Characterization of Staphylococcus intermedius Group Isolates Associated with Animals from Antarctica and Emended Description of Staphylococcus delphini
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Their Biochemical Characterization
2.2. Partial 16S rRNA and RNA polymerase beta-subunit (rpoB) Gene Sequencing and Phylogenetic Analysis
2.3. Rep-PCR
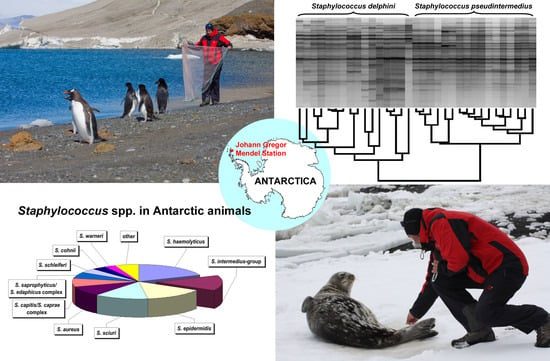
2.4. MALDI-TOF MS
2.5. Whole Genome Sequencing and Bioinformatic Analyses
3. Results
3.1. Bacterial Strain Collection and Identification of Staphylococci
3.2. rpoB Gene Sequencing
3.3. Repetitive Sequence-Based PCR (Rep-PCR) Fingerprinting
3.4. MALDI-TOF MS
3.5. Biochemical Identification
3.6. Comparative Genomic Analysis of Staphylococcus delphini Isolates
4. Discussion
5. Emended Description of Staphylococcus delphini (Varaldo et al., 1988)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Götz, F.; Bannerman, T.; Schleifer, K.-H. The Genera Staphylococcus and Macrococcus. In The Prokaryotes. Volume 4: Bacteria: Firmicutes, Cyanobacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer Science: New York, NY, USA, 2006; Volume 4, pp. 5–75. [Google Scholar]
- Pantůček, R.; Sedláček, I.; Indráková, A.; Vrbovská, V.; Mašlaňová, I.; Kovařovic, V.; Švec, P.; Králová, S.; Krištofová, L.; Kekláková, J.; et al. Staphylococcus edaphicus sp. nov., isolated in Antarctica, harbors the mecC gene and genomic islands with a suspected role in adaptation to extreme environments. Appl. Env. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Shivaji, S.; Begum, Z.; Shiva Nageswara Rao, S.S.; Vishnu Vardhan Reddy, P.V.; Manasa, P.; Sailaja, B.; Prathiba, M.S.; Thamban, M.; Krishnan, K.P.; Singh, S.M.; et al. Antarctic ice core samples: Culturable bacterial diversity. Res. Microbiol. 2013, 164, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Peeters, K.; Hodgson, D.A.; Convey, P.; Willems, A. Culturable diversity of heterotrophic bacteria in Forlidas Pond (Pensacola Mountains) and Lundstrom Lake (Shackleton Range), Antarctica. Microbiol. Ecol. 2011, 62, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Arenas, F.A.; Pugin, B.; Henriquez, N.A.; Arenas-Salinas, M.A.; Diaz-Vasquez, W.A.; Pozo, M.F.; Munoz, C.M.; Chasteen, T.G.; Perez-Donoso, J.M.; Vasquez, C.C. Isolation, identification and characterization of highly tellurite-resistant, tellurite-reducing bacteria from Antarctica. Polar Sci. 2014, 8, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Leiva, S.; Alvarado, P.; Huang, Y.; Wang, J.; Garrido, I. Diversity of pigmented Gram-positive bacteria associated with marine macroalgae from Antarctica. FEMS Microbiol. Lett. 2015, 362, fnv206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieburth, J.M. Gastrointestinal microflora of Antarctic birds. J. Bacteriol. 1959, 77, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, S.C.; Merino, L.N.R.; Maccormack, W.P.; Fraile, E.R. Protease-producing psychrotrophic bacteria isolated from Antarctica. Polar Biol. 1995, 15, 131–135. [Google Scholar] [CrossRef]
- Nievas, V.F.; Leotta, G.A.; Vigo, G.B. Subcutaneous clostridial infection in Adelie penguins in Hope Bay, Antarctica. Polar Biol. 2007, 30, 249–252. [Google Scholar] [CrossRef]
- Mellish, J.; Tuomi, P.; Hindle, A.; Jang, S.; Horning, M. Skin microbial flora and effectiveness of aseptic technique for deep muscle biopsies in Weddell seals (Leptonychotes weddellii) in McMurdo Sound, Antarctica. J. Wildlife Dis. 2010, 46, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Bannoehr, J.; Ben Zakour, N.L.; Waller, A.S.; Guardabassi, L.; Thoday, K.L.; van den Broek, A.H.M.; Fitzgerald, J.R. Population genetic structure of the Staphylococcus intermedius group: Insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 2007, 189, 8685–8692. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Reclassification of phenotypically identified Staphylococcus intermedius strains. J. Clin. Microbiol. 2007, 45, 2770–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hájek, V. Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Bacteriol. 1976, 26, 401–408. [Google Scholar] [CrossRef]
- Devriese, L.A.; Vancanneyt, M.; Baele, M.; Vaneechoutte, M.; De Graef, E.; Snauwaert, C.; Cleenwerck, I.; Dawyndt, P.; Swings, J.; Decostere, A.; et al. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 2005, 55, 1569–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varaldo, P.E.; Kilpperbalz, R.; Biavasco, F.; Satta, G.; Schleifer, K.H. Staphylococcus delphini sp. nov., a coagulase-positive species isolated from dolphins. Int. J. Syst. Bacteriol. 1988, 38, 436–439. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.K.; Lee, J.; Bendall, R.; Zhang, L.; Sunde, M.; Schau Slettemeas, J.; Gaze, W.; Page, A.J.; Vos, M. Staphylococcus cornubiensis sp. nov., a member of the Staphylococcus intermedius group (SIG). Int. J. Syst. Evol. Microbiol. 2018, 68, 3404–3408. [Google Scholar] [CrossRef]
- Bond, R.; Loeffler, A. What’s happened to Staphylococcus intermedius? Taxonomic revision and emergence of multi-drug resistance. J. Small Anim. Pr. 2012, 53, 147–154. [Google Scholar] [CrossRef]
- Viau, R.; Hujer, A.M.; Hujer, K.M.; Bonomo, R.A.; Jump, R.L.P. Are Staphylococcus intermedius infections in humans cases of mistaken identity? A case series and literature review. Open Forum Infect. Dis. 2015, 2, ofv110. [Google Scholar] [CrossRef] [Green Version]
- Decristophoris, P.; Fasola, A.; Benagli, C.; Tonolla, M.; Petrini, O. Identification of Staphylococcus intermedius group by MALDI-TOF MS. Syst. Appl. Microbiol. 2011, 34, 45–51. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Walther, B.; Stamm, I.; Abou-Elnaga, Y.; Brueggemann-Schwarze, S.; Vincze, S.; Wieler, L.H.; Lubke-Becker, A.; Semmler, T.; Roesler, U. Species differentiation within the Staphylococcus intermedius group using a refined MALDI-TOF MS database. Clin. Microbiol. Infect. 2014, 20, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Murray, A.; Bendall, R.; Gaze, W.; Zhang, L.H.; Vos, M. Improved detection of Staphylococcus intermedius group in a routine diagnostic laboratory. J. Clin. Microbiol. 2015, 53, 961–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slettemeas, J.S.; Mikalsen, J.; Sunde, M. Further diversity of the Staphylococcus intermedius group and heterogeneity in the MboI restriction site used for Staphylococcus pseudintermedius species identification. J. Vet. Diagn. Invest. 2010, 22, 756–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solyman, S.M.; Black, C.C.; Duim, B.; Perreten, V.; van Duijkeren, E.; Wagenaar, J.A.; Eberlein, L.C.; Sadeghi, L.N.; Videla, R.; Bemis, D.A.; et al. Multilocus sequence typing for characterization of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2013, 51, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Zakour, N.L.; Beatson, S.A.; van den Broek, A.H.M.; Thoday, K.L.; Fitzgerald, J.R. Comparative genomics of the Staphylococcus intermedius group of animal pathogens. Front. Cell. Infect. Microbiol. 2012, 2, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstappen, K.M.; Huijbregts, L.; Spaninks, M.; Wagenaar, J.A.; Fluit, A.C.; Duim, B. Development of a real-time PCR for detection of Staphylococcus pseudintermedius using a novel automated comparison of whole-genome sequences. PLoS ONE 2017, 12, e0189520. [Google Scholar] [CrossRef] [Green Version]
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J.R. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009, 47, 469–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borjesson, S.; Gomez-Sanz, E.; Ekstrom, K.; Torres, C.; Gronlund, U. Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur. J. Clin. Microbiol. 2015, 34, 839–844. [Google Scholar] [CrossRef]
- Lainhart, W.; Yarbrough, M.L.; Burnham, C.A. The brief case: Staphylococcus intermedius group-look what the dog dragged in. J. Clin. Microbiol. 2018, 56, e00839-17. [Google Scholar] [CrossRef] [Green Version]
- Hanselman, B.A.; Kruth, S.A.; Rousseau, J.; Weese, J.S. Coagulase positive staphylococcal colonization of humans and their household pets. Can. Vet. J. 2009, 50, 954–958. [Google Scholar]
- Ishihara, K.; Shimokubo, N.; Sakagami, A.; Ueno, H.; Muramatsu, Y.; Kadosawa, T.; Yanagisawa, C.; Hanaki, H.; Nakajima, C.; Suzuki, Y.; et al. Occurrence and molecular characteristics of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius in an academic veterinary hospital. Appl. Env. Microbiol. 2010, 76, 5165–5174. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Methicillin-resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. J. Clin. Microbiol. 2007, 45, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Soedarmanto, I.; Kanbar, T.; Ulbegi-Mohyla, H.; Hijazin, M.; Alber, J.; Lammler, C.; Akineden, O.; Weiss, R.; Moritz, A.; Zschock, M. Genetic relatedness of methicillin-resistant Staphylococcus pseudintermedius (MRSP) isolated from a dog and the dog owner. Res. Vet. Sci. 2011, 91, E25–E27. [Google Scholar] [CrossRef] [PubMed]
- Van Hoovels, L.; Vankeerberghen, A.; Boel, A.; Van Vaerenbergh, K.; De Beenhouwer, H. First case of Staphylococcus pseudintermedius infection in a human. J. Clin. Microbiol. 2006, 44, 4609–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardabassi, L.; Schmidt, K.R.; Petersen, T.S.; Espinosa-Gongora, C.; Moodley, A.; Agerso, Y.; Olsen, J.E. Mustelidae are natural hosts of Staphylococcus delphini group A. Vet. Microbiol. 2012, 159, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Magleby, R.; Bemis, D.A.; Kim, D.; Carroll, K.C.; Castanheira, M.; Kania, S.A.; Jenkins, S.G.; Westblade, L.F. First reported human isolation of Staphylococcus delphini. Diagn. Microbiol. Infect. Dis. 2019, 94, 274–276. [Google Scholar] [CrossRef]
- Nikolaisen, N.K.; Lassen, D.C.K.; Chriel, M.; Larsen, G.; Jensen, V.F.; Pedersen, K. Antimicrobial resistance among pathogenic bacteria from mink (Neovison vison) in Denmark. Acta. Vet. Scand. 2017, 59, 60. [Google Scholar] [CrossRef] [Green Version]
- Novakova, D.; Sedlacek, I.; Pantucek, R.; Stetina, V.; Svec, P.; Petras, P. Staphylococcus equorum and Staphylococcus succinus isolated from human clinical specimens. J. Med. Microbiol. 2006, 55, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Mellmann, A.; Becker, K.; von Eiff, C.; Keckevoet, U.; Schumann, P.; Harmsen, D. Sequencing and staphylococci identification. Emerg. Infect. Dis. 2006, 12, 333–336. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Švec, P.; Pantůček, R.; Petráš, P.; Sedláček, I.; Nováková, D. Identification of Staphylococcus spp. using (GTG)5-PCR fingerprinting. Syst. Appl. Microbiol. 2010, 33, 451–456. [Google Scholar] [CrossRef]
- Freiwald, A.; Sauer, S. Phylogenetic classification and identification of bacteria by mass spectrometry. Nat. Protoc. 2009, 4, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000 Research 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J. Comput. Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushnell, B. BBMap Short-Read Aligner, and Other Bioinformatics Tools; Lawrence Berkeley National Lab.: Berkeley, CA, USA; Available online: http://sourceforge.net/projects/bbmap/ (accessed on 1 March 2019).
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Yu, C.S.; Cheng, C.W.; Su, W.C.; Chang, K.C.; Huang, S.W.; Hwang, J.K.; Lu, C.H. CELLO2GO: A web server for protein subCELlular LOcalization prediction with functional gene ontology annotation. PLoS ONE 2014, 9, e99368. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef] [Green Version]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [Green Version]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkau, A.; Stuart-Edwards, M.; Stothard, P.; Van Domselaar, G. Interactive microbial genome visualization with GView. Bioinformatics 2010, 26, 3125–3126. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, Y.O.; Yoon, S.H.; Ha, S.M.; Baek, I.; Chun, J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018, 56, 280–285. [Google Scholar] [CrossRef]
- Cole, K.; Foster, D.; Russell, J.E.; Golubchik, T.; Llewelyn, M.; Wilson, D.J.; Crook, D.; Paul, J.; Modernising Medical Microbiology Consortium. Draft genome sequences of 64 type strains of 50 species and 25 subspecies of the genus Staphylococcus Rosenbach 1884. Microbiol. Resour. Announc. 2019, 8, e00062-19. [Google Scholar] [CrossRef] [Green Version]
- Stepan, J.; Pantucek, R.; Doskar, J. Molecular diagnostics of clinically important staphylococci. Folia Microbiol. 2004, 49, 353–386. [Google Scholar] [CrossRef]
- Richter, M.; Rossello-Mora, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Harmsen, D.; Mellmann, A.; Meier, C.; Schumann, P.; Peters, G.; von Eiff, C. Development and evaluation of a quality-controlled ribosomal sequence database for 16S ribosomal DNA-based identification of Staphylococcus species. J. Clin. Microbiol. 2004, 42, 4988–4995. [Google Scholar] [CrossRef] [Green Version]
- Ah Tow, L.; Cowan, D.A. Dissemination and survival of non-indigenous bacterial genomes in pristine Antarctic environments. Extremophiles 2005, 9, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Elk, C.E.; Boelens, H.A.M.; van Belkum, A.; Foster, G.; Kuiken, T. Indications for both host-specific and introduced genotypes of Staphylococcus aureus in marine mammals. Vet. Microbiol. 2012, 156, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Wayne, R.K. Molecular evolution of the dog family. Trends Genet. 1993, 9, 218–224. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Comparative ribotyping of Staphylococcus intermedius isolated from members of the Canoidea gives possible evidence for host-specificity and co-evolution of bacteria and hosts. Int. J. Syst. Evol. Microbiol. 2001, 51, 1343–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantůček, R.; Sedláček, I.; Petráš, P.; Koukalová, D.; Švec, P.; Štětina, V.; Vancanneyt, M.; Chrastinová, L.; Vokurková, J.; Růžičková, V.; et al. Staphylococcus simiae sp. nov., isolated from South American squirrel monkeys. Int. J. Syst. Evol. Microbiol. 2005, 55, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Lefebure, T.; Bitar, P.P.; Stanhope, M.J. Comparative genomic analysis of the genus Staphylococcus including Staphylococcus aureus and its newly described sister species Staphylococcus simiae. BMC Genom. 2012, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: the non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef]
- Fitzgerald, J.R. The Staphylococcus intermedius group of bacterial pathogens: species re-classification, pathogenesis and the emergence of methicillin resistance. Vet. Derm. 2009, 20, 490–495. [Google Scholar] [CrossRef]
- Canver, M.C.; Tekle, T.; Compton, S.T.; Callan, K.; Burd, E.M.; Zimmer, B.L.; Bemis, D.A.; Carroll, K.C.; Westblade, L.F. Performance of five commercial identification platforms for identification of Staphylococcus delphini. J. Clin. Microbiol. 2019, 57, e00721-19. [Google Scholar] [CrossRef]
- Chua, K.Y.; Stinear, T.P.; Howden, B.P. Functional genomics of Staphylococcus aureus. Brief Funct. Genom. 2013, 12, 305–315. [Google Scholar] [CrossRef]
- Ingmer, H.; Gerlach, D.; Wolz, C. Temperate phages of Staphylococcus aureus. Microbiol. Spectr. 2019, 7. [CrossRef]
- Maslanova, I.; Stribna, S.; Doskar, J.; Pantucek, R. Efficient plasmid transduction to Staphylococcus aureus strains insensitive to the lytic action of transducing phage. FEMS Microbiol. Lett. 2016, 363, fnw211. [Google Scholar] [CrossRef] [PubMed]
- Pires Dos Santos, T.; Damborg, P.; Moodley, A.; Guardabassi, L. Systematic review on global epidemiology of methicillin-resistant Staphylococcus pseudintermedius: Inference of population structure from multilocus sequence typing data. Front. Microbiol. 2016, 7, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novick, R.P.; Christie, G.E.; Penades, J.R. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 2010, 8, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Fillol-Salom, A.; Martinez-Rubio, R.; Abdulrahman, R.F.; Chen, J.; Davies, R.; Penades, J.R. Phage-inducible chromosomal islands are ubiquitous within the bacterial universe. ISME J. 2018, 12, 2114–2128. [Google Scholar] [CrossRef]
- Becker, K.; Keller, B.; von Eiff, C.; Bruck, M.; Lubritz, G.; Etienne, J.; Peters, G. Enterotoxigenic potential of Staphylococcus intermedius. Appl. Env. Microbiol. 2001, 67, 5551–5557. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, A.; Schuberth, H.-J.; Schueler, K.; Lloyd, D.H. Frequency of superantigen-producing Staphylococcus intermedius isolates from canine pyoderma and proliferation-inducing potential of superantigens in dogs. Res. Vet. Sci. 2002, 73, 273–277. [Google Scholar] [CrossRef]
- Iyori, K.; Hisatsune, J.; Kawakami, T.; Shibata, S.; Murayama, N.; Ide, K.; Nagata, M.; Fukata, T.; Iwasaki, T.; Oshima, K.; et al. Identification of a novel Staphylococcus pseudintermedius exfoliative toxin gene and its prevalence in isolates from canines with pyoderma and healthy dogs. FEMS Microbiol. Lett. 2010, 312, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Futagawa-Saito, K.; Makino, S.; Sunaga, F.; Kato, Y.; Sakurai-Komada, N.; Ba-Thein, W.; Fukuyasu, T. Identification of first exfoliative toxin in Staphylococcus pseudintermedius. FEMS Microbiol. Lett. 2009, 301, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Nishifuji, K.; Sugai, M.; Amagai, M. Staphylococcal exfoliative toxins: “Molecular scissors” of bacteria that attack the cutaneous defense barrier in mammals. J. Derm. Sci. 2008, 49, 21–31. [Google Scholar] [CrossRef]






| Species | Strain | Source | Locality | Conventional Test Results | API 50 CH | API ZYM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COA | ARG | URE | TWE | GEL | Cas | E-Y | C45 | GLY | MAN | SOR | ARB | MDG | LAC | TRE | GEN | TUR | N-PH | α-FU | ||||
| S. delphini | CCM 4115T | dolphin | Italy | + | + | + | - | - | - | + | + | w | + | - | - | - | + | - | - | w | - | - |
| S. delphini | CCM 4184 | dolphin | Italy | + | w | + | - | - | - | + | + | w | + | - | - | - | + | - | - | w | - | - |
| S. delphini | P5747 | penguin | Antarctica | w | - | - | w | + | + | - | + | - | w | - | - | - | - | + | - | w | - | - |
| S. delphini | P5749 | penguin | Antarctica | w | w | + | + | + | + | - | + | w | w | - | - | - | + | + | - | w | - | - |
| S. delphini | P5833 | penguin | Antarctica | w | - | + | + | + | + | - | + | w | + | - | - | - | + | + | - | w | - | - |
| S. delphini | P5835 | penguin | Antarctica | - | - | + | + | + | + | - | + | w | + | - | - | - | + | + | - | w | - | - |
| S. delphini | P6070 | penguin | Antarctica | - | - | - | + | + | + | - | + | - | + | - | - | - | + | + | - | w | - | - |
| S. delphini | P6456 | penguin | Antarctica | w | - | + | - | + | + | - | w | w | - | - | - | - | w | + | - | - | - | - |
| S. delphini | P12456 | camel | France | w | w | + | - | + | + | - | - | + | + | - | w | - | + | + | + | w | + | - |
| S. delphini | P12457 | horse | UK | + | w | + | - | + | + | - | - | w | + | + | - | - | w | + | + | w | w | w |
| S. delphini | P12458 | horse | UK | w | w | + | + | + | + | - | + | w | w | - | - | - | + | + | - | - | w | - |
| S. delphini | CCM 2618 | mink | Czechia | + | - | + | + | + | + | - | + | + | + | - | - | - | + | + | - | w | - | w |
| S. delphini | P12548 | mink | Denmark | w | - | + | - | + | + | - | + | w | - | - | - | - | + | + | - | w | - | w |
| S. delphini | P12549 | mink | Denmark | w | - | + | + | + | + | + | + | w | + | - | - | - | + | + | - | w | - | - |
| S. delphini | P12550 | mink | Denmark | w | w | + | + | + | + | - | + | w | + | - | - | - | + | + | - | w | - | w |
| S. delphini | CCM 8998 | human | USA | + | + | + | + | + | - | - | + | w | + | - | - | - | + | + | - | + | - | - |
| S. pseudintermedius | CCM 7315T | cat | Belgium | + | + | + | - | + | - | - | + | + | + | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P7945 | seal | Antarctica | w | w | + | - | + | + | - | - | w | - | - | - | - | + | + | - | - | - | - |
| S. pseudintermedius | P8480 | seal | Antarctica | + | - | + | - | + | + | - | + | + | - | - | - | w | + | + | - | w | - | - |
| S. pseudintermedius | P8688 | seal | Antarctica | + | - | + | - | + | + | - | - | w | - | - | - | - | + | + | - | - | w | - |
| S. pseudintermedius | P8720 | seal | Antarctica | w | + | + | - | + | + | - | - | w | - | - | - | - | + | + | - | - | - | - |
| S. pseudintermedius | P8807 | seal | Antarctica | + | + | + | - | + | + | - | w | + | - | - | - | w | + | + | - | w | - | - |
| S. pseudintermedius | P9111 | seal | Antarctica | + | w | + | - | + | + | - | w | + | + | - | - | w | + | + | - | w | - | - |
| S. pseudintermedius | P12459 | seal | Antarctica | w | - | + | - | + | + | - | + | w | - | - | - | w | + | + | - | - | - | - |
| S. pseudintermedius | P12460 | seal | Antarctica | - | w | + | - | + | + | - | + | w | + | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P12461 | seal | Antarctica | w | w | + | - | + | + | - | + | + | - | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P12462 | seal | Antarctica | - | - | + | - | + | + | - | + | + | + | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P12463 | seal | Antarctica | - | w | + | - | + | + | - | + | + | - | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P12464 | seal | Antarctica | - | w | + | - | + | + | - | + | + | + | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P12465 | seal | Antarctica | w | w | + | - | + | + | - | + | + | - | - | - | - | + | + | - | + | - | - |
| S. pseudintermedius | P12466 | seal | Antarctica | w | w | + | - | + | + | - | w | + | + | - | - | w | + | + | - | + | - | - |
| S. pseudintermedius | P12467 | seal | Antarctica | + | w | + | - | + | + | - | + | + | - | - | - | w | + | + | - | - | - | - |
| S. pseudintermedius | P10574 | seal | Antarctica | - | + | + | - | + | + | - | - | w | - | - | - | - | + | + | - | - | - | - |
| S. intermedius | CCM 5739T | pigeon | Czechia | w | - | + | - | + | + | - | + | w | + | - | - | w | + | + | + | w | - | - |
| S. cornubiensis | CCM 8997T | human | UK | + | + | + | + | + | + | + | + | + | + | - | - | - | + | + | - | + | - | - |
| Species | Strains | TWE | E-Y | MAN | MDG |
|---|---|---|---|---|---|
| S. delphini | 16 strains | d (63%) | - (6%) | + (88%) | - (0%) |
| S. pseudintermedius | 17 strains | - (0%) | - (0%) | (-) (29%) | (+) (71%) |
| S. intermedius | CCM 5739T | - | - | + | w |
| S. cornubiensis | CCM 8997T | + | + | + | - |
| Strain | P5747 | P6456 | NCTC 12225T | 215100905101-2 | 8086 | NCTC 11048T | LMG 22219T | NW1T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GGD | ANI | GGD | ANI | GGD | ANI | GGD | ANI | GGD | ANI | GGD | ANI | GGD | ANI | GGD | ANI | |
| S. delphini P5747 | - | - | 70.2 | 96.3 | 70.5 | 96.5 | 77.4 | 97.6 | 85.6 | 98.4 | 35.8 | 88.6 | 55.1 | 93.9 | 33.5 | 87.4 |
| S. delphini P6456 | 70.2 | 96.3 | - | - | 87.5 | 98.7 | 69.5 | 96.3 | 69.0 | 96.3 | 35.5 | 88.4 | 51.3 | 93.1 | 33.2 | 87.2 |
| S. delphini NCTC 12225T | 70.5 | 96.5 | 87.5 | 98.7 | - | - | 70.4 | 96.6 | 70.1 | 96.4 | 35.9 | 88.6 | 51.2 | 93.1 | 33.5 | 87.4 |
| S. delphini 215100905101-2 | 77.4 | 97.6 | 69.5 | 96.3 | 70.4 | 96.6 | - | - | 78.4 | 97.6 | 35.7 | 88.6 | 54.7 | 93.7 | 33.6 | 87.8 |
| S. delphini 8086 | 85.6 | 98.4 | 69.0 | 96.3 | 70.1 | 96.4 | 78.4 | 97.6 | - | - | 35.6 | 88.6 | 54.9 | 93.9 | 33.6 | 87.5 |
| S. intermedius NCTC 11048T | 35.8 | 88.6 | 35.5 | 88.4 | 35.9 | 88.6 | 35.7 | 88.6 | 35.6 | 88.7 | - | - | 34.9 | 88.0 | 36.2 | 88.6 |
| S. pseudintermedius LMG 22219T | 55.1 | 93.9 | 51.3 | 93.1 | 51.2 | 93.1 | 54.7 | 93.7 | 54.9 | 93.9 | 34.9 | 88.0 | - | - | 32.9 | 87.2 |
| S. cornubiensis NW1T | 33.5 | 87.4 | 33.2 | 87.2 | 33.5 | 87.4 | 33.6 | 87.8 | 33.6 | 87.6 | 36.2 | 88.6 | 32.9 | 87.2 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrbovská, V.; Sedláček, I.; Zeman, M.; Švec, P.; Kovařovic, V.; Šedo, O.; Laichmanová, M.; Doškař, J.; Pantůček, R. Characterization of Staphylococcus intermedius Group Isolates Associated with Animals from Antarctica and Emended Description of Staphylococcus delphini. Microorganisms 2020, 8, 204. https://doi.org/10.3390/microorganisms8020204
Vrbovská V, Sedláček I, Zeman M, Švec P, Kovařovic V, Šedo O, Laichmanová M, Doškař J, Pantůček R. Characterization of Staphylococcus intermedius Group Isolates Associated with Animals from Antarctica and Emended Description of Staphylococcus delphini. Microorganisms. 2020; 8(2):204. https://doi.org/10.3390/microorganisms8020204
Chicago/Turabian StyleVrbovská, Veronika, Ivo Sedláček, Michal Zeman, Pavel Švec, Vojtěch Kovařovic, Ondrej Šedo, Monika Laichmanová, Jiří Doškař, and Roman Pantůček. 2020. "Characterization of Staphylococcus intermedius Group Isolates Associated with Animals from Antarctica and Emended Description of Staphylococcus delphini" Microorganisms 8, no. 2: 204. https://doi.org/10.3390/microorganisms8020204