Bioaccumulation of Selenium, Heavy Metals and Rare Earth Elements with Different Rice Cultivars Grown on Seleniferous Soils in Lianchen County, Fujian Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Descriptions of Lianchen (LC) County
2.2. Rice Cultivation Trial Design
2.2.1. Cultivars
2.2.2. Main Cultivar Field Trial
2.2.3. Verify Trial
2.3. Laboratory Analyses
2.3.1. Harvest and Analysis of Rice Plant Tissues
2.3.2. Soil Analyses
2.3.3. Sequential Extraction of Soil Se, Heavy Metals and Rare Earth Elements
2.4. Statistical Analyses
3. Results
3.1. Total Soil Se, Heavy Metals, and Rare Earth Elements
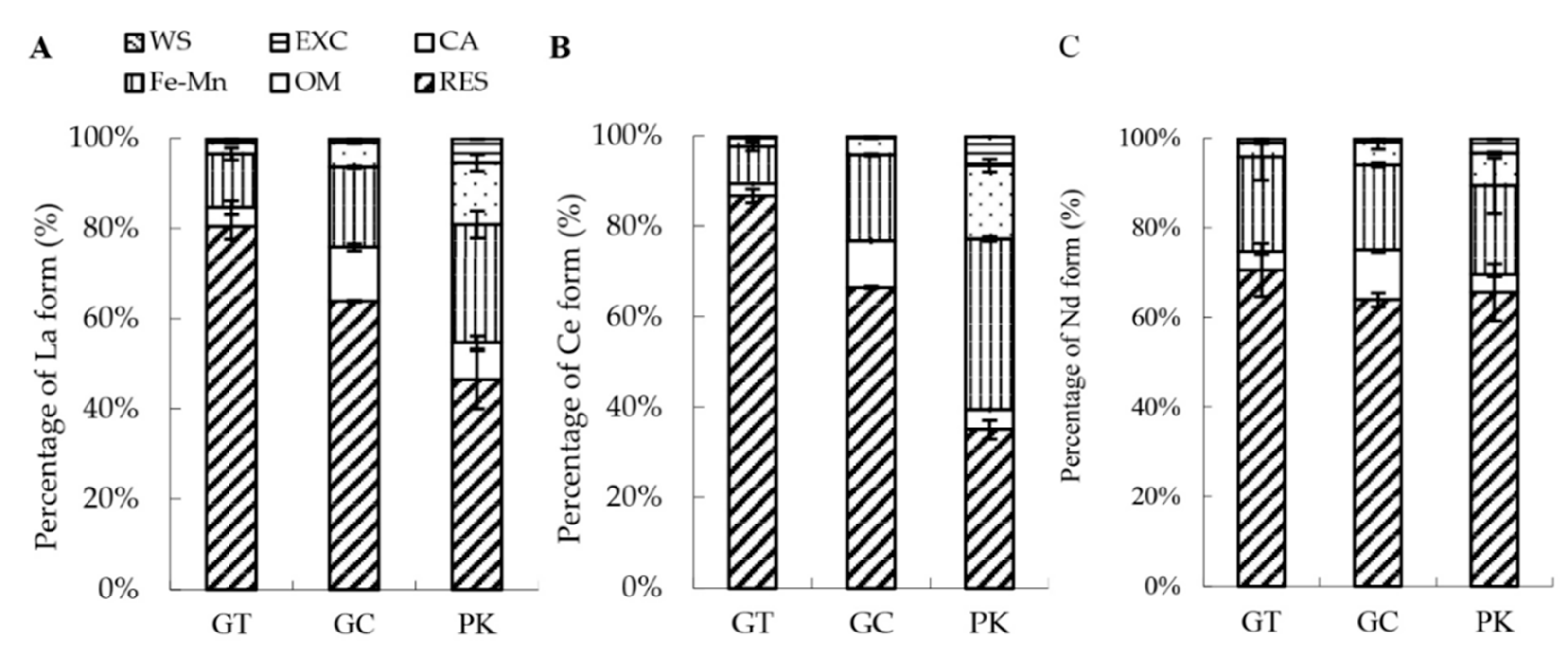
3.2. Sequential Extraction of Soil Se, Heavy Metals, and Rare Earth Elements
3.3. Se, Heavy Metals, and Rare Earth Elements in the Rice Tissues
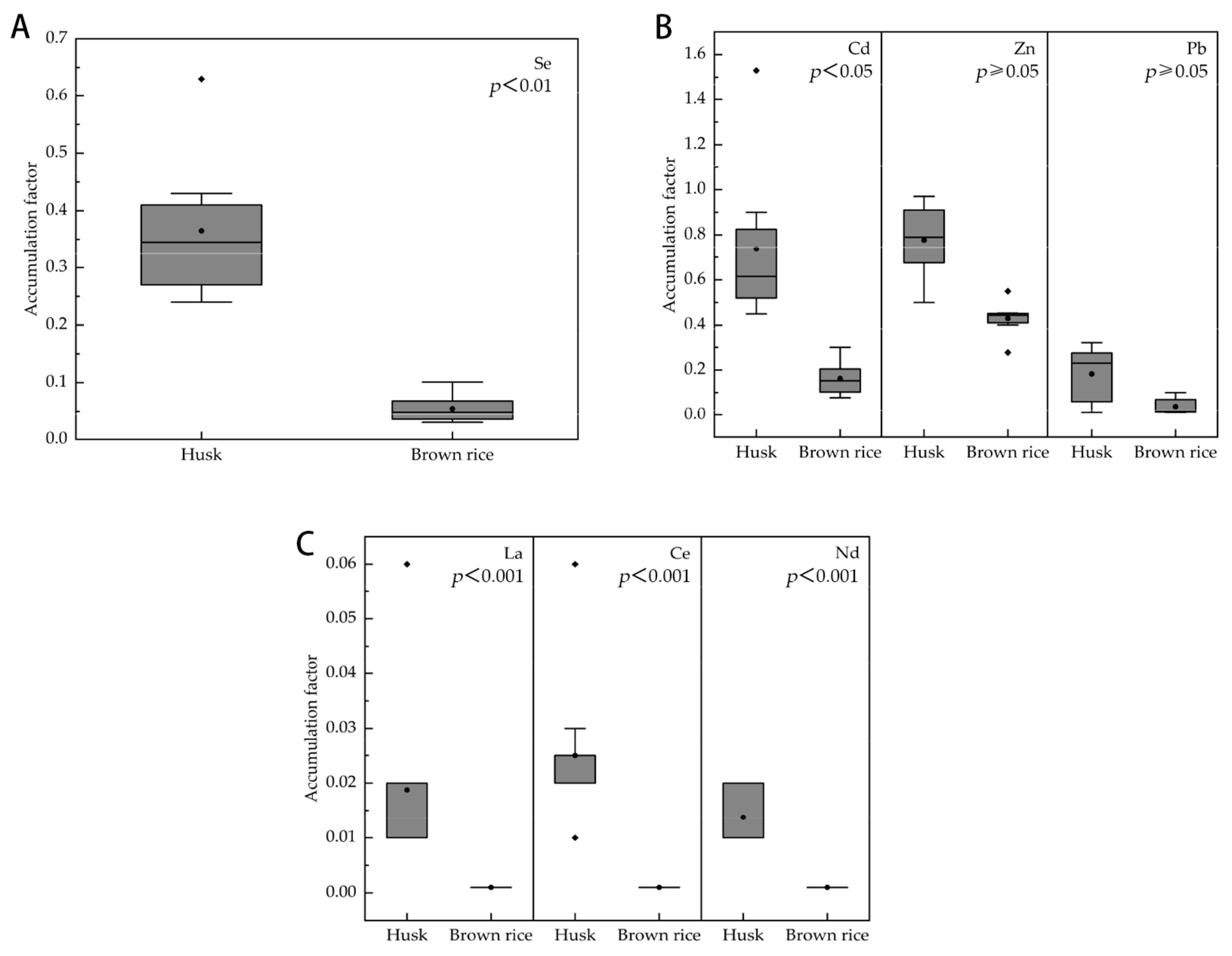
3.4. Accumulation of Se, Heavy Metals, and Rare Earth Elements in the Rice Tissues
4. Discussion
4.1. Se, Cd, Zn, Pb, La, Ce, and Nd Concentrations in Soils and Plant Tissues
4.2. Bioaccumulation of Se, Cd, Zn, Pb, La, Ce, and Nd within Different Plants and Soils
4.3. Sequential Extraction of Soil Se, Heavy Metals, and Rare Earth Elements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, M.S.; Lakin., H.W.; Beeson, K.C.; Smith, F.F.; Thacker, E. Selenium in agriculture. In USDA Agric. Handbook 200; US Gov. Print Office: Washington, DC, USA, 1961. [Google Scholar]
- Carey, A.M.; Lombi, E.; Donner, E.; De Jonge, M.D.; Punshon, T.; Jackson, B.P.; Guerinot, M.L.; Price, A.H.; Meharg, A.A. A review of recent developments in the speciation and location of arsenic and selenium in rice grain. Analy. Bioanaly. Chem. 2012, 402, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.S.; Noron, G.J.; Price, A.H.; Meharg, A.A. Grain accumulation of selenium species in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.S.; Dhillon, S.K. Selenium accumulation by sequentially grown wheat and rice as influenced by gypsum application in a seleniferous soil. Plant. Soil. 2000, 227, 243–248. [Google Scholar] [CrossRef]
- National Academy of Science-National Research Council (NAS-NRC). Selenium Assembly of Life Sciences; NAS-NRC: Washington, DC, USA, 1983. [Google Scholar]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology; Springer: Berlin, Germany, 2013; pp. 375–416. [Google Scholar]
- Fordyce, F.M. Selenium geochemistry and health. Ambio 2007, 36, 94–97. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.; Tack, F.M.G.; Kwon, E.E.; Rinklebe, J.; Yin, R. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Hooda, P.S. Assessing bioavailability of soil trace elements. In Trace Elements in Soils; Chapter 11; Hooda, P.S., Ed.; John Wiley and Sons Ltd. Publication: Wiltshire, UK, 2010; pp. 229–265. [Google Scholar]
- Luo, J.M.; Ye, Y.J.; Gao, Z.G.; Wang, Y.J.; Wang, W.F. Trace element (Pb, Cd and As) contamination in the sediments and organisms in Zhalong Wetland, Northeastern China. Soil. Sedim. Contamin. 2016, 25, 395–407. [Google Scholar] [CrossRef]
- Mihajlovic, J.; Bauriegel, A.; Stark, H.J.; Roßkopf, N.; Zeitz, J.; Milbert, G.; Rinklebe, J. Rare earth elements in soil profiles of various ecosystems across Germany. Appl. Geochem. 2019, 102, 197–217. [Google Scholar] [CrossRef]
- Feitosa, M.M.; da Silva, Y.J.A.B.; Biondi, C.M.; Alcantara, V.A.; do Nascimento, C.W.A. Rare Earth elements in rocks and soil profiles of a tropical volcanic archipelago in the Southern Atlantic. Catena 2020, 194, 104674. [Google Scholar] [CrossRef]
- Wu, C.; Hseu, Z. Pedochemical behaviors of rare earth elements in soil profiles along a lithosequence in eastern Taiwan. Catena 2023, 225, 107047. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.R.; Zhao, G.W. Assessment of the bioavailability of rare earth elements in soils by chemical fractionation and multiple regression analysis. Chemosphere 2000, 40, 23–28. [Google Scholar] [PubMed]
- Chen, H.M. Behavior of Chemical Matter and Environmental Mass of Soils; Scientific Publishers: Beijing, China, 2002; p. 663, (In Chinese with English abstract). [Google Scholar]
- Zhao, F.J.; Ma, Y.B.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Soil processes and the behavior of metals. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie Academic and Professional: Glasgow, UK, 1990; pp. 11–37. [Google Scholar]
- Huang, J.H.; Wang, S.L.; Lin, J.H.; Chen, Y.M.; Wang, M.K. Dynamics of cadmium concentration in contaminated rice paddy soils with submerging time. Paddy Water Environ. 2013, 11, 483–491. [Google Scholar] [CrossRef]
- Krishnamuri, G.S.R.; McArthur, D.E.F.; Wang, M.K.; Kozak, L.M.; Huang, P.M. Biogeochemistry of soil cadmium and the impact on terrestrial food chain contamination. In Biogeochemistry of Trace Elements in the Rhizosphere; Chapter, 7; Huang, P.M., Gobran, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 197–257. [Google Scholar]
- Kuwada, T.; Suzuku, S. A review on the cadmium content of rice, daily cadmium intake, and accumulation on the kidneys. J. Occup. Health 1998, 40, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilization within the plant and root Cd uptake post-flowering. Plant Soil. 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Zeng, F.R.; Ali, S.F.; Zhang, H.T.; Ouyang., Y.N.; Qju, B.Y.; Wu, F.B. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, X.M.; Xu, J.M.; Selim, H.M. Heavy metal contaminations in soil-rice system: Identification of spatial dependence in relation to soil properties of paddy fields. J. Hazard. Mater. 2010, 181, 778–787. [Google Scholar] [CrossRef]
- Roman, M. Selenium: Properties and determination. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 734–743. [Google Scholar]
- James, L.F.; Panter, K.E.; Mayland, H.F.; Miller, M.R.; Baker, D.C. Selenium poisoning in livestock: A review and Progress. In Selenium in Agriculture and the Environment; Chapter, 7; Jacobs, L.W., Chang, A.C., Sommers, L.E., Dowdy, R.H., Volk, V.V., Severson, R.C., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; pp. 123–131. [Google Scholar]
- Zhuan, W.M. Map of Fujian Province; Map Publisher: Fuzhou, China, 2008. [Google Scholar]
- Meharg, A.A.; Rahman, M. Arsenic contamination of Bangladesh paddy soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 220–234. [Google Scholar] [CrossRef]
- Rhoades, J.D. Cation-exchange capacity. In Methods of Soil Analysis. Part 2—Chemical and Microbiological Properties; Page, M., Keeney, D.R., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Course, A., Ed.; University of Wisconsin: Madison, WI, USA, 1979. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; pp. 383–412. [Google Scholar]
- Tessier, A.; Cambell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Munsell Soil Color Charts. Macbeth; Division of Kollmorgen Instruments Corp.: New York, NY, USA, 1952. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture and Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Fan, J.; Ren, J.; Liao, Q.; Ge, L.; Xu, H.; Cui, X.; Li, W.; Wang, Z. Antagonism between Se and Cd in typical farmland soil in southern Jiangsu Province. Soils 2021, 53, 1023–1032. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Q.; Han, T.; Ding, Y.; Sun, J.; Wang, F.; Zhu, C. Heavy metal pollution in a soil-rice system in the Yangtze River Region of China. Int. J. Environ. Res. Public Health 2016, 13, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Ma, Y.; Xu, R.; Yan, W. Environmental biogeochemical characteristics of rare earth elements in soil and soil-grown plants of the Hetai goldfield, Guangdong Province, China. Environ. Earth Sci. 2011, 63, 501–511. [Google Scholar] [CrossRef]
- Chapela, L.M.; Buss, H.L.; Pett-Ridge, J.C. The effects of lithology on trace element and REE behavior during tropical weathering. Chem. Geol. 2018, 500, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.; Wu, X.; Ren, Y.; Sun, C.; Liao, X.; Li, X.; Zhu, B. Geochemistry of trace and rare earth elements during weathering of black shale profiles in Northeast Chongqing, Southwestern China: Their mobilization, redistribution, and fractionation. Geochemistry 2015, 75, 403–417. [Google Scholar] [CrossRef]
- Kong, X.; Liu, T.; Yu, Z.; Chen, Z.; Lei, D.; Wang, Z.; Zhang, H.; Li, Q.; Zhang, S. Heavy metal bioaccumulation in rice from a high geological background area in Guizhou province, China. Int. J. Environ. Res. Public Health 2018, 15, 2281. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Chen, X.; Zhu, T.; Zhang, Z.; Fan, J. Effects of Selenium Fertilizer Application on Yield and Selenium Accumulation Characteristics of Different Japonica Rice Varieties. Sustainability 2021, 13, 10284. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Wu, J.; Gao, X.; Nyasha, N.K.; Cai, G.; Zhang, W. Bi-directional pollution characteristics and ecological health risk assessment of heavy metals in soil and crops in Wanjiang economic zone, Anhui province, China. Int. J. Environ. Res. Public Health 2022, 19, 9669. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, C.; Yan, Y.; Zu, C. Selenium distribution and translocation in rice (Oryza sativa L.) under different naturally seleniferous soils. Sustainability 2019, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.W.; Chang, A.C.; Sommers, L.E.; Dowdy, R.H.; Volk, V.V.; Severson, R.C. Selenium in Agriculture and the Environment; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; p. 233. [Google Scholar]
- Fedotov, P.S.; Savonina, E.Y.; Spivakov, B.Y.; Wennrich, R. Possibilities for the harmonization of methods of the dynamic fractionation of elements in soils and bottom sediments. J. Anal. Chem. 2012, 67, 851–861. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soil using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Yocubal, L.; Akyol, N.H. Adsorption and transport of arsenate in carbonate-rich soils: Coupled effects of nonlinear and rate–limited sorption. Chemosphere 2008, 73, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.Y.; Lin, K.C.; Lin, S.C.; Chang, T.K.; Wang, M.K. Arsenic and lead (beudantite) contamination of agricultural rice soils in the Guandu Plain of northern Taiwan. J. Hazard. Mater. 2010, 181, 1066–1071. [Google Scholar] [CrossRef]
- Liang, Y.C.; Sun, W.C.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshed, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Safe 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, Y.T.; Chen, Y.H.; Wang, S.L.; Wang, M.K.; Xie, T.H.; Wang, G. Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci. Rep. 2016, 6, 31616. [Google Scholar] [CrossRef]

| Elemental Form | Dissolvent | Disposal Method |
|---|---|---|
| F1: Water soluble (WS) | Deionized water | 1 h shaking, 20 °C |
| F2: Exchangeable (EXC) | 1 mol L−1 NH4OAc (pH = 7.0) | 2 h shaking, 20 °C |
| F3: Bound to carbonate and weakly specifically adsorbed (CA) | 1 mol L−1 NaOAc (adjusted to pH = 5.0 with HOAc) | 2 h shaking, 20 °C |
| F4: Bound to Fe–Mn oxides (Fe-Mn) | 0.04 mol L−1 NH2OH·HCl in 25% HOAc (v/v) | 6 h shaking, water bath, 60 °C |
| F5: Bound to organic matter (OM) | 15 mL 30% H2O2 (adjusted to pH = 2.0 with HNO3); after cooling, 5 mL 3.2 mol L−1 NH4OAc in 20% (v/v) HNO3, diluted to 20 mL | 5.5 h shaking, 80 °C |
| F6: Residual (RES) | 9 mL HNO3 and 3 mL HClO4 | 0.5 h shaking, microwave digestion |
| Sample | Soil Colour | pH | OM | TN | C/N | CEC |
|---|---|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (cmol kg−1) | ||||
| GT | 2.5YR 4/1 | 6.1 ± 0.0 | 43.5 ± 0.1 | 2.1 ± 0.0 | 12.2 ± 0.0 | 8.8 ± 0.0 |
| GC | 5YR 6/2 | 6.3 ± 0.0 | 33.3 ± 0.0 | 1.6 ± 0.0 | 12.3 ± 0.2 | 8.3 ± 0.0 |
| PK | 2.5YR 5/4 | 5.3 ± 0.0 | 32.0 ± 0.0 | 1.7 ± 0.0 | 11.0 ± 0.0 | 6.9 ± 0.0 |
| Sample | Exchangeable Cation | Base Saturation | Texture | |||
| K | Na | Ca | Mg | |||
| (mmol kg−1) | (%) | |||||
| GT | 9.1 ± 0.0 | 0.7 ± 0.0 | 26.3 ± 0.0 | 4.4 ± 0.0 | 46.2 ± 0.8 | Silty loam |
| GC | 7.5 ± 0.0 | 0.8 ± 0.0 | 25.8 ± 0.7 | 5.6 ± 0.2 | 47.8 ± 0.8 | Silty clay loam |
| PK | 3.8 ± 0.0 | 0.4 ± 0.0 | 12.6 ± 0.0 | 2.8 ± 0.0 | 28.4 ± 0.2 | Silty loam |
| Location | Se | Cd | Zn | Pb | La | Ce | Nd |
|---|---|---|---|---|---|---|---|
| GT | 0.8 ± 0.1 | 0.5 ± 0.1 | 76.9 ± 4.6 | 53.8 ± 3.4 | 320.0 ± 10.3 | 187.0 ± 55.2 | 438.1 ± 8.2 |
| GC | 0.8 ± 0.1 | 0.5 ± 0.0 | 48.3 ± 0.1 | 20.3 ± 2.3 | 40.2 ± 0.2 | 33.4 ± 0.1 | 69.8 ± 0.1 |
| PK | 0.8 ± 0.1 | 0.4 ± 0.0 | 52.6 ± 1.7 | 29.0 ± 3.7 | 95.5 ± 4.2 | 97.2 ± 4.8 | 187.9 ± 10.4 |
| Location /Cultivar | Stage /Plant Tissue | Se | Cd | Zn | Pb | La | Ce | Nd |
|---|---|---|---|---|---|---|---|---|
| GT/JZY | Booting stage/Root | 0.2 ± 0.1 cd | 0.7 ± 0.0 c | 60.2 ± 2.0 b | 21.4 ± 2.0 a | 60.6 ± 4.1 a | 43.8 ± 4.2 a | 74.0 ± 5.6 a |
| /Stem | 0.3 ± 0.0 d | 2.3 ± 0.0 e | 37.6 ± 2.7 c | 4.3 ± 0.1 e | 7.1 ± 0.1 c | 4.9 ± 0.0 c | 8.9 ± 0.1 c | |
| /Leaf | 0.3 ± 0.1 bc | 0.3 ± 0.1 e | 24.1 ± 1.5 d | 3.0 ± 0.0 e | 1.5 ± 0.0 d | 0.8 ± 0.0 d | 2.4 ± 1.4 de | |
| Filling stage/Root | 0.2 ± 0.0 cd | 1.2 ± 0.1 f | 59.4 ± 6.6 ef | 24.4 ± 1.8 b | 33.0 ± 2.6 b | 31.9 ± 2.5 b | 62.9 ± 5.3 b | |
| /Stem | 0.2 ± 0.1 de | 0.3 ± 0.0 if | 52.0 ± 0.4 f | 2.8 ± 0.3 f | 2.4 ± 0.1 e | 1.9 ± 0.1 e | 3.7 ± 0.2 de | |
| /Leaf | 0.2 ± 0.0 cd | 0.2 ± 0.0 ijk | 32.3 ± 2.1 h | 2.3 ± 0.1 fg | 1.4 ± 0.1 efg | 0.9 ± 0.0 efg | 1.3 ± 0.1 ef | |
| /Husk | 0.2 ± 0.0 cd | 0.1 ± 0.0 jk | 23.6 ± 1.0 ij | 1.1 ± 0.0 ghi | 0.8 ± 0.0 fg | 0.5 ± 0.0 fg | 0.7 ± 0.0 f | |
| /Brown rice | 0.0 ± 0.0 e | 0.1 ± 0.0 k | 19.8 ± 0.1 j | 0.6 ± 0.0 hi | 0.0 ± 0.0 g | 0.0 ± 0.0 g | <0.001 | |
| GC /WSSM | Booting stage/Root | 0.3 ± 0.0 bc | 1.8 ± 0.4 a | 101.7 ± 3.0 a | 15.4 ± 1.6 b | 3.0 ± 0.1 e | 2.5 ± 0.1 c | 7.5 ± 0.1 c |
| /Stem | 0.4 ± 0.1 a | 0.7 ± 0.0 bc | 60.1 ± 10.1 b | 8.9 ± 0.0 d | 1.0 ± 0.0 d | 0.7 ± 0.0 de | 2.8 ± 0.6 de | |
| /Leaf | 0.3 ± 0.0 abc | 0.3 ± 0.0 de | 36.2 ± 1.0 c | 4.1 ± 0.0 e | 1.1 ± 0.0 d | 0.7 ± 0.0 de | 2.2 ± 0.0 de | |
| Filling stage/Root | 0.5 ± 0.2 a | 2.3 ± 0.0 b | 158.4 ± 3.3 a | 18.3 ± 2.5 c | 9.7 ± 0.1 c | 7.4 ± 0.1 c | 18.0 ± 0.8 c | |
| /Stem | 0.3 ± 0.0 b | 2.1 ± 0.0 c | 134.2 ± 6.2 b | 14.4 ± 0.0 d | 1.2 ± 0.1 efg | 0.8 ± 0.0 efg | 1.7 ± 0.4 ef | |
| /Leaf | 0.3 ± 0.0 b | 1.4 ± 0.2 e | 61.0 ± 1.7 e | 4.5 ± 0.0 e | 1.0 ± 0.0 efg | 0.7 ± 0.0 fg | 0.6 ± 0.0 f | |
| /Husk | 0.3 ± 0.0 b | 0.7 ± 0.2 h | 29.1 ± 0.3 hi | 2.0 ± 0.0 fgh | 0.6 ± 0.0 fg | 0.6 ± 0.0 fg | 0.6 ± 0.0 f | |
| /Brown rice | 0.0 ± 0.0 e | 0.0 ± 0.0 i | 20.7 ± 1.6 j | 1.0 ± 0.0 ghi | 0.0 ± 0.0 g | 0.0 ± 0.0 g | <0.001 | |
| PK /HBS | Booting stage/Root | 0.3 ± 0.1 bc | 0.7 ± 0.0 c | 59.6 ± 1.9 b | 16.0 ± 0.0 b | 17.0 ± 0.9 b | 15.9 ± 0.8 b | 31.9 ± 2.6 b |
| /Stem | 0.3 ± 0.0 bc | 1.0 ± 0.2 b | 57.0 ± 0.9 b | 16.7 ± 0.6 b | 2.6 ± 0.1 d | 2.2 ± 0.1 d | 5.4 ± 0.2 cd | |
| /Leaf | 0.2 ± 0.0 cd | 1.3 ± 0.3 cd | 39.5 ± 0.1 c | 14.2 ± 0.2 c | 1.3 ± 0.0 d | 1.1 ± 0.0 e | 1.5 ± 0.1 e | |
| Filling stage/Root | 0.5 ± 0.1 a | 0.7 ± 0.0 a | 103.9 ± 1.8 a | 44.3 ± 1.6 a | 62.4 ± 2.6 a | 42.0 ± 1.2 a | 80.4 ± 2.8 a | |
| /Stem | 0.3 ± 0.0 cd | 2.0 ± 0.3 d | 79.4 ± 10.3 d | 5.3 ± 0.0 e | 5.4 ± 0.7 d | 3.7 ± 0.4 d | 6.6 ± 1.0 d | |
| /Leaf | 0.2 ± 0.0 cd | 1.0 ± 0.0 g | 42.1 ± 9.4 g | 4.8 ± 0.4 e | 2.1 ± 0.0 ef | 1.4 ± 0.0 ef | 1.9 ± 0.0 ef | |
| /Husk | 0.2 ± 0.0 cd | 0.6 ± 0.0 h | 22.1 ± 3.4 ij | 0.4 ± 0.0 i | 0.0 ± 0.0 g | 0.3 ± 0.0 fg | 0.0 ± 0.0 f | |
| /Brown rice | 0.0 ± 0.0 e | 0.2 ± 0.0 ijk | 7.5 ± 0.1 k | 0.0 ± 0.0 i | <0.001 # | <0.001 | <0.001 |
| Location /Cultivar | Stage /Plant Tissue | Se | Cd | Zn | Pb | La | Ce | Nd |
|---|---|---|---|---|---|---|---|---|
| GT/JZY | Booting stage/Root | 0.29 | 1.38 | 0.78 | 0.40 | 0.19 | 0.26 | 0.18 |
| /Stem | 0.34 | 4.85 | 0.49 | 0.49 | 0.02 | 0.03 | 0.02 | |
| /Leaf | 0.43 | 0.64 | 0.31 | 0.05 | 0.05 | 0.04 | 0.06 | |
| Filling stage/Root | 0.28 | 2.60 | 0.77 | 0.45 | 0.10 | 0.17 | 0.14 | |
| /Stem | 0.19 | 0.60 | 0.68 | 0.05 | 0.08 | 0.01 | 0.01 | |
| /Leaf | 0.25 | 0.40 | 0.42 | 0.04 | 0.05 | 0.05 | 0.03 | |
| /Husk | 0.19 | 0.26 | 0.31 | 0.02 | 0.23 | 0.03 | 0.05 | |
| /Brown rice | 0.04 | 0.13 | 0.26 | 0.01 | <0.001 # | <0.001 | <0.001 | |
| GC/WSSM | Booting stage/Root | 0.40 | 4.00 | 2.11 | 0.76 | 0.07 | 0.07 | 0.01 |
| /Stem | 0.48 | 1.50 | 1.25 | 0.44 | 0.03 | 0.02 | 0.03 | |
| /Leaf | 0.39 | 0.70 | 0.75 | 0.20 | 0.03 | 0.02 | 0.03 | |
| Filling stage/Root | 0.59 | 4.96 | 3.28 | 0.90 | 0.24 | 0.22 | 0.25 | |
| /Stem | 0.35 | 4.49 | 2.78 | 0.71 | 0.03 | 0.02 | 0.02 | |
| /Leaf | 0.34 | 3.09 | 1.26 | 0.22 | 0.02 | 0.02 | 0.09 | |
| /Husk | 0.36 | 1.52 | 0.60 | 0.10 | 0.02 | 0.02 | 0.02 | |
| /Brown rice | 0.02 | 0.07 | 0.42 | 0.05 | <0.001 | <0.001 | <0.001 | |
| PK/HBS | Booting stage/Root | 0.41 | 1.55 | 1.13 | 0.54 | 0.18 | 0.16 | 0.17 |
| /Stem | 0.41 | 2.20 | 1.08 | 0.56 | 0.02 | 0.02 | 0.03 | |
| /Leaf | 0.32 | 2.86 | 0.75 | 0.48 | 0.01 | 0.01 | 0.01 | |
| Filling stage/Root | 0.68 | 1.55 | 1.97 | 1.50 | 0.63 | 0.43 | 0.43 | |
| /Stem | 0.34 | 4.50 | 1.51 | 0.18 | 0.06 | 0.04 | 0.04 | |
| /Leaf | 0.32 | 2.20 | 0.80 | 0.16 | 0.02 | 0.02 | 0.01 | |
| /Husk | 0.31 | 1.30 | 0.42 | 0.01 | <0.001 | <0.001 | <0.001 | |
| /Brown rice | 0.04 | 0.39 | 0.01 | 0.02 | <0.001 | <0.001 | <0.001 |
| Cultivar /Location | Tissue | Se | Cd | Zn | Pb | La | Ce | Nd |
|---|---|---|---|---|---|---|---|---|
| JLY289/BT | Husk | 0.24 | 0.23 | 46.05 | 0.33 | 0.58 | 0.60 | 0.86 |
| Brown rice | 0.02 | 0.05 | 22.69 | 0.30 | 0.03 | <0.001 # | 0.01 | |
| HHZ/BT | Husk | 0.41 | 0.25 | 43.54 | 8.16 | 0.56 | 0.58 | 1.16 |
| Brown rice | 0.02 | 0.06 | 27.61 | 0.41 | 0.03 | <0.001 | <0.001 | |
| HBS/GT | Husk | 0.19 | 0.28 | 38.82 | 4.85 | 0.58 | 0.58 | 0.93 |
| Brown rice | 0.05 | 0.04 | 21.33 | 0.84 | 0.02 | <0.001 | 0.13 | |
| XYZ/GC | Husk | 0.21 | 0.25 | 32.50 | 6.15 | 0.67 | 0.65 | 10.74 |
| Brown rice | 0.04 | 0.04 | 21.70 | 0.98 | 0.02 | <0.001 | 0.04 | |
| YXZ/GC | Husk | 0.32 | 0.18 | 43.53 | 5.05 | 0.59 | 0.60 | 0.75 |
| Brown rice | 0.06 | 0.12 | 20.29 | 2.03 | 0.03 | <0.001 | 0.22 | |
| WSSM/GC | Husk | 0.27 | 0.61 | 34.49 | 6.49 | 0.57 | 0.61 | 0.59 |
| Brown rice | 0.03 | 0.07 | 21.90 | 0.41 | 0.03 | <0.001 | <0.001 | |
| JN313/GC | Husk | 0.24 | 0.36 | 46.55 | 4.52 | 0.59 | 0.59 | 1.34 |
| Brown rice | 0.38 | 0.17 | 19.35 | 1.86 | 0.02 | <0.001 | 0.10 | |
| XYZ/PK | Husk | 0.32 | 0.33 | 35.77 | 7.16 | 0.57 | 0.60 | 0.56 |
| Brown rice | 0.08 | 0.10 | 23.02 | 0.34 | 0.02 | <0.001 | 0.12 | |
| Soil/Location | ||||||||
| /BT | 0.65 | 0.46 | 50.23 | 30.84 | 40.84 | 34.73 | 116.60 | |
| /GT | 0.80 | 0.47 | 76.87 | 53.81 | 319.98 | 187.01 | 438.08 | |
| /GC | 0.83 | 0.46 | 48.25 | 20.29 | 40.80 | 33.36 | 69.78 | |
| /PK | 0.74 | 0.44 | 52.61 | 29.63 | 95.47 | 97.17 | 187.88 | |
| Index | Soil | Booting Stage | Filling Stage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Root | Stem | Leaf | Husk | Brown Rice | |||
| Soil | 1.00 | −0.93 | −0.90 | 0.00 | −1.00 ** | −0.98 * | −0.95 * | −0.85 | −0.87 | |
| Booting stage | Root | 1.00 | 1.00 ** | 0.36 | 0.91 | 0.99 * | 1.00 ** | 0.98 * | −0.99 * | |
| Stem | 1.00 | 0.43 | 0.87 | 0.97 * | 0.99 * | 0.99 ** | −1.00 ** | |||
| Leaf | 1.00 | −0.06 | 0.20 | 0.30 | 0.53 | −0.50 | ||||
| Filling stage | Root | 1.00 | 0.97 * | 0.93 | 0.81 | −0.83 | ||||
| Stem | 1.00 | 0.99 ** | 0.94 | −0.95 | ||||||
| Leaf | 1.00 | 0.97 * | −0.98 * | |||||||
| Husk | 1.00 | −1.00 ** | ||||||||
| Brown rice | 1.00 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Chen, J.; Wang, H.; Lu, X.; Luo, X.; Wang, M. Bioaccumulation of Selenium, Heavy Metals and Rare Earth Elements with Different Rice Cultivars Grown on Seleniferous Soils in Lianchen County, Fujian Province, China. Environments 2023, 10, 114. https://doi.org/10.3390/environments10070114
Chen M, Chen J, Wang H, Lu X, Luo X, Wang M. Bioaccumulation of Selenium, Heavy Metals and Rare Earth Elements with Different Rice Cultivars Grown on Seleniferous Soils in Lianchen County, Fujian Province, China. Environments. 2023; 10(7):114. https://doi.org/10.3390/environments10070114
Chicago/Turabian StyleChen, Minjian, Jinying Chen, Huangping Wang, Xianyong Lu, Xuhui Luo, and Mingkuang Wang. 2023. "Bioaccumulation of Selenium, Heavy Metals and Rare Earth Elements with Different Rice Cultivars Grown on Seleniferous Soils in Lianchen County, Fujian Province, China" Environments 10, no. 7: 114. https://doi.org/10.3390/environments10070114




