Proton Adsorption Selectivity of Zeolites in Aqueous Media: Effect of Exchangeable Cation Species of Zeolites
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Li+, K+, Rb+, and Cs+ Saturated Zeolites
2.2. Determination of CEC of Zeolites at Different pH Levels
3. Results and Discussion
3.1. Structural Stability of Zeolites with CEC Measurement
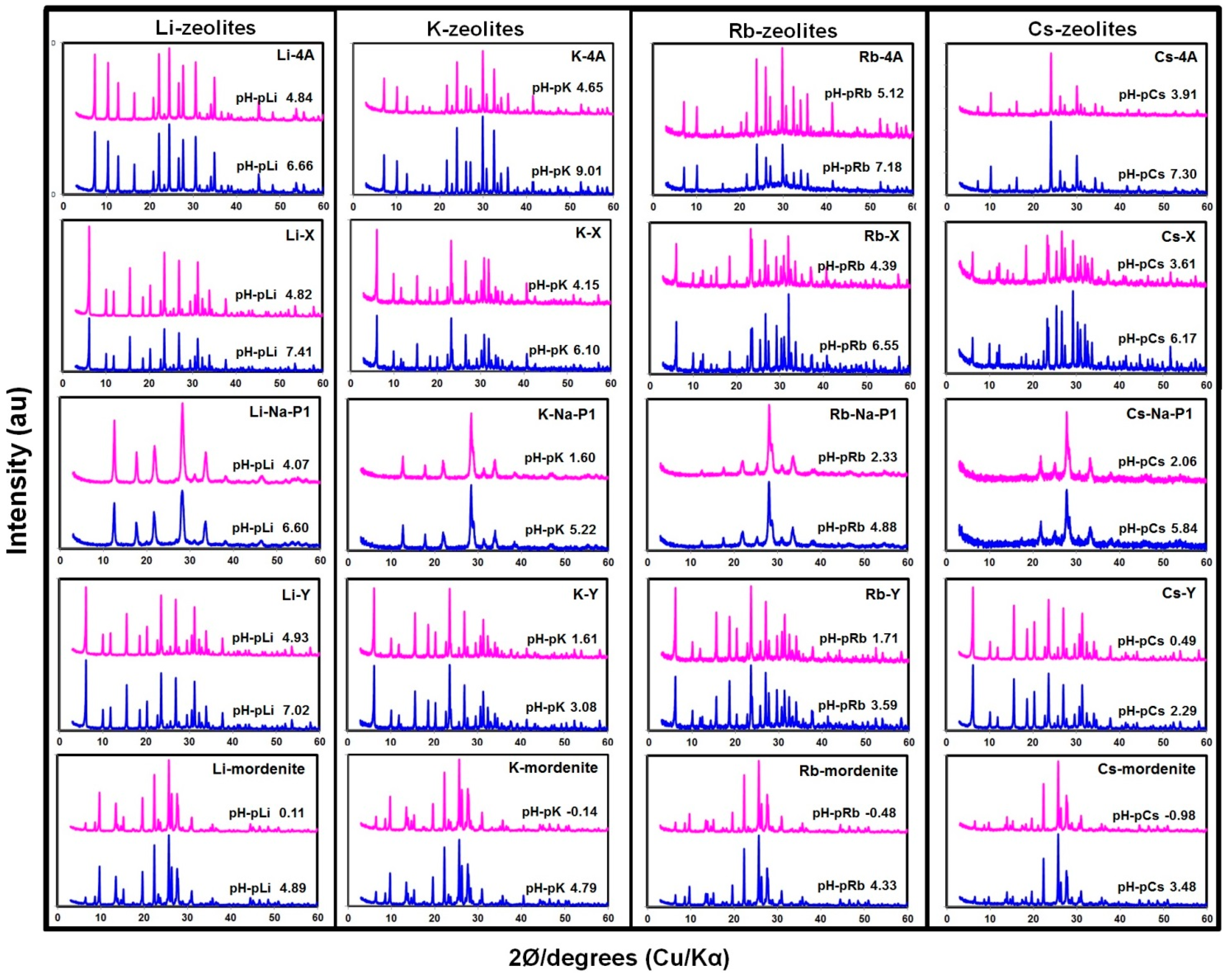

3.2. Effect of pH-pM on CEC of Zeolites

3.3. Effect of Cation and Zeolite Species on Proton Adsorption
| Zeolite | (pH-pLi)90 | (pH-pNa)90 | (pH-pK)90 | (pH-pRb)90 | (pH-pCs)90 |
|---|---|---|---|---|---|
| Linde-type A | 5.61 | 5.38 | 5.64 | 6.23 | 3.80 |
| faujasite X | 5.90 | 5.75 | 4.30 | 5.59 | 3.21 |
| Na-P1 | 4.34 | 3.64 | 2.47 | 4.42 | 2.53 |
| faujasite Y | 4.79 | 3.06 | 2.02 | 2.71 | 0.57 |
| mordenite | 0.51 | 0.92 | 0.18 | −0.33 | −0.42 |
| Saturation Cation | H+ Adsorption Selectivity Sequence |
|---|---|
| Li+ | faujasite X > Linde-type A > faujasite Y > Na-P1 > mordenite |
| Na+ | faujasite X > Linde-type A > Na-P1 > faujasite Y > mordenite |
| K+ | Linde-type A > faujasite X > Na-P1 > faujasite Y > mordenite |
| Rb+ | Linde-type A > faujasite X > Na-P1 > faujasite Y > mordenite |
| Cs+ | Linde-type A > faujasite X > Na-P1 > faujasite Y > mordenite |
| Zeolite | H+ Adsorption Selectivity Sequence |
|---|---|
| Linde-type A | Rb+-type > K+-type > Li+-type > Na+-type > Cs+-type |
| faujasite X | Li+-type > Na+-type > Rb+-type > K+-type > Cs+-type |
| Na-P1 | Rb+-type > Li+-type > Na+-type > Cs+-type > K+-type |
| faujasite Y | Li+-type > Na+-type > Rb+-type > K+-type > Cs+-type |
| mordenite | Na+-type > Li+-type > K+-type > Rb+-type > Cs+-type |
3.4. Factors Affecting Proton Adsorption Selectivity of Zeolites
3.4.1. Si/Al Ratio and Charge Density
3.4.2. Nature of Cations and Zeolite Pore Sizes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Global inorganic geochemistry. In Frontiers in Geochemistry: Konrad Krauskopf; Ernst, W.G. (Ed.) The Sheridan Press: Hanover, PA, USA, 2002; Volume 1.
- Mon, J.; Deng, Y.; Flury, M.; Harsh, J.B. Cesium incorporation and diffusion in cancrinite, sodalite, zeolite and allophane. Microporous Mesoporous Mater. 2005, 86, 277–286. [Google Scholar]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Tehcnol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Kuronen, M.; Harjula, R.; Jernstrom, J.; Vestenius, M.; Lehto, J. Effect of the framework charge density on zeolite ion exchange selectivities. Phys. Chem. Chem. Phys. 2000, 2, 2655–2659. [Google Scholar]
- Su, H.; Kim, H.S.; Seo, S.M.; Ko, S.O.; Suh, J.M.; Kim, G.H.; Lim, W.T. Location of Na+ ions in fully dehydrated Na+-saturated zeolite Y (FAU, Si/Al-1.56). Bull. Kor. Chem. Soc. 2012, 33, 2785–2788. [Google Scholar]
- Mortier, W.J.; Bosmans, H.J.; Uytterhoeven, J.B. Location of univalent cations in synthetic zeolites of the Y and X type with varying Silicon to aluminum ratio. II dehydrated potassium exchanged forms. J. Phys. Chem. 1972, 1, 650–656. [Google Scholar]
- Katada, N.; Suzuki, K.; Noda, T.; Sastre, G.; Niwa, M. Correlation between BrØnsted Acid strength and local structure in zeolites. J. Phys. Chem. 2009, 113, 19208–19217. [Google Scholar]
- Bonelli, B.; Civalleri, B.; Fubini, B.; Ugliengo, P.; Areán, C.O.; Garrone, E. Experimental and quantum chemical studies on the adsorption of carbondioxide on alkali-metal-exchanged ZSM-5 zeolites. J. Phys. Chem. 2000, 104, 10978–10988. [Google Scholar]
- Abdel-Rahman, R.O.; Ibrahium, H.A.; Yung-Tse, H. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar]
- Kim, J. Li+- and H+-exchanged low-silica X zeolite as selective N adsorbent for air separation. Bull. Korean Chem. Soc. 2003, 24, 1814–1818. [Google Scholar]
- Nilchi, A.; Maalek, B.; Khanchi, A.; Ghanadi, M.M.; Bagheri, A.; Savoji, K. Ion exchangers in radioactive waste management: Natural Iranian zeolites. Appl. Radiat. Isot. 2006, 64, 138–143. [Google Scholar]
- Busca, G. Acid catalysts in industrial hydrocarbon chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar]
- Degnan, T.F. The implications of the fundamentals of shape selectivity for the development of catalysts for the petroleum and petrochemical industries. J. Catal. 2003, 216, 32–46. [Google Scholar]
- Pickering, H.W.; Menzies, N.W.; Hunter, M.N. Zeolite/rock phosphate—A novel slow release phosphorus fertiliser for potted plant production. Sci. Hortic. 2002, 94, 333–343. [Google Scholar]
- Rabai, K.A.; Ahmed, O.H.; Kasim, S. Use of formulated nitrogen, phosphorus, and potassium compound fertilizer using clinoptilolite zeolite in maize (Zea mays L.) cultivation. Emir. J. Food Agric. 2013, 25, 713–722. [Google Scholar]
- Aono, H.; Tamura, K.; Johan, E.; Yamauchi, R.; Yamamoto, T.; Matsue, N.; Henmi, T. Preparation of composite material of Na-P1-type zeolite and magnetic for Cs decontamination. Che. Lett. 2013, 42, 589–591. [Google Scholar]
- Macasek, F.; Navratil, J.D.; Dulanska, S. Magnetic sorbent for soil remediation—A new waste for waste treatment. Separ. Sci Technol. 2002, 37, 3673–3692. [Google Scholar]
- Dyer, A.; Miki-iail, K.Y. The use of zeolites for the treatment of radioactive waste. Mineral. Mag. 1985, 49, 203–210. [Google Scholar]
- Kabwadza-Corner, P.; Munthali, M.W.; Johan, E.; Matsue, N. Comparative study of copper adsorptivity and selectivity toward zeolites. Amer. J. Analyt. Chem. 2014, 5, 395–405. [Google Scholar]
- Fruijtier-Pölloth, C. The safety of synthetic zeolites used in detergents. Arch. Toxicol. 2009, 83, 23–35. [Google Scholar]
- Dyer, A. An Introduction to Zeolite Molecular Sieves; John Wiley and Sons, Bath Press Ltd.: Bath, Avon, UK, 1988. [Google Scholar]
- Yousef, R.; El-Eswed, B. The effect of pH on the adsorption of phenol and chlorophenols onto natural zeolite. Physicochem. Eng. Aspects 2009, 334, 92–99. [Google Scholar]
- Seifert, R.; Rytz, R.; Calzaferri, G. Colors of Ag+-Exchanged Zeolite A. J. Phys. Chem. A 2009, 104, 7473–7483. [Google Scholar]
- Chen, W.; Wang, Z.; Lin, L.; Lin, J.; Su, M. Photostimulated luminescence of silver clusters in zeolite-Y. Physics Lett. A 1997, 232, 391–394. [Google Scholar]
- Menezes, R.A.; Paz, S.P.R.; Angélica, R.S.; Neves, R.F.; Pergher, S.B.C. Color and shade parameters of ultramarine zeolitic pigments synthesized from kaolin waste. Mater. Res. 2014, 17 (Suppl. 1), 23–27. [Google Scholar]
- Jankowska, A.; Kowalak, S. Cesium bearing ultramarine prepared from zeolites. Stud. Surf. Sci. Catal. 2008, 174, 193–196. [Google Scholar]
- Calzaferri, G.; Leiggener, C.; Glaus, S.; Schürcha, D.; Kuge, K. The electronic structure of Cu+, Ag+, and Au+ zeolites. Chem. Soc. Rev. 2003, 32, 29–37. [Google Scholar]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2001, 363, 1–24. [Google Scholar]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar]
- Munthali, M.W.; Kabwadza-Corner, P.; Johan, E.; Matsue, N. Decrease in cation exchange capacity of zeolites at neutral pH: Examples and proposal of a determination method. J. Mater. Sci. Chem. Eng. 2014, 2, 1–5. [Google Scholar]
- Santen, A.R.V.; Neurock, M. Molecular Heterogeneous Catalysis: A Conceptual and Computational Approach; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- McBride, M.B. Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 35–88. [Google Scholar]
- Kirov, G.; Filizova, L. Cationic hydration impact on zeolite formation and properties: A review and discussion. Geochem. Miner. Petrol. Sofia 2012, 49, 65–82. [Google Scholar]
- Barrer, R.M.; Klinowski, J. Influence of framework charge density on ion exchange properties of zeolites. J. Chem. Soc. Faraday Trans. 1 1972, 68, 1956–1963. [Google Scholar]
- Wada, S.; Kawabata, K. Ion adsorption on variable charge materials and thermodynamics of ion exchange. Soil Sci. Plant. Nutr. 1991, 37, 191–200. [Google Scholar]
- Fogg, A.M. Simple and mixed metal oxides and hydroxides: Solids with extended structures of different dimensionalities and porosity. In The group 13 metals, Aluminium, Gallium, Indium and Thallium: Chemical patterns and peculiarities; Aldridge, S., Downs, A.J., Eds.; John Wiley & Sons: New York, NY, USA, 2011; pp. 503–506. [Google Scholar]
- Munthali, M.W.; Elsheik, M.A.; Johan, E.; Matsue, N. Proton adsorption selectivity of zeolites in aqueous media: Effect of Si/Al ratio of zeolites. Molecules 2014, 19, 20468–20481. [Google Scholar]
- Noda, T.; Suzuki, K.; Katada, N.; Niwa, M. Combined study of IRMS-TPD measurement and DFT calculation on Brønstedacidity and catalytic cracking activity of cation-exchanged Y zeolites. J. Catalysis. 2008, 259, 203–210. [Google Scholar]
- Sastre, G.; Katada, N.; Niwa, M. Computational study of BrØnsted acidity of mordenite. Effect of the electric field on the infrared OH stretching frequencies. J. Phys. Chem. 2010, 114, 15424–15431. [Google Scholar]
- Hiromi, Y.; Masakazi, A. Photofunctional Zeolites: Synthesis, Characterization, Photocatalytic Reactions, Light Harvesting; Nova Science publishers: Huntington, NY, USA, 2000. [Google Scholar]
- van Santen, R.A.; Neurock, M. Molecular Heterogeneous Catalysis: A Conceptual and Computational Approach; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Beran, S.; Dubskÿ, J. Quantum chemical study of the electronic structure of Na-X and Na-Y zeolites. J. Phys. Chem. 1979, 83, 2538–2543. [Google Scholar]
- Kim, H.S.; Choi, S.Y.; Lim, W.T. Complete Li+ exchange into zeolite X (FAU, Si/Al=1.09) from undried methanol solution. J. Porous Mater. 2013, 20, 1449–1456. [Google Scholar]
- Herron, N.; Corbin, D.R. Inclusion Chemistry with Zeolites: Nanoscale Materials by Design; Kluwer Academic Publishers: Boston, MA, USA, 1995. [Google Scholar]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Separ. Purif. Tech. 2012, 86, 119–126. [Google Scholar]
- Querol, X.; Moreno, N.; Umana, J.C.; Alastuey, A.; Hernandez, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar]
- Sherry, H.S. The ion-exchange properties of zeolites. In Ion Exchange; Manisky, J.A., Dekker, M., Eds.; Marcel Dekker: New York, NY, USA, 1969; pp. 89–133. [Google Scholar]
- Sienko, M.; Plan, R.; Hester, R. Structural Inorganic Chemistry; Mir Publishers: Moscow, Russia, 1968; p. 344. (In Russian) [Google Scholar]
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar]
- Binder, H.; Zschornig, O. The effect of metal cations on the phase behavior and hydration characteristics of phospholipid membranes. Chem. Phys. Lipids 2002, 115, 39–61. [Google Scholar]
- Woods, R.M.; Gunter, M.E. Na-and Cs-exchange in a clinoptilolite-rich rock: Analysis of the outgoing cations in solution. Am. Mineral. 2001, 86, 424–430. [Google Scholar]
- Breck, D.W. Zeolites Molecular Sieves. Structure, Chemistry, and Use; Wiley-Interscience Publication: New York, NY, USA, 1974; p. 82. [Google Scholar]
- Loera, S.; Llewellyn, P.L.; Lima, E. Na+ charge tuning through encapsulation of sulfur chromophores in zeolite A and the consequences in adsorbent properties. J. Phys. Chem. 2010, 114, 7880–7887. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Ko, D.; Siriwardane, R.; Biegler, L.T. Optimization of pressure swing adsorption and fractionated vacuum pressure swing adsorption processes for CO2 capture. Ind. Eng. Chem. Res. 2005, 44, 8084–8094. [Google Scholar]
- Yang, S.; Navrotsky, A.; Wilkin, R. Thermodynamics of ion exchanged and natural clinoptilolite. Am. Mineral. 2001, 86, 438–447. [Google Scholar]
- Wilson, J.N.; Curtis, R.M. Dipole polarizabilities of ions in alkali halide crystals. J. Phys. Chem. 1970, 74, 187–196. [Google Scholar]
- Legras, B.; Polaert, I.; Estel, L. Effect of alcaline cations in zeolites on their dielectric properties. J. Microw. Power Electromagn. Energy 2012, 46, 2011–2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munthali, M.W.; Johan, E.; Matsue, N. Proton Adsorption Selectivity of Zeolites in Aqueous Media: Effect of Exchangeable Cation Species of Zeolites. Environments 2015, 2, 91-104. https://doi.org/10.3390/environments2010091
Munthali MW, Johan E, Matsue N. Proton Adsorption Selectivity of Zeolites in Aqueous Media: Effect of Exchangeable Cation Species of Zeolites. Environments. 2015; 2(1):91-104. https://doi.org/10.3390/environments2010091
Chicago/Turabian StyleMunthali, Moses Wazingwa, Erni Johan, and Naoto Matsue. 2015. "Proton Adsorption Selectivity of Zeolites in Aqueous Media: Effect of Exchangeable Cation Species of Zeolites" Environments 2, no. 1: 91-104. https://doi.org/10.3390/environments2010091





