decomposition of salicylic acid | [8] |
| Dutoit et al. | 1995 | sol-gel | TTIP | TMOS | Epoxidation of olefins. | [9] |
| Fu et al. | 1996 | sol−gel | TTIP | TEOS | Oxidation of ethylene | [10] |
| Aguado et al. | 2006 | sol-gel | TTIP | TEOS | Oxidation of potassium cyanide | [11] |
| Kochkar et al. | 1997 | sol-gel | TTIP | TEOS | Epoxidation of cyclohexene | [12] |
| Li et al. | 2013 | sol-gel | TTIP | TEOS | Degradation of MB | [13] |
| Guo et al. | 2014 | sol-gel | TBOT | TEOS | Degradation of azo dyes and phenol | [14] |
| Dong et al. | 2007 | sol-gel | TTIP | TEOS | Degradation of RhB | [15] |
| Li et al. | 2005 | sol-gel | TiCl4 | Na2SiO3 | Degradation of benzene | [16] |
| Beyers et al. | 2009 | sol-gel | TTIP | TEOS | Degradation of Rh6G | [17] |
| Van Grieken | 2002 | sol-gel | TTIP | SiO2,TEOS | Cyanide oxidation | [18] |
| Itoh et al. | 1974 | sol-gel | TiCl4 | TEOS | amination of phenol and hydration of ethylene. | [19] |
| Nilchi et al. | 2010 | sol-gel | TiCl4 | TEOS | degradation of Congo Red (CR) azo dye | [20] |
| Yang et al. | 2016 | sol-gel, hydro-calcination, co-precipitation, solid-phase synthesis | TBOT | TEOS | Degradation of MB, RhB, methyl violet, naphthol green B, basic fuchsin, malachite green, and methyl red | [21] |
| Zhang et al. | 2005 | sol-gel | TTIP | TEOS | Degradation of methyl orange | [22] |
| Xie et al. | 2004 | sol-gel | TBOT | TEOS | Oxidation of heptane | [23] |
| Bellardita et al. | 2010 | Impregnation | TiCl4 | SiO2,flyash | NOx abatement and for 4-nitrophenol photodegradation | [24] |
| Belhekar et al. | 2002 | Impregnation | TBOT | TEOS | Degradation of MB, | [25] |
| Leboda et al. | 1999 | CVD | TiCl4 | SiO2 | [26] | |
| Zhijie et al. | 2005 | hydrothermal | TBOT | TEOS | Decomposition of MB | [27] |
| Anpo et al. | 1986 | coprecipitation | TiCl4 | TEOS | [28] | |
| He et al. | 2010 | templating method | TBOT | TEOS | Degradation of Rh6G | [29] |
| Inumaru et al. | 2005 | precipitation | P25 | TEOS | Decomposition of alkylphenols | [30] |
| Paušová et al. | 2014 | Wet mixing | TiCl4 | SiO2 | decomposition of Acid, MB, hexane | [31] |
| Smitha et al. | 2010 | sol-gel | TiOSO4 | TEOS | Degradation of MB | [32] |
| Alaoui et al. | 2009 | SiO2-entrapment method | P25 | Sodium silicate | Degradation of indigo carmine | [33] |
| Hirano et al. | 2004 | hydrothermal | TiOSO4 | TEOS | Degradation of MB, NO | [34] |
| Montes et al. | 1997 | Precipitation, hydrolysis | TiCl3 | TEOS | Catalyst of n-butane-hydrogen reactions | [35] |
| Texture Properties | Water Vapor Adsorption Kinetic | ||||
|---|---|---|---|---|---|
| Samples | BET Surface Area | Pore Volume | Average Pore Diameter | ||
| m2 g−1 | cm3 g−1 | nm | mg g−1 | mg g−1 min−1 | |
| SiO2 | 327 | 0.74 | 9 | 222 | 24 |
| TiO2 | 54 | 0.30 | 22 | 42 | 15 |
| A3 | 258 | 0.64 | 10 | 98 | 13 |
| A7 | 241 | 0.62 | 10.4 | 165 | 15 |
| A12 | 210 | 0.57 | 10.9 | 117 | 27 |
| W3 | 314 | 0.77 | 9.8 | 165 | 30 |
| W7 | 302 | 0.76 | 10 | 111 | 26 |
| W12 | 288 | 0.72 | 10 | 152 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierpaoli, M.; Zheng, X.; Bondarenko, V.; Fava, G.; Ruello, M.L. Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite. Environments 2019, 6, 87. https://doi.org/10.3390/environments6080087
Pierpaoli M, Zheng X, Bondarenko V, Fava G, Ruello ML. Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite. Environments. 2019; 6(8):87. https://doi.org/10.3390/environments6080087
Chicago/Turabian StylePierpaoli, Mattia, Xu Zheng, Vladimir Bondarenko, Gabriele Fava, and Maria Letizia Ruello. 2019. "Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite" Environments 6, no. 8: 87. https://doi.org/10.3390/environments6080087







