Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism Selection and Storage
2.2. Bacterial Inoculum Preparation
2.3. Characterization of Bacterial Strains under Normal Condition
2.3.1. Motility Test
2.3.2. Qualitative Characterizations of Bacteria for Phosphate Solubilization on Plates
2.3.3. Quantitative Estimation of Phosphate Solubilization in Liquid Broth
2.4. Characterization of Bacterial Strains under Salt Stress
2.4.1. Bacterial Tolerance to Salt
2.4.2. Motility Test under Salt Stress
2.4.3. Phosphate Solubilization under Salt Stress
2.5. Plant Inoculation Experiment
2.6. Statistical Analyses
3. Results
3.1. Characterization of Bacterial Strains under Normal Condition
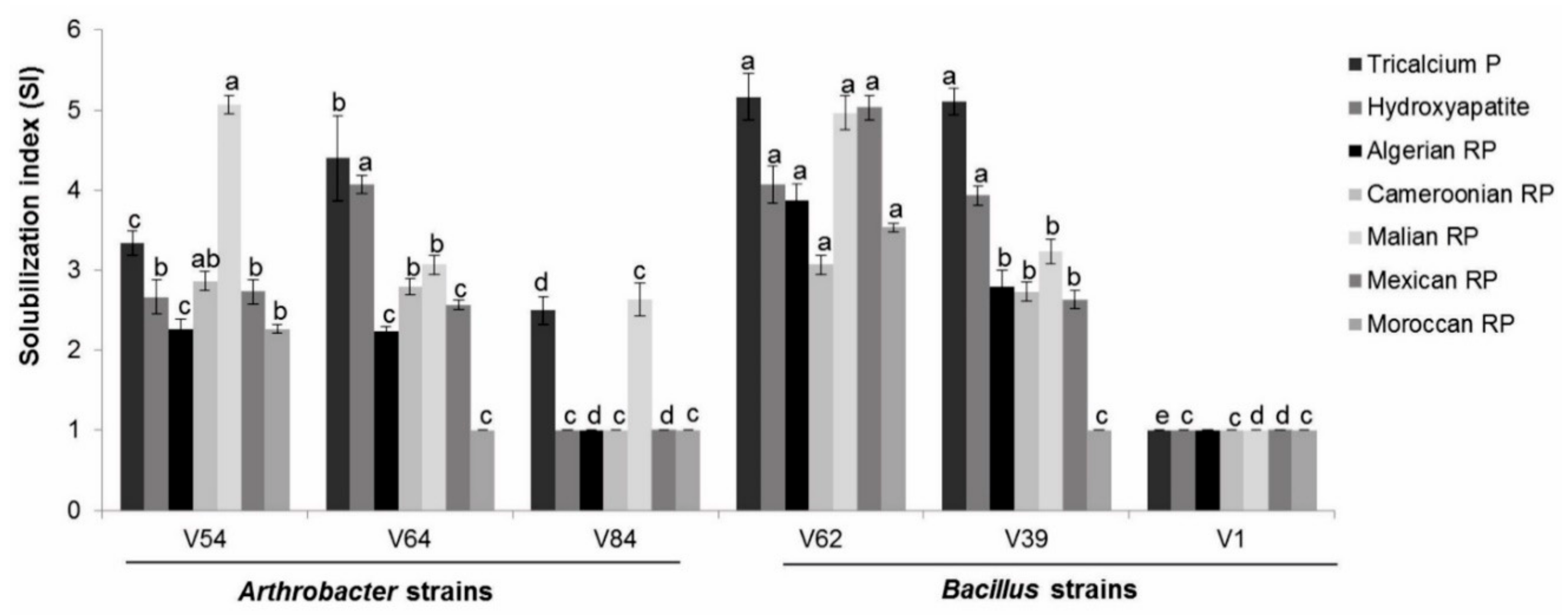
3.1.1. Phosphate Source and Strain-Dependent Phosphate-Solubilizing Efficacy on Agar Plates
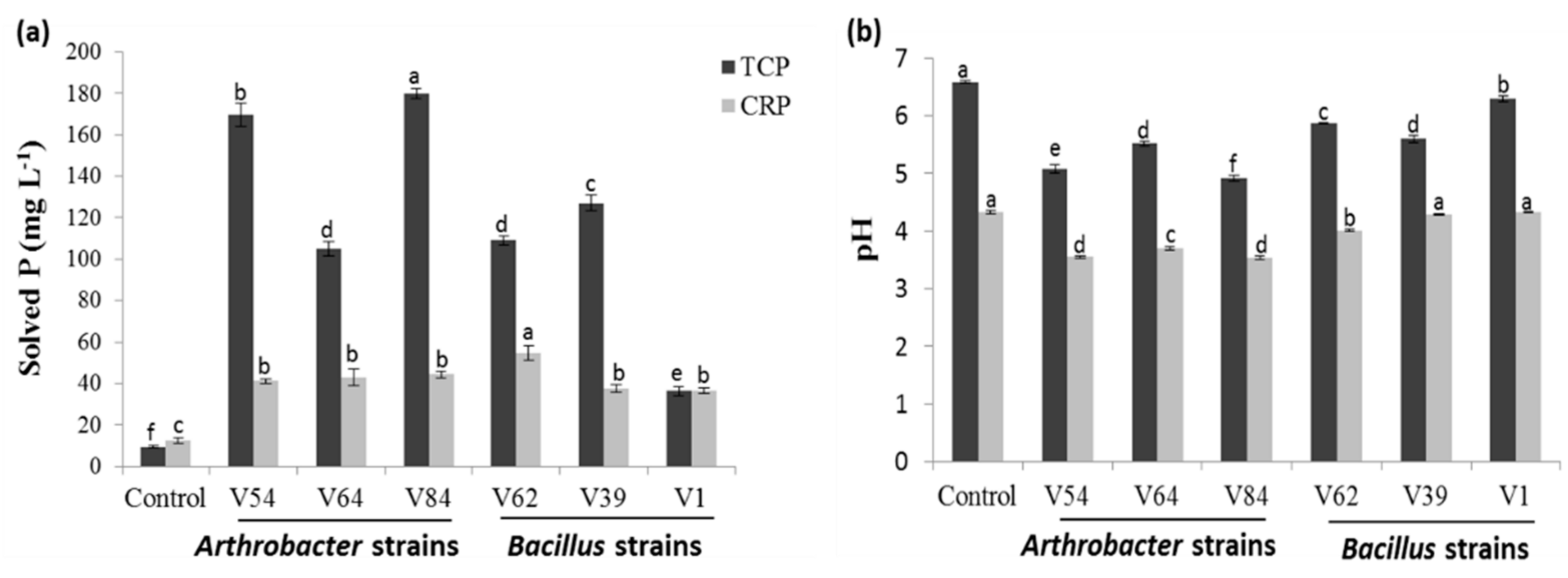
3.1.2. Phosphate Source and Strain-Dependent Phosphate-Solubilizing Efficiency in Liquid Culture under Non-Stress Conditions
3.2. Characterization of Bacterial Strains under Salt Stress
3.2.1. Bacillus Strains Tolerated Higher Salt than Arthrobacter Strains and Reveal Best Swarming and Swimming Abilities under Salt Stress Conditions
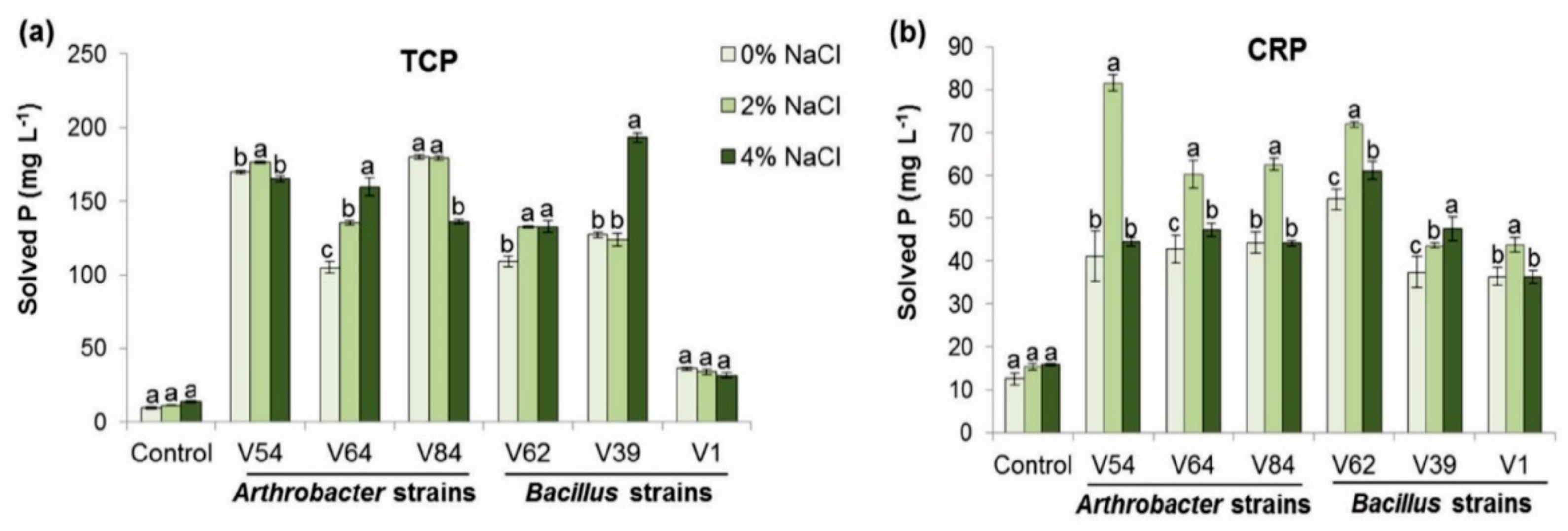
3.2.2. Effect of Phosphate Source and Salt on Phosphate-Solubilizing Activity
3.3. Bacterial Inoculations Promoted Tomato Plant Growth and P Uptake Even More Efficiently under Increased Salt Stress and P Deficient Conditions
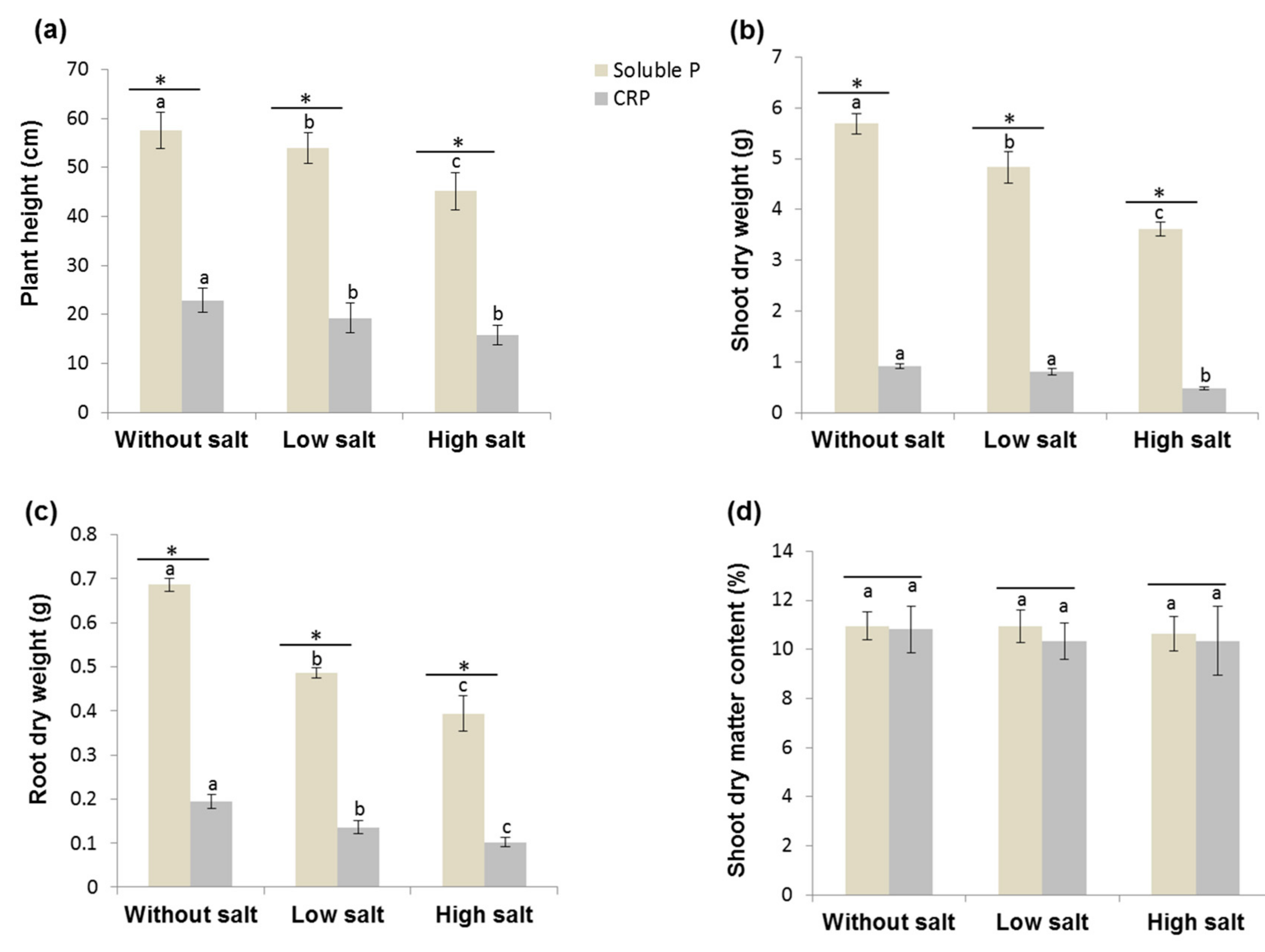
3.3.1. Verification of Phosphorus Deficiency and Salinity Effects on the Growth and P Uptake of Tomato Plants
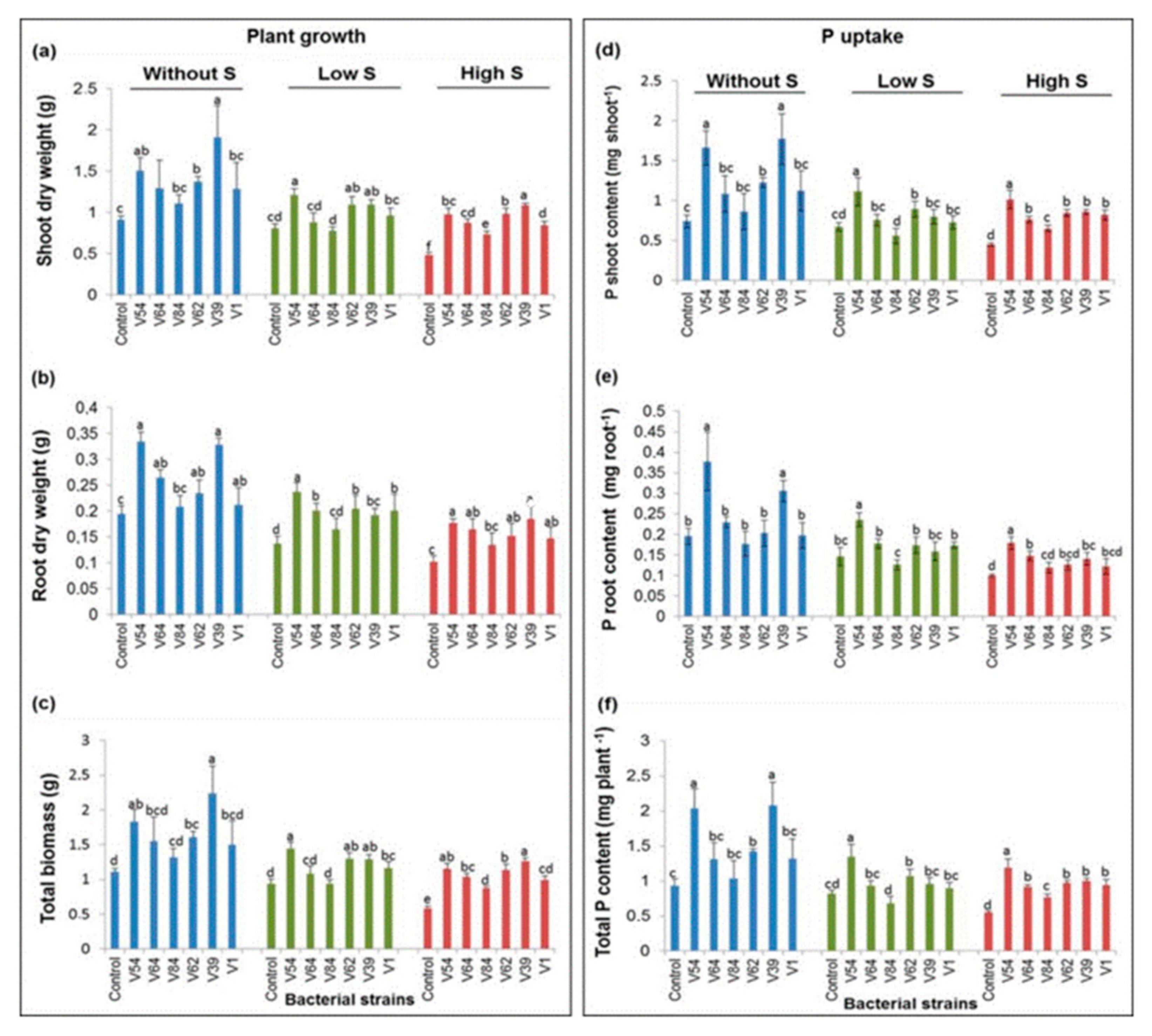
3.3.2. Selected Bacterial Strains Promoted Tomato Plant Growth Parameters even under Combined P and Salt Stress Conditions
3.3.3. Effect of Bacteria on P Uptake of the Tomato Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yadav, A.N.; Verma, D.P.; Singh, B.; Singh Chauhan, V.; Suman, A.; Saxena, A. Plant growth promoting bacteria: Biodiversity and multifunctional attributes for sustainable agriculture. Adv. Biotechnol. Microbiol. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Talbi Zribi, O.; Abdelly, C.; Debez, A. Interactive effects of salinity and phosphorus availability on growth, water relations, nutritional status and photosynthetic activity of barley (Hordeum vulgare L.). Plant Biol. 2011, 13, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Qi, P.; Wang, T.; Wang, M.; Chen, M.; Chen, N.; Pan, L.; Chi, X. Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. 3 Biotech 2018, 8, 461. [Google Scholar] [CrossRef]
- Srinivasan, R.; Yandigeri, M.S.; Kashyap, S.; Alagawadi, A.R. Effect of salt on survival and P-solubilization potential of phosphate solubilizing microorganisms from salt affected soils. Saudi J. Biol. Sci. 2012, 19, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Soni, A.; Rokad, S.; Sharma, P. Screening of efficient halotolerant phosphate solubilizing bacteria and their effect on seed germination under saline conditions. J. Sci. Innov. Res. 2013, 2, 932–937. [Google Scholar]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [Green Version]
- Cherif-Silini, H.; Silini, A.; Ghoul, M.; Yahiaoui, B.; Arif, F. Solubilization of phosphate by the Bacillus under salt stress and in the presence of osmoprotectant compounds. Afr. J. Microbiol. Res. 2013, 7. [Google Scholar] [CrossRef]
- Venieraki, A.; Tsalgatidou, P.C.; Georgakopoulos, D.G.; Dimou, M.; Katinakis, P. Swarming motility in plant-associated bacteria. Hell. Plant Prot. J. 2016, 9, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, S.K.; Singh, D.P.; Saikia, R. Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr. Microbiol. 2009, 59, 489–496. [Google Scholar] [CrossRef]
- Fankem, H.; Tchuisseu Tchakounte, G.V.; Ngo, N.L.; Nguesseu, N.G.; Nwaga, D.; Etoa, F.-X. Maize (Zea mays) growth promotion by rock-phosphate solubilising bacteria isolated from nutrient deficient soils of Cameroon. Afr. J. Microbiol. Res. 2014, 8, 3570–3579. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, G.; Ma, P.; Lin, Y.; Yang, X.; Cao, C. Isolation and characterization of a phosphorus-solubilizing bacterium from rhizosphere soils and its colonization of chinese cabbage (Brassica campestris ssp. chinensis). Front. Microbiol. 2017, 8, 1270. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. A halotolerant bacterium Bacillus licheniformis hsw-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front. Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef] [PubMed]
- Tchuisseu, T.G.V.; Berger, B.; Patz, S.; Becker, M.; Turečková, V.; Novák, O.; Tarkowská, D.; Henri, F.; Ruppel, S. The Response of maize to inoculation with Arthrobacter sp. and Bacillus sp. in phosphorus-deficient, salinity-affected soil. Microorganisms 2020, 8, 1005. [Google Scholar]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Tchuisseu Tchakounté, G.V.; Berger, B.; Patz, S.; Fankem, H.; Ruppel, S. Community structure and plant growth-promoting potential of cultivable bacteria isolated from Cameroon soil. Microbiol. Res. 2018, 214, 47–59. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Ahmad, Z.A.; Akhtar, N.; Iqbal, A.; Mujeeb, F.; Shakir, M. Role of phosphate solubilizing bacteria (PSB) in enhancing P availability and promoting cotton growth. J. Anim. Plant Sci. 2012, 22, 204–210. [Google Scholar]
- Bae, H.D.; Yanke, L.J.; Cheng, K.J.; Selinger, L.B. A novel staining method for detecting phytase activity. J. Microbiol. Methods 1999, 39, 17–22. [Google Scholar] [CrossRef]
- Raun, W.R.; Olson, R.A.; Sander, D.H.; Westerman, R.L. Alternative procedure for total phosphorus determination in plant tissue. Commun. Soil Sci. Plant Anal. 1987, 18, 543–557. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, M.; Patz, S.; Becker, Y.; Berger, B.; Drungowski, M.; Bunk, B.; Overmann, J.; Spröer, C.; Reetz, J.; Tchuisseu Tchakounte, G.V.; et al. Comparative genomics reveal a flagellar system, a type vi secretion system and plant growth-promoting gene clusters unique to the endophytic bacterium Kosakonia radicincitans. Front. Microbiol. 2018, 9, 1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicario, J.C.; Dardanelli, M.S.; Giordano, W. Swimming and swarming motility properties of peanut-nodulating rhizobia. FEMS Microbiol. Lett. 2015, 362, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Kpomblekou-A, K.; Tabatabai, M.A. Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci. 1994, 158, 442–453. [Google Scholar] [CrossRef]
- Kim, K.Y.; Jordan, D.; McDonald, G.A. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol. Fertil. Soils 1997, 26, 79–87. [Google Scholar] [CrossRef]
- Rosado, A.; Azevedo, D.; da Cruz, D.W.; Elsas, V.; Seldin, L. Phenotypic and genetic diversity of Paenibacillus azotofixans strains from the rhizoplane or rhizosphere soil of different grasses. J. Appl. Microbiol. 2002, 84, 216–226. [Google Scholar] [CrossRef]
- Shenker, M.; Ben-Gal, A.; Shani, U. Sweet corn response to combined nitrogen and salinity environmental stresses. Plant Soil 2003, 256, 139–147. [Google Scholar] [CrossRef]
- Yousfi, S.; Wissal, M.; Mahmoudi, H.; Abdelly, C.; Gharsalli, M. Effect of salt on physiological responses of barley to iron deficiency. Plant Physiol. Biochem. 2007, 45, 309–314. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, M.M.; Estañ, M.T.; Moyano, E.; Garcia-Abellan, J.O.; Flores, F.B.; Campos, J.F.; Al-Azzawi, M.J.; Flowers, T.J.; Bolarín, M.C. The effectiveness of grafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Environ. Exp. Bot. 2008, 63, 392–401. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, X.; Chen, S.; He, X.; Zhang, F.; Feng, G. Reducing carbon: Phosphorus ratio can enhance microbial phytin mineralization and lessen competition with maize for phosphorus. J. Plant Interact. 2014, 9, 850–856. [Google Scholar] [CrossRef]
- Fankem, H.; Tchuisseu Tchakounte, G.V.; Ngo Nkot, L.; Mafokoua, H.L.; Tchouomo, D.D.; Simo, C.; Nwaga, D.; Etoa, F.X. Common bean (Phaseolus vulgaris L.) and soya bean (Glycine max) growth and nodulation as influenced by rock phosphate solubilising bacteria under pot grown conditions. Int. J. Agric. Policy Res. 2015, 3, 242–250. [Google Scholar] [CrossRef]
- Gomes, E.; Silva, U.; Marriel, I.; De Oliveira, C.; Lana, U. Rock phosphate solubilizing microorganisms isolated fom maize rhizosphere soil. Braz. J. Maize Sorghum 2014, 13, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Ullah, S. Drought stress effects on plant water relations, growth, fruit quality and osmotic adjustment of tomato (Solanum lycopersicum) under subtropical condition. Asian J. Agric. Hortic. Res. 2018, 1, 1–14. [Google Scholar] [CrossRef]
| Bacterial Isolates | Salinity Tolerance (% NaCl) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | ||
| Arthrobacter strains | V54 | + | + | + | - | - | - | - | - | - |
| V64 | + | + | + | - | - | - | - | - | - | |
| V84 | + | + | + | - | - | - | - | - | - | |
| Bacillus strains | V62 | + | + | + | + | + | + | - | - | - |
| V39 | + | + | + | + | + | + | + | + | - | |
| V1 | + | + | - | - | - | - | - | - | - | |
| Bacterial Isolates | 0% NaCl | 2% NaCl | 4% NaCl | 8% NaCl | 10% NaCl | 12% NaCl | 14% NaCl | 16% NaCl | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | 0.5% Agar | 0.3% Agar | ||
| Arthrobacter strains | V54 | +++ | +++ | +++ | +++ | ++ | +++ | - | - | - | - | - | - | - | - | - | - |
| V64 | +++ | +++ | +++ | +++ | ++ | ++ | - | - | - | - | - | - | - | - | - | - | |
| V84 | +++ | +++ | ++ | ++ | ++ | ++ | - | - | - | - | - | - | - | - | - | - | |
| Bacillus strains | V62 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | - | - | - | - | - | - |
| V39 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - | - | - | |
| V1 | +++ | +++ | ++ | ++ | - | - | - | - | - | - | - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchakounté, G.V.T.; Berger, B.; Patz, S.; Becker, M.; Fankem, H.; Taffouo, V.D.; Ruppel, S. Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses. Microorganisms 2020, 8, 1844. https://doi.org/10.3390/microorganisms8111844
Tchakounté GVT, Berger B, Patz S, Becker M, Fankem H, Taffouo VD, Ruppel S. Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses. Microorganisms. 2020; 8(11):1844. https://doi.org/10.3390/microorganisms8111844
Chicago/Turabian StyleTchakounté, Gylaine Vanissa Tchuisseu, Beatrice Berger, Sascha Patz, Matthias Becker, Henri Fankem, Victor Désiré Taffouo, and Silke Ruppel. 2020. "Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses" Microorganisms 8, no. 11: 1844. https://doi.org/10.3390/microorganisms8111844





