Enumeration of Escherichia coli in Probiotic Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Escherichia coli Probiotic Products
2.2. Product Testing
2.3. Analytical Methods
2.4. Statistical Methods
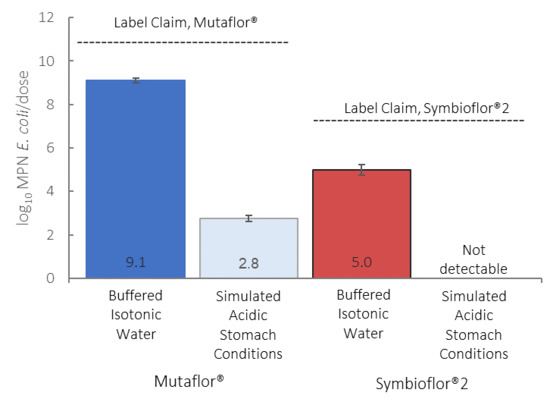
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Caillard, R.; Lapointe, N. In vitro gastric survival of commercially available probiotic strains and oral dosage forms. Int. J. Pharm. 2017, 519, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.T.; Shah, S.; Winkler, J.T. Public health issues arising from microbiological and labelling quality of foods and supplements containing probiotic microorganisms. Public Health Nutr. 1999, 2, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff, B.A. Caveat emptor. ‘Probiotics’ might not be what they seem. Can. Fam. Physician 2004, 50, 583–587. [Google Scholar] [PubMed]
- Lin, W.H.; Hwang, C.F.; Chen, L.W.; Tsen, H.Y. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Int. J. Food Microbiol. 2006, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, C.G.; Reinheimer, J.A. Enumeration of Lactobacillus casei in the presence of L. acidophilus, bifidobacteria and lactic starter bacteria in fermented dairy products. Int. Dairy J. 2000, 10, 271–275. [Google Scholar] [CrossRef]
- Möllenbrink, M.; Bruckschen, E. Treatment of chronic constipation with physiologic Escherichia coli bacteria. Results of a clinical study of the effectiveness and tolerance of microbiological therapy with the E. coli Nissle 1917 strain (Mutaflor). Med. Klin. (Munich) 1994, 89, 587–593. [Google Scholar] [PubMed]
- Enck, P.; Zimmermann, K.; Menke, G.; Klosterhalfen, S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z. Gastroenterol. 2009, 47, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schütz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M. Insights from 100 Years of Research with Probiotic E. Coli. Eur. J. Microbiol. Immunol. 2016, 6, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Beimfohr, C.A. Review of Research Conducted with Probiotic E. coli Marketed as Symbioflor. Int. J. Bacteriol. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, Examples, and Specific Applications. Cold Spring Harb. Perspect. Med. 2013, 3, a010074. [Google Scholar] [CrossRef] [PubMed]
- A Bacterium Becomes a Medicinal Product. Available online: https://tinyurl.com/yywbkx9s (accessed on 17 May 2019).
- Sonnenborn, U.; Schulze, J. The non-pathogenic Escherichia coli strain Nissle 1917—Features of a versatile probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar] [CrossRef]
- Symbioflor® 2—For Irritable Bowel Syndrome. Available online: https://tinyurl.com/yyk26qvf (accessed on 4 September 2019).
- Brashears, M.M.; Jaroni, D.; Trimble, J. Isolation, Selection, and Characterization of Lactic Acid Bacteria for a Competitive Exclusion Product to Reduce Shedding of Escherichia coli O157:H7 in Cattle. J. Food Protect. 2003, 66, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of Lactic Acid Bacteria in the Human Stomach and Adhesion to Intestinal Cells. Int. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Colilert—IDEXX, US. Available online: https://tinyurl.com/y6gyd97h (accessed on 10 July 2018).
- Ding, W.K.; Shah, N.P. Effect of Various Encapsulating Materials on the Stability of Probiotic Bacteria. J. Food Sci. 2009, 74, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, C.C.; Wang, J.; Basit, A.W.; Stapleton, P.; Gaisford, S. Targeted delivery of probiotics to enhance gastrointestinal stability and intestinal colonization. Int. J. Pharm. 2017, 530, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Health Canada released its guidance document on The Use of Probiotic Microorganisms in Food in April 2009.
- German Collection of Microorganisms and Cell Cultures GmbH: Welcome to the Leibniz Institute DSMZ. Available online: https://www.dsmz.de/ (accessed on 23 September 2019).
- Hill, M.J.; Drasar, B.S. The normal colonic bacterial flora. Gut 1975, 16, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Canganella, F.; Paganini, S.; Ovidi, M.; Vettraino, A.M.; Bevilacqua, L.; Massa, S.; Trovatelli, L.D. A microbiological investigation on probiotic pharmaceutical products used for human health. Microbiol. Res. 1997, 152, 171–179. [Google Scholar] [CrossRef]
- Temmerman, R.; Pot, B.; Huys, G.; Swings, J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int. J. Food Microbiol. 2003, 81, 1–10. [Google Scholar] [CrossRef]
- Reuter, G. Present and Future of Probiotics in Germany and in Central Europe. Biosci. Microflora 1997, 16, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis in’t Veld, J.H.J. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Prilassnig, M.; Wenisch, C.; Daxboeck, F.; Feierl, G. Are probiotics detectable in human feces after oral uptake by healthy volunteers? Wien. Klin. Wochenschr. 2007, 119, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Joeres-Nguyen-Xuan, T.H.; Boehm, S.K.; Joeres, L.; Schulze, J.; Kruis, W. Survival of the probiotic Escherichia coli Nissle 1917 (EcN) in the gastrointestinal tract given in combination with oral mesalamine to healthy volunteers. Inflamm. Bowel Dis. 2010, 16, 256–262. [Google Scholar] [CrossRef]

| Product Name | Format | Dose 1 | Manufacturer Claim of E. coli Count per Dose 1 | Lot Number | Purchase Date | Expiry Date 1 |
|---|---|---|---|---|---|---|
| Mutaflor | Powder inside a capsule | minimum 4 capsules per day | >1.0 × 1011 per 4 capsules | 730160 | 10 February 2018 | 11 August 2018 |
| 74170 | 10 February 2018 | 11 August 2018 | ||||
| 810180 | 21 June 2018 | 25 January 2019 | ||||
| Symbioflor 2 | Liquid suspension | 1 mL | 1.5–4.5 × 107 | 2564 | 14 July 2018 | November 2018 |
| 2582 | 14 July 2018 | June 2019 | ||||
| 2584 | 14 July 2018 | August 2019 |
| Product Name | Total Degrees of Freedom | p-Value |
|---|---|---|
| Mutaflor | 28 | <0.01 1 |
| Symbioflor 2 | 29 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmer, C.; Dorea, C. Enumeration of Escherichia coli in Probiotic Products. Microorganisms 2019, 7, 437. https://doi.org/10.3390/microorganisms7100437
Zimmer C, Dorea C. Enumeration of Escherichia coli in Probiotic Products. Microorganisms. 2019; 7(10):437. https://doi.org/10.3390/microorganisms7100437
Chicago/Turabian StyleZimmer, Camille, and Caetano Dorea. 2019. "Enumeration of Escherichia coli in Probiotic Products" Microorganisms 7, no. 10: 437. https://doi.org/10.3390/microorganisms7100437