Assessment of Fecal Contamination in Oklahoma Water Systems through the Use of Sterol Fingerprints
Abstract
:1. Introduction
2. Materials and Methods
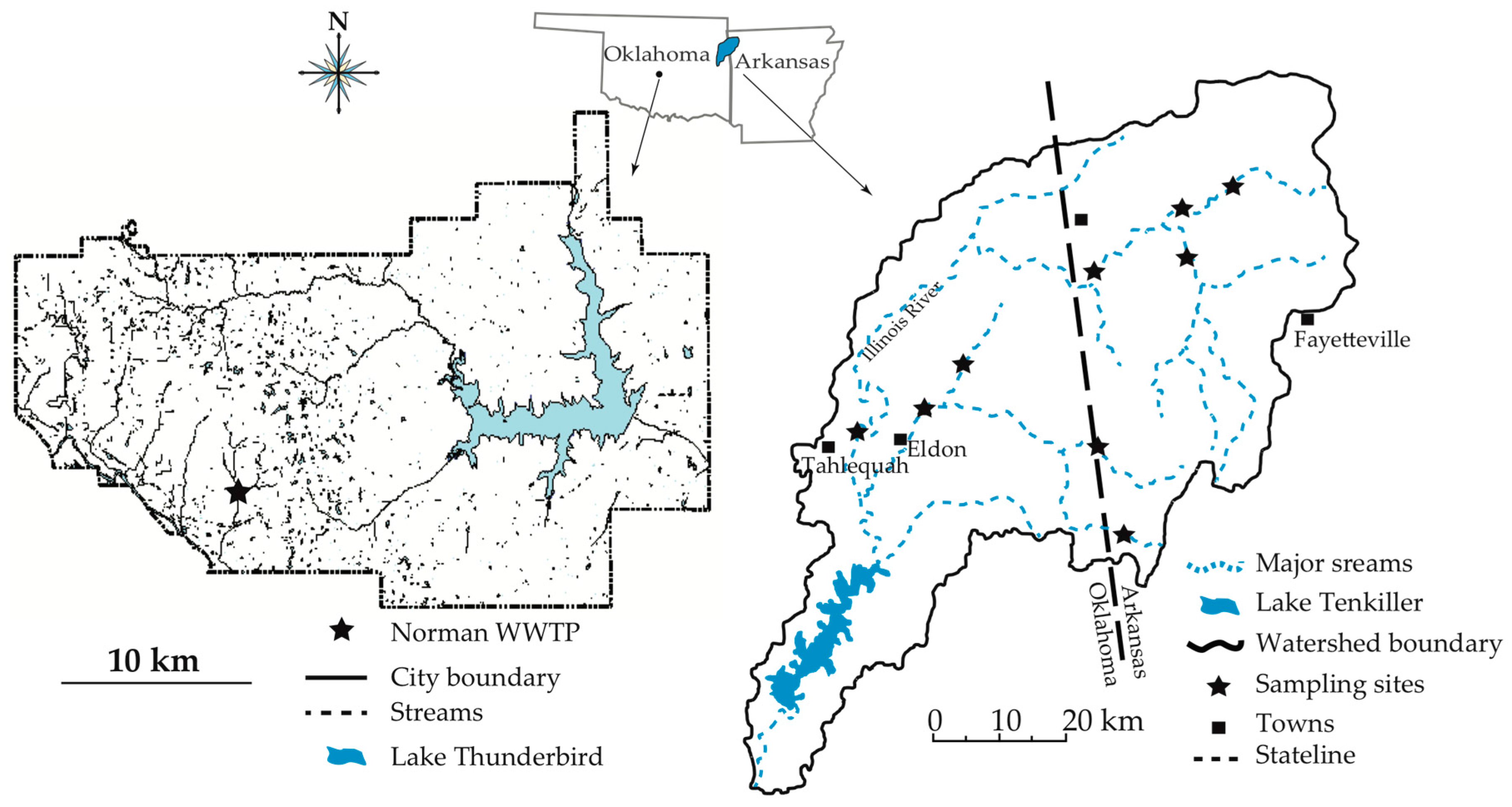
2.1. Samples
2.2. Sample Treatments and Analysis
2.2.1. Pretreatments
2.2.2. Extraction and Fractionation
2.3. Sterol Quantification and Compound Specific Isotope Analysis (CSIA)
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Norman WWTP
3.2. The Illinois River Basin
3.3. Sterol Fingerprint as Source of Fecal Contamination Indicator
4. Conclusions
Author Contributions
Conflicts of Interest
References
- UN-Water. Coping with water scarcity: Challenge of the twenty-first century. In 2007 World Water Day; FAO Publishing: Rome, Italy, 2007. [Google Scholar]
- World Water Assessment Programme. The United Nations World Water Development Report 2014: Water and Energy; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2014. [Google Scholar]
- Field, K.G.; Samadpour, M. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007, 41, 3517–3538. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health-Based Monitoring of Recreational Waters: The Feasibility of a New Approach; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Simpson, J.M.; Santo Domingo, J.W.; Reasoner, D.J. Microbial source tracking: State of the science. Environ. Sci. Technol. 2002, 36, 5279–5288. [Google Scholar] [CrossRef] [PubMed]
- Mainstone, C.P.; Parr, W. Phosphorus in rivers—Ecology and management. Sci. Total Environ. 2002, 282, 25–47. [Google Scholar] [CrossRef]
- Glibert, P.; Burkholder, J. The complex relationships between increases in fertilization of the earth, coastal eutrophication and proliferation of harmful algal blooms. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 341–354. [Google Scholar]
- Gerba, C.P.; Smith, J.E. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 2005, 34, 42–48. [Google Scholar] [PubMed]
- Soller, J.A.; Schoen, M.E.; Bartrand, T.; Ravenscroft, J.E.; Ashbolt, N.J. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 2010, 44, 4674–4691. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Rose, J.B.; Jenkins, T.M.; Farrah, S.R.; Lukasik, J. Microbial source tracking: Current methodology and future directions. Appl. Environ. Microbiol. 2002, 68, 5796–5803. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.B.; van de Werfhorst, L.C.; Griffith, J.F.; Holden, P.A.; Jay, J.A.; Shanks, O.C.; Wang, D.; Weisberg, S.B. Performance of forty-one microbial source tracking methods: A twenty-seven lab evaluation study. Water Res. 2013, 47, 6812–6828. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, M.; Caprais, M.-P.; Mieszkin, S.; Marti, R.; Wery, N.; Jardé, E.; Derrien, M.; Jadas-Hécart, A.; Communal, P.; Jaffrezic, A.; et al. Development of microbial and chemical mst tools to identify the origin of the faecal pollution in bathing and shellfish harvesting waters in France. Water Res. 2010, 44, 4812–4824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ervin, J.S.; van de Werfhorst, L.C.; Murray, J.L.; Holden, P.A. Microbial source tracking in a coastal california watershed reveals canines as controllable sources of fecal contamination. Environ. Sci. Technol. 2014, 48, 9043–9052. [Google Scholar] [CrossRef] [PubMed]
- Biache, C.; Philp, R.P. The use of sterol distributions combined with compound specific isotope analyses as a tool to identify the origin of fecal contamination in rivers. Water Res. 2013, 47, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Sinigalliano, C.D.; Fleisher, J.M.; Gidley, M.L.; Solo-Gabriele, H.M.; Shibata, T.; Plano, L.R.; Elmir, S.M.; Wanless, D.; Bartkowiak, J.; Boiteau, R.; et al. Traditional and molecular analyses for fecal indicator bacteria in non-point source subtropical recreational marine waters. Water Res. 2010, 44, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Layton, B.A.; Boehm, A.B.; Holden, P.A.; Jay, J.A.; Hagedorn, C.; McGee, C.D.; Weisberg, S.B. The California Microbial Source Identification Manual: A Tiered Approach to Identifying Fecal Pollution Sources to Beaches; Technical Report 804; Southern California Coastal Water Research Project: Costa Mesa, CA, USA, 2013. [Google Scholar]
- Volkman, J.K. A review of sterol markers for marine and terrigenous organic matter. Organ. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Jardé, E.; Gruau, G.; Mansuy-Huault, L.; Peu, P.; Martinez, J. Using sterols to detect pig slurry contribution to soil organic matter. Water Air Soil Pollut. 2007, 178, 169–178. [Google Scholar] [CrossRef]
- Benveniste, P. Sterol biosynthesis. Annu. Rev. Plant Physiol. 1986, 37, 275–308. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Fernandez, P.; Bayona, J.M.; Albaiges, J. Assessment of fecal sterols and ketones as indicators of urban sewage inputs to coastal waters. Environ. Sci. Technol. 1990, 24, 357–363. [Google Scholar] [CrossRef]
- Leeming, R.; Ball, A.; Ashbolt, N.; Nichols, P. Using faecal sterols from humans and animals to distinguish faecal pollution in receiving waters. Water Res. 1996, 30, 2893–2900. [Google Scholar] [CrossRef]
- Standley, L.J.; Kaplan, L.A.; Smith, D. Molecular tracers of organic matter sources to surface water resources. Environ. Sci. Technol. 2000, 34, 3124–3130. [Google Scholar] [CrossRef]
- Carreira, R.S.; Wagener, A.L.R.; Readman, J.W. Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): Space–time variations. Estuar. Coast. Shelf Sci 2004, 60, 587–598. [Google Scholar] [CrossRef]
- Leeming, R.; Stark, J.S.; Smith, J.J. Novel use of faecal sterols to assess human faecal contamination in antarctica: A likelihood assessment matrix for environmental monitoring. Antarct. Sci. 2015, 27, 31–43. [Google Scholar] [CrossRef]
- Isobe, K.O.; Tarao, M.; Zakaria, M.P.; Chiem, N.H.; Minh, L.Y.; Takada, H. Quantitative application of fecal sterols using gas chromatography-mass spectrometry to investigate fecal pollution in tropical waters: Western Malaysia and Mekong delta, Vietnam. Environ. Sci. Technol. 2002, 36, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Ferezou, J.; Gouffier, E.; Coste, T.; Chevallier, F. Daily elimination of fecal neutral sterols by humans. Digestion 1978, 18, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.A.; Xian, G.; Jin, S.; Dewitz, J.A.; Homer, C.G.; Limin, Y.; Barnes, C.A.; Herold, N.D.; Wickham, J.D. Completion of the 2006 national land cover database for the conterminous United States. Photogramm. Eng. Remote Sens. 2011, 77, 858–864. [Google Scholar]
- Sims, J.T.; Wolf, D.C. Poultry waste management: Agricultural and environmental issues. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1994; Volume 52, pp. 2–72. [Google Scholar]
- Biache, C.; Frómeta, A.E.N.; Czechowski, F.; Lu, Y.; Philp, R.P. Thiosteranes in samples impacted by fecal materials and their potential use as marker of sewage input. Environ. Pollut. 2015, 196, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Pardo, R.; Barrado, E.; Debán, L. Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 1998, 32, 3581–3592. [Google Scholar] [CrossRef]
- Wenning, R.J.; Erickson, G.A. Interpretation and analysis of complex environmental data using chemometric methods. TrAC Trends Anal. Chem. 1994, 13, 446–457. [Google Scholar] [CrossRef]
- Badin, A.-L.; Faure, P.; Bedell, J.-P.; Delolme, C. Distribution of organic pollutants and natural organic matter in urban storm water sediments as a function of grain size. Sci. Total Environ. 2008, 403, 178–187. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.V.; Cooke, M.; Nickless, G. Effect of sewage treatment on faecal sterols. Water Res. 1981, 15, 1019–1025. [Google Scholar] [CrossRef]
- Subbiah, M.T.R.; Kottke, B.A.; Zollman, P.E. Fecal sterols of some avian species. Comp. Biochem. Physiol. Part B Comp. Biochem. 1972, 41, 695–704. [Google Scholar] [CrossRef]
- Burczak, J.D.; McNaughton, J.L.; Kellogg, T.F. Cholesterol metabolism in poultry, gallus domesticus: Fecal neutral sterol and bile acid excretion. Comp. Biochem. Physiol. Part B Comp. Biochem. 1980, 66, 385–389. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—An overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Philp, R.P. The emergence of stable isotopes in environmental and forensic geochemistry studies: A review. Environ. Chem. Lett. 2007, 5, 57–66. [Google Scholar] [CrossRef]
- Park, R.; Epstein, S. Carbon isotope fractionation during photosynthesis. Geochim. Cosmochim. Acta 1960, 21, 110–126. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Werner, R.A. Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: A review and synthesis. New Phytol. 2004, 161, 371–385. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E.; Helliker, B.R. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 1997, 112, 285–299. [Google Scholar] [CrossRef]
- Bender, M.M. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 1971, 10, 1239–1244. [Google Scholar] [CrossRef]
- Collatz, G.J.; Berry, J.A.; Clark, J.S. Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: Present, past, and future. Oecologia 1998, 114, 441–454. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Naraoka, H.; Poulson, S.R. Hydrogen and carbon isotopic fractionations of lipid biosynthesis among terrestrial (C3, C4 and CAM) and aquatic plants. Phytochemistry 2004, 65, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Bull, I.D.; Lockheart, M.J.; Elhmmali, M.M.; Roberts, D.J.; Evershed, R.P. The origin of faeces by means of biomarker detection. Environ. Int. 2002, 27, 647–654. [Google Scholar] [CrossRef]
- Shah, V.G.; Hugh Dunstan, R.; Geary, P.M.; Coombes, P.; Roberts, T.K.; von Nagy-Felsobuki, E. Evaluating potential applications of faecal sterols in distinguishing sources of faecal contamination from mixed faecal samples. Water Res. 2007, 41, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-H.; Lam, M.H.W.; Poon, K.-F.; Yeung, H.-Y.; Chiu, T.K.T. Application of sedimentary fecal stanols and sterols in tracing sewage pollution in coastal waters. Water Res. 1998, 32, 225–235. [Google Scholar] [CrossRef]
- Reeves, A.; Patton, D. Faecal sterols as indicators of sewage contamination in estuarine sediments of the Tay Estuary, Scotland: An extended baseline survey. Hydrolo. Earth Syst. Sci. 2005, 9, 81–94. [Google Scholar] [CrossRef]
- Derrien, M.; Jardé, E.; Gruau, G.; Pierson-Wickmann, A.-C. Extreme variability of steroid profiles in cow feces and pig slurries at the regional scale: Implications for the use of steroids to specify fecal pollution sources in waters. J. Agric. Food Chem. 2011, 59, 7294–7302. [Google Scholar] [CrossRef] [PubMed]
- Leeming, R.; Latham, V.; Rayner, M.; Nichols, P. Detecting and distinguishing sources of sewage pollution in australian inland and coastal waters and sediments. In Molecular Markers in Environmental Geochemistry; American Chemical Society: Washington, DC, USA, 1997; Volume 671, pp. 306–319. [Google Scholar]
- Dutka, B.J.; Chau, A.S.Y.; Coburn, J. Relationship between bacterial indicators of water pollution and fecal sterols. Water Res. 1974, 8, 1047–1055. [Google Scholar] [CrossRef]
- Leeming, R.; Nichols, P.D. Concentrations of coprostanol that correspond to existing bacterial indicator guideline limits. Water Res. 1996, 30, 2997–3006. [Google Scholar] [CrossRef]
- Isobe, K.O.; Tarao, M.; Chiem, N.H.; Minh, L.Y.; Takada, H. Effect of environmental factors on the relationship between concentrations of coprostanol and fecal indicator bacteria in tropical (Mekong delta) and temperate (Tokyo) freshwaters. Appl. Environ. Microbiol. 2004, 70, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Savichtcheva, O.; Okabe, S. Alternative indicators of fecal pollution: Relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res. 2006, 40, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Farnleitner, A.H.; Field, K.G.; Green, H.C.; Shanks, O.C.; Boehm, A.B. Enterococcus and Escherichia coli fecal source apportionment with microbial source tracking genetic markers—Is it feasible? Water Res. 2013, 47, 6849–6861. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K. Fate of coprostanol, an index of fecal pollution in Tokyo Bay. Geochemistry 1983, 17, 68–75. (In Japanese) [Google Scholar]


| Trivial Name | Systematic Name | Number of Carbon | M.W. | m/z for Quantification and Identification | Standard Used for Quantification |
|---|---|---|---|---|---|
| Cholestane-d6 | Cholestane-2.2.3.3.4.4-d6 | 27 | 378 | 223; 363; 115; 378 | Cholestane-d6 |
| Coprostanol | 5β-cholestan-3β-ol | 27 | 460 | 370; 355; 75; 215; 95; 207 | Coprostanol |
| Epi-coprostanol | 5β-cholestan-3α-ol | 27 | 460 | 370; 215; 75; 355; 257 | Epicoprostanol |
| Cholesterol-d6 | Cholesterol-2.2.3.4.4.6-d6 | 27 | 464 | 333; 374; 131; 359; 73; 464 | Cholesterol-d6 |
| Cholesterol | Cholest-5-en-3β-ol | 27 | 458 | 129; 329; 368; 353; 458 | Cholesterol |
| Cholestanol | 5α-cholestan-3β-ol | 27 | 460 | 215; 75; 445; 355; 460; 405 | Cholestanol |
| Campesterol | 24-methylcholest-5-en-3β-ol | 28 | 472 | 129; 95; 82; 69; 343; 204; 109; 472 | β-Sitosterol |
| Campestanol | 24-methyl-5α-cholestan-3β-ol | 28 | 474 | 215; 75; 459; 369; 474; 305 | Sitostanol |
| Stigmasterol | 24-ethylcholesta-5,22(E)-dien-3β-ol | 29 | 484 | 129; 83; 484; 255; 394 | Stigmasterol |
| Stigmastanol | 24-ethyl-5α-cholesta-5,22(E)-dien-3β-ol | 29 | 486 | 257; 75; 55; 486; 353; 345 | Sitostanol |
| β-Sitosterol | 24-ethylcholest-5-en-3β-ol | 29 | 486 | 129; 357; 396; 73; 381; 486 | β-Sitosterol |
| Sitostanol | 24-ethyl-5α-cholestan-3β-ol | 29 | 488 | 215; 75; 473; 383; 488; 305 | Sitostanol |
| 24-Ethylcoprostanol | 24-ethyl-5β-cholestan-3β-ol | 29 | 488 | 398; 383; 215; 75; 257 | Epicoprostanol |
| / | 5α-cholestane-3β-thiol | 27 | 404 | 249; 250; 389; 404 | Coprostanol |
| / | 24-ethyl-5α-cholestane-3β-thiol | 30 | 432 | 249; 250; 417; 432 | Coprostanol |
| Compounds | Incoming Water (µg/L) | Primary Effluent (µg/L) | Effluent (µg/L) | Digested Sludge from Primary Clarification (µg/L) | Activated Sludge (µg/L) | Sludge from Final Clarification (µg/L) | Cake (µg/g) |
|---|---|---|---|---|---|---|---|
| Coprostanol | 385 | 376 | 25 | 23,533 | 2594 | 7734 | 1489 |
| Epicholestanol a | 10 | <L.D. | 9 | 1872 | <L.D. | 1503 | 129 |
| Epicoprostanol b | <L.D. | <L.D. | <L.D. | 12,485 | 581 | 6272 | 813 |
| Coprostanone c | <L.D. | <L.D. | <L.D. | 369 | 0 | 246 | 87 |
| Cholesterol | 547 | 545 | 42 | 5797 | 3074 | 2896 | 313 |
| Cholestanol | 40 | 43 | 11 | 348 | 806 | 3859 | 430 |
| 5β-stigmastanol d | 17 | <L.D. | 5 | 1317 | 214 | 620 | 95 |
| 24-ethylcoprostanol | 140 | 140 | 17 | 9226 | 1183 | 3695 | 601 |
| Campesterol | 82 | 81 | 15 | 1760 | 681 | 894 | 102 |
| 24-ethylepicoprostanol e | <L.D. | <L.D. | 7 | 2755 | 231 | 1161 | 178 |
| Campestanol | 55 | 55 | 10 | 4820 | 382 | 1815 | 271 |
| Stigmasterol | 24 | 23 | 8 | 875 | 412 | 517 | 54 |
| Stigmastanol f | <L.D. | <L.D. | <L.D. | 406 | 103 | 314 | 27 |
| β-Sitosterol | 292 | 293 | 38 | 5953 | 2062 | 3227 | 364 |
| Sitostanol | 23 | 25 | 8 | 2582 | 400 | 1648 | 165 |
| Sum steroids | 1615 | 1580 | 194 | 74,099 | 12,724 | 36,401 | 5117 |
| 5α-cholestane-3β-thiol g | <L.D. | <L.D. | <L.D. | 181 | <L.D. | 138 | 18 |
| 24-ethyl-5α-cholestane-3β-thiol h | <L.D. | <L.D. | <L.D. | 61 | <L.D. | 46 | 6 |
| Ratio | Sterol Ratios | Incoming Water | Digested Sludge from Primary Clarification | Primary Effluent | Activated Sludge | Sludge from Final Clarification | Effluent | Cake | Reference |
|---|---|---|---|---|---|---|---|---|---|
| R1 | Cpr/(Cpr + Chnl) | 0.9 | 1.0 | 0.9 | 0.8 | 0.7 | 0.7 | 0.8 | [20] |
| R2 | (Cpr + Epi)/(Cpr + Epi + Chnl) | / | 1.0 | / | 0.8 | 0.8 | / | 0.8 | [44] |
| R3 | Cpr/Chtl | 0.7 | 4.1 | 0.7 | 0.8 | 2.7 | 0.6 | 4.8 | [23] |
| R4 | Cpr/Chnl | 9.6 | 67.6 | 8.7 | 3.2 | 2.0 | 2.3 | 3.5 | [45] |
| R5 | Cpr/(Chnl + Chtl) | 0.7 | 3.8 | 0.6 | 0.7 | 1.1 | 0.5 | 2.0 | [46] |
| R6 | Cprn/Cpr | / | 0.0 | / | 0.0 | 0.0 | / | 0.1 | [20] |
| R7 | Cpr/Epi | / | 1.9 | / | 4.5 | 1.2 | / | 1.8 | [47] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Philp, R.P.; Biache, C. Assessment of Fecal Contamination in Oklahoma Water Systems through the Use of Sterol Fingerprints. Environments 2016, 3, 28. https://doi.org/10.3390/environments3040028
Lu Y, Philp RP, Biache C. Assessment of Fecal Contamination in Oklahoma Water Systems through the Use of Sterol Fingerprints. Environments. 2016; 3(4):28. https://doi.org/10.3390/environments3040028
Chicago/Turabian StyleLu, Yueming, R. Paul Philp, and Coralie Biache. 2016. "Assessment of Fecal Contamination in Oklahoma Water Systems through the Use of Sterol Fingerprints" Environments 3, no. 4: 28. https://doi.org/10.3390/environments3040028






