The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments
Abstract
1. Introduction
2. Community Assembly at the Mineral-Air Interface
| Sequenced Genomes | |
|---|---|
| Biological Soil Crusts | Microvirga sp. Strain BSC39 [47] Aquincola tertiaricarbonis [48] Microcoleus vaginatus FGP-2 [49] Massilia sp. Strain BSC265 [50] Bacillus sp. Strain BSC154 [51] |
| Desert Rocks | Knufia petricola [52] Sphingomonas sp. strain AntH11 [53] Rachicladosporium antarcticum CCFEE 5527 and Rachicladosporium sp. CCFEE 5018 [54] Halorubrum sp. SAH-A6 [55] Nakamurella lactea [56] Cryomyces antarcticus [57] |
| Stone Surfaces | Hassallia byssoidea strain VB512170 [58] Scytonema millei VB511283 [59] Tolypothrix boutellei strain VB521301 [60] Blastococcus saxobsidens DD2 [61] Modestobacter marinus strain BC501 [62] |
3. Biological Interactions in SABs: A Symbiotic Playground
4. Stress Resistance and Resilience of SABs
4.1. Physical Stresses
4.2. Chemical Stresses
5. Lab-Scale Systems and Mathematical Models: Methods to Study SABs
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uroz, S.; Kelly, L.C.; Turpault, M.P.; Lepleux, C.; Frey-Klett, P. The mineralosphere concept: Mineralogical control of the distribution and function of mineral-associated bacterial communities. Trends Microbiol. 2015, 23, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Stewart, P.S.; Klapper, I.; Jacob, J.M.; Cappitelli, F. Subaerial biofilms on outdoor stone monuments: Changing the perspective toward an ecological framework. BioScience 2016, 66, 285–294. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Chiquoine, L.P.; Abella, S.R.; Bowker, M.A. Rapidly restoring biological soil crusts and ecosystem functions in a severely disturbed desert ecosystem. Ecol. Appl. 2016, 26, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, D.; Villa, F.; Cappitelli, F.; Toniolo, L. Biofilm colonization of metamorphic lithotypes of a renaissance cathedral exposed to urban atmosphere. Sci. Total Environ. 2018, 639, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, X.; Zheng, K.; Zhang, C.; Liu, Y.; Lu, K.; Jia, R.; Zhao, C. Ecohydrological effects of biocrust type on restoration dynamics in drylands. Sci. Total. Environ. 2019, 687, 527–534. [Google Scholar] [CrossRef]
- Wu, H.; Fang, Y.; Yu, J.; Zhang, Z. The quest for a unified view of bacterial land colonization. ISME J. 2014, 8, 1358–1369. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Broughton, W.J. Microbiology of the atmosphere–rock interface: How biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 2009, 63, 431–450. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Yang, X.; Ge, Q. Deterioration-associated microbiome of stone monuments: Structure, variation, and assembly. Appl. Environ. Microbiol. 2018, 84, e02680-17. [Google Scholar] [CrossRef]
- Zanardini, E.; May, E.; Purdy, K.J.; Murrell, J.C. Nutrient cycling potential within microbial communities on culturally important stoneworks. Environ. Microbiol. Rep. 2019, 11, 147–154. [Google Scholar] [CrossRef]
- Villa, F.; Vasanthakumar, A.; Mitchell, R.; Cappitelli, F. RNA-based molecular survey of biodiversity of limestone tombstone microbiota in response to atmospheric sulfur pollution. Lett Appl Microbiol. 2015, 60, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.J.; Pace, N.R. Endolithic microbial ecosystems. Annu. Rev. Microbiol. 2007, 61, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Casanova Municchia, A.; Percario, Z.; Caneva, G. Detection of endolithic spatial distribution in marble stone. J. Microsc. 2014, 256, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Geomicrobiology of the built environment. Nat Microbiol. 2017, 28, 16275. [Google Scholar] [CrossRef] [PubMed]
- Wierzchos, J.; Cámara, B.; de Los Ríos, A.; Davila, A.F.; Sánchez Almazo, I.M.; Artieda, O.; Wierzchos, K.; Gómez-Silva, B.; McKay, C.; Ascaso, C. Microbial colonization of Ca-sulfate crusts in the hyperarid core of the Atacama Desert: Implications for the search for life on Mars. Geobiology 2011, 9, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Mogul, R.; Vaishampayan, P.; Bashir, M.; McKay, C.P.; Schubert, K.; Bornaccorsi, R.; Gomez, E.; Tharayil, S.; Payton, G.; Capra, J.; et al. Microbial community and biochemical dynamics of biological soil crusts across a gradient of surface coverage in the central Mojave Desert. Front. Microbiol. 2017, 8, 1974. [Google Scholar] [CrossRef] [PubMed]
- Swenson, T.L.; Karaoz, U.; Swenson, J.M.; Bowen, B.P.; Northen, T.R. Linking soil biology and chemistry in biological soil crust using isolate exometabolomics. Nat. Commun. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Steven, B.; Belnap, J.; Kuske, C.R. Chronic physical disturbance substantially alters the response of biological soil crusts to a wetting pulse, as characterized by metatranscriptomic sequencing. Front. Microbiol. 2018, 9, 2382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, L.; Wang, Z.; Liu, L.; Zhang, P.; Sun, J.; Wang, B.; Guang Song, G.; Li, X. Changes in functional gene structure and metabolic potential of the microbial community in biological soil crusts along a revegetation chronosequence in the Tengger Desert. Soil Biol. Biochem. 2018, 126, 40–48. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Yuan, S.; Liu, Y. Shifts in community structure and function of ammonia-oxidizing archaea in biological soil crusts along a revegetation chronosequence in the Tengger Desert. Sci. Cold Arid Reg. 2019, 11, 139–149. [Google Scholar]
- Nunes da Rocha, U.; Cadillo-Quiroz, H.; Karaoz, U.; Rajeev, L.; Klitgord, N.; Dunn, S.; Truong, V.; Buenrostro, M.; Bowen, B.P.; Garcia-Pichel, F.; et al. Isolation of a significant fraction of non-phototroph diversity from a desert Biological Soil Crust. Front. Microbiol. 2015, 6, 277. [Google Scholar] [CrossRef] [PubMed]
- El Moustaid, F.; Carlson, R.P.; Villa, F.; Klapper, I. Photorespiration and rate synchronization in a phototroph–heterotroph microbial consortium. Processes 2017, 5, 11. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Bar-Eyal, L.; Eisenberg, I.; Faust, A.; Raanan, H.; Nevo, R.; Rappaport, F.; Krieger–Liszkay, A.; Sétif, P.; Thurotte, A.; Reich, Z.; et al. An easily reversible structural change underlies mechanisms enabling desert crust cyanobacteria to survive desiccation. Biochim. Biophys Acta 2015, 1847, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Holzinger, A. Green algae in alpine biological soil crust communities: Acclimation strategies against ultraviolet radiation and dehydration. Biodivers. Conserv. 2014, 23, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Ahmed, E.; Ciccazzo, S.; Sikorski, J.; Overmann, J.; Holmström, S.J.; Brusetti, L. Comparison of rock varnish bacterial communities with surrounding non-varnished rock surfaces: Taxon-specific analysis and morphological description. Microb. Ecol. 2015, 70, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Lang-Yona, N.; Maier, S.; Macholdt, D.S.; Müller-Germann, I.; Yordanova, P.; Rodriguez-Caballero, E.; Jochum, K.P.; Al-Amri, A.; Andreae, M.O.; Fröhlich-Nowoisky, J.; et al. Insights into microbial involvement in desert varnish formation retrieved from metagenomic analysis. Environ. Microbiol. Rep. 2018, 10, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Le, P.T.; Makhalanyane, T.P.; Guerrero, L.D.; Vikram, S.; Van de Peer, Y.; Cowan, D.A. Comparative metagenomic analysis reveals mechanisms for stress response in hypoliths from extreme hyperarid deserts. Genome Biol. Evol. 2016, 8, 2737–2747. [Google Scholar] [CrossRef]
- Alnaimat, S.; Shattal, S.A.; Althunibat, O.; Alsbou, E.; Amasha, R. Iron (II) and other heavy-metal tolerance in bacteria isolated from rock varnish in the arid region of Al-jafer basin, Jordan. Biodiversitas 2017, 18, 1250–1257. [Google Scholar] [CrossRef]
- Krinsley, D.H.; DiGregorio, B.; Dorn, R.I.; Razink, J.; Fisher, R. Mn-Fe-Enhancing budding bacteria in century-old rock varnish, Erie Barge Canal, New York. J. Geol. 2017, 125, 317–336. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Wiens, M.; Schloßmacher, U.; Jochum, K.P.; Schröder, H.C.; Müller, W.E.G. Evidence for a biogenic, microorganismal origin of rock varnish from the Gangdese Belt of Tibet. Micron 2011, 42, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, K.; Tesei, D.; Marzban, G.; Dijksterhuis, J.; Wyatt, T.; Sterflinger, K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia 2013, 175, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, G.; Piredda, R.; Pepe, G.; van der Werf, I.D.; Sabbatini, L.; Crecchio, C.; Ricciuti, P.; D’Erchia, A.M.; Manzari, C.; Pesole, G. Profile of microbial communities on carbonate stones of the medieval church of San Leonardo di Siponto (Italy) by Illumina-based deep sequencing. Appl. Microbiol. Biotechnol. 2016, 100, 8537–8548. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, B.; He, Z.; Yang, X. Distribution and diversity of bacteria and fungi colonization in stone monuments analyzed by high–throughput sequencing. PLOS ONE 2016, 11, e0163287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ge, Q.; Zhu, Z.; Dengc, Y.; Gu, J.D. Microbiological community of the Royal Palace in Angkor Thom and Beng Mealea of Cambodia by Illumina sequencing based on 16S rRNA gene. Int. Biodeterior. Biodegradation 2018, 134, 127–135. [Google Scholar] [CrossRef]
- Brewer, T.E.; Fierer, N. Tales from the tomb: The microbial ecology of exposed rock surfaces. Environ. Microbiol. 2018, 20, 958–970. [Google Scholar] [CrossRef]
- Pointing, S.B. Hypolithic Communities. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 199–213. [Google Scholar]
- Wierzchos, J.; Casero, M.C.; Artieda, O.; Ascaso, C. Endolithic microbial habitats as refuges for life in polyextreme environment of the Atacama Desert. Curr. Opin. Microbiol. 2018, 43, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Tamm, A.; Hassenrück, C.; Al-Rawahi, A.N.; Rodríguez-Caballero, E.; Fiedler, S.; Maier, S.; Weber, B. Habitat-dependent composition of bacterial and fungal communities in biological soil crusts from Oman. Sci. Rep. 2019, 9, 6468. [Google Scholar] [CrossRef]
- Gaylarde, C.; Baptista-Neto, J.A.; Ogawa, A.; Kowalski, M.; Celikkol-Aydin, S.; Beech, I. Epilithic and endolithic microorganisms and deterioration on stone church facades subject to urban pollution in a sub-tropical climate. Biofouling 2017, 33, 113–127. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the past: Find the guilty bug-microorganisms involved in the biodeterioration of archeological and historical artifacts. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Creuzé-des-Châtelliers, C.; Trabac, T.; Dubost, A.; Moënne-Loccoz, Y.; Pommier, T. Rock substrate rather than black stain alterations drives microbial community structure in the passage of Lascaux Cave. Microbiome 2018, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Archer, S.D.J.; Boyle, R.H.; Lacap-Bugler, D.C.; Belnap, J.; Pointing, S.B. Niche filtering of bacteria in soil and rock habitats of the Colorado Plateau Desert, Utah, USA. Front. Microbiol. 2016, 7, 1489. [Google Scholar] [CrossRef] [PubMed]
- Tait, A.W.; Gagen, E.J.; Wilson, S.A.; Tomkins, A.G.; Southam, G. Microbial populations of stony meteorites: Substrate controls on first colonizers. Front. Microbiol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Casero, M.C.; Dailey, M.; Wierzchos, J.; Ascaso, C.; Artieda, O.; McCullough, P.R.; DiRuggiero, J. Fundamental drivers for endolithic microbial community assemblies in the hyperarid Atacama Desert. Environ. Microbiol. 2018, 20, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.C.; Kellom, M.; Poret-Peterson, A.T.; Noonan, K.; Hartnett, H.E.; Raymond, J. Draft genome sequence of Microvirga sp. Strain BSC39, isolated from biological soil crust of Moab, Utah. Genome Announc. 2014, 2, e01197-14. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Yuan, B.; Zeng, Y.; Jia, L.; Feng, F. Draft genome sequence of Aquincola tertiaricarbonis MIMtkpLc11, an aerobic anoxygenic phototrophic bacterial strain isolated from biological soil crusts. Microbiol. Resour. Announc. 2018, 7, e01085-18. [Google Scholar] [CrossRef] [PubMed]
- Starkenburg, S.R.; Reitenga, K.G.; Freitas, T.; Johnson, S.; Chain, P.S.; Garcia-Pichel, F.; Kuske, C.R. Genome of the cyanobacterium Microcoleus vaginatus FGP-2, a photosynthetic ecosystem engineer of arid land soil biocrusts worldwide. J. Bacteriol. 2011, 193, 4569–4570. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.C.; Kellom, M.; Poret-Peterson, A.T.; Noonan, K.; Hartnett, H.E.; Raymond, J. Draft genome sequence of Massilia sp. strain BSC265, isolated from biological soil crust of Moab, Utah. Genome Announc. 2014, 2, e01199-14. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.C.; Kellom, M.; Poret-Peterson, A.T.; Noonan, K.; Hartnett, H.E.; Raymond, J. Draft genome sequence of Bacillus sp. strain BSC154, isolated from biological soil crust of Moab, Utah. Genome Announc. 2014, 2, e01198-14. [Google Scholar] [CrossRef] [PubMed]
- Tesei, D.; Tafer, H.; Poyntner, C.; Piñar, G.; Lopandic, K.; Sterflinger, K. Draft genome sequences of the black rock fungus Knufia petricola and its spontaneous nonmelanized mutant. Genome Announc. 2017, 5, e01242-17. [Google Scholar] [CrossRef] [PubMed]
- Gunnigle, E.; Ramond, J.B.; Guerrero, L.D.; Makhalanyane, T.P.; Cowan, D.A. Draft genomic DNA sequence of the multi-resistant Sphingomonas sp. strain AntH11 isolated from an Antarctic hypolith. FEMS Microbiol. Lett. 2015, 362, 37. [Google Scholar]
- Coleine, C.; Masonjones, S.; Selbmann, L.; Zucconi, L.; Onofri, S.; Pacelli, C.; Stajich, J.E. Draft genome sequences of the Antarctic endolithic fungi Rachicladosporium antarcticum CCFEE 5527 and Rachicladosporium sp. CCFEE 5018. Genome Announc. 2017, 5, e00397-17. [Google Scholar] [CrossRef] [PubMed]
- Gibtan, A.; Woo, M.; Oh, D.; Park, K.; Lee, H.S.; Sohn, J.H.; Lee, D.W.; Shin, J.K.; Lee, S.J. Draft genome sequence of the extremely halophilic Halorubrum sp. SAH-A6 isolated from rock salts of the Danakil depression, Ethiopia. Genom. Data 2016, 10, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Nouioui, I.; Göker, M.; Carro, L.; Montero-Calasanz, M.D.; Rohde, M.; Woyke, T.; Kyrpides, N.C.; Klenk, H.P. High quality draft genome of Nakamurella lactea type strain, a rock actinobacterium, and emended description of Nakamurella lactea. Stand. Genomic. Sci. 2017, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Lopandic, K.; Pandey, R.V.; Blasi, B.; Kriegner, A. Nothing special in the specialist? Draft genome sequence of Cryomyces antarcticus, the most extremophilic fungus from Antarctica. PLoS ONE 2014, 9, e109908. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Chandrababunaidu, M.M.; Singh, D.; Sanghi, N.; Ghorai, A.; Mishra, G.P.; Madduluri, M.; Adhikary, S.P.; Tripathy, S. Draft genome sequence of the terrestrial cyanobacterium Scytonema millei VB511283, isolated from Eastern India. Genome Announc. 2015, 3, e00009–e00015. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chandrababunaidu, M.M.; Panda, A.; Sen, D.; Bhattacharyya, S.; Adhikary, S.P.; Tripathy, S. Draft genome sequence of cyanobacterium Hassallia byssoidea Strain VB512170, Isolated from Monuments in India. Genome Announc. 2015, 3, e00064-15. [Google Scholar] [CrossRef] [PubMed]
- Chandrababunaidu, M.M.; Singh, D.; Sen, D.; Bhan, S.; Das, S.; Gupta, A.; Adhikary, S.P.; Tripathy, S. Draft genome sequence of Tolypothrix boutellei strain VB521301. Genome Announc. 2015, 3, e00001–e00015. [Google Scholar] [CrossRef] [PubMed]
- Chouaia, B.; Crotti, E.; Brusetti, L.; Daffonchio, D.; Essoussi, I.; Nouioui, I.; Sbissi, I.; Ghodhbane-Gtari, F.; Gtari, M.; Vacherie, B.; et al. Genome sequence of Blastococcus saxobsidens DD2, a stone-inhabiting bacterium. J. Bacteriol. 2012, 194, 2752–2753. [Google Scholar] [CrossRef]
- Normand, P.; Gury, J.; Pujic, P.; Chouaia, B.; Crotti, E.; Brusetti, L.; Daffonchio, D.; Vacherie, B.; Barbe, V.; Médigue, C.; et al. Genome sequence of radiation-resistant Modestobacter marinus strain BC501, a representative actinobacterium that thrives on calcareous stone surfaces. J. Bacteriol. 2012, 194, 4773–4774. [Google Scholar] [CrossRef]
- Kim, M.; Or, D. Hydration status and diurnal trophic interactions shape microbial community function in desert biocrusts. Biogeosciences 2017, 14, 5403–5424. [Google Scholar] [CrossRef]
- Ortiz, M.; Legatzki, A.; Neilson, J.W.; Fryslie, B.; Nelson, W.M.; Wing, R.A.; Soderlund, C.A.; Pryor, B.M.; Maier, R.M. Making a living while starving in the dark: Metagenomic insights into the energy dynamics of a carbonate cave. ISME J. 2014, 8, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Vikram, S.; Guerrero, L.D.; Makhalanyane, T.P.; Le, P.T.; Seely, M.; Cowan, D.A. Metagenomic analysis provides insights into functional capacity in a hyperarid desert soil niche community. Environ. Microbiol. 2016, 18, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Goordial, J.; Davila, A.; Greer, C.W.; Cannam, R.; DiRuggiero, J.; McKay, C.P.; Whyte, L.G. Comparative activity and functional ecology of permafrost soils and lithic niches in a hyper–arid polar desert. Environ. Microbiol. 2016, 19, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, D.M.; Paterson, G.A.; Zavarzin, W.E. Fossil and Recent Biofilms: A Natural History of Life on Earth; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding microbial multi–species symbioses. Front. Microbiol. 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Wedin, M. Lichenized fungi and the evolution of symbiotic organization. Microbiol. Spectr. 2016, 4, 6. [Google Scholar]
- Grube, M.; Cernava, T.; Soh, J.; Fuchs, S.; Aschenbrenner, I.; Lassek, C.; Wegner, U.; Becher, D.; Riedel, K.; Sensen, C.W.; et al. Exploring functional contexts of symbiotic sustain within lichen–associated bacteria by comparative omics. ISME J. 2015, 9, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Eymann, C.; Lassek, C.; Wegner, U.; Bernhardt, J.; Fritsch, O.A.; Fuchs, S.; Otto, A.; Albrecht, D.; Schiefelbein, U.; Cernava, T.; et al. Symbiotic interplay of fungi, algae, and bacteria within the lung lichen Lobaria pulmonaria L. Hoffm. as assessed by state–of–the–art metaproteomics. J. Proteome Res. 2017, 16, 2160–2173. [Google Scholar] [CrossRef]
- Cole, J.K.; Hutchison, J.R.; Renslow, R.S.; Kim, Y.-M.; Chrisler, W.B.; Engelmann, H.E.; Dohnalkova, A.C.; Hu, D.; Metz, T.O.; Fredrickson, J.K.; et al. Phototrophic biofilm assembly in microbial–mat–derived unicyanobacterial consortia: Model systems for the study of autotroph–heterotroph interactions. Front. Microbiol. 2014, 5, 109. [Google Scholar] [CrossRef]
- Couradeau, E.; Giraldo-Silva, A.; De Martini, F.; Garcia-Pichel, F. Spatial segregation of the biological soil crust microbiome around its foundational cyanobacterium, Microcoleus vaginatus, and the formation of a nitrogen-fixing cyanosphere. Microbiome 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Pitts, B.; Lauchnor, E.; Cappitelli, F.; Stewart, P.S. Development of a laboratory model of a phototroph–heterotroph mixed–species biofilm at the stone/air interface. Front. Microbiol. 2015, 6, 1251. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Makhalanyane, T.P.; Seely, M.; Cowan, D.A. Cyanobacteria drive community composition and functionality in rock–soil interface communities. Mol. Ecol. 2015, 24, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Sharif, D.I.; Gallon, J.; Smith, C.J.; Dudley, E. Quorum sensing in Cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME J. 2008, 2, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Gantner, S.; Schmid, M.; Dürr, C.; Schuhegger, R.; Steidle, A.; Hutzler, P.; Langebartels, C.; Eberl, L.; Hartmann, A.; Dazzo, F.B. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol. Ecol. 2006, 56, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.C.; Carlson, R.P. Microbial consortia engineering for cellular factories: In vitro to in silico systems. Comput. Struct. Biotechnol. J. 2012, 3, e201210017. [Google Scholar] [CrossRef]
- Beliaev, A.S.; Romine, M.F.; Serres, M.; Bernstein, H.C.; Linggi, B.E.; Markillie, L.M.; Isern, N.G.; Chrisler, W.B.; Kucek, L.A.; Hill, E.A.; et al. Inference of interactions in cyanobacterial–heterotrophic co–cultures via transcriptome sequencing. ISME J. 2014, 8, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Coe, A.; Chisholm, S.W. Torn apart and reunited: Impact of a heterotroph on the transcriptome of Prochlorococcus. ISME J. 2016, 10, 2831–2843. [Google Scholar] [CrossRef]
- Bernstein, H.C.; McClure, R.S.; Thiel, V.; Sadler, N.C.; Kim, Y.M.; Chrisler, W.B.; Hill, E.A.; Bryant, D.A.; Romine, M.F.; Jansson, J.K.; et al. Indirect interspecies regulation: Transcriptional and physiological responses of a cyanobacterium to heterotrophic partnership. mSystems 2017, 2, e00181-16. [Google Scholar] [CrossRef]
- Ohad, I.; Raanan, H.; Keren, N.; Tchernov, D.; Kaplan, A. Light–Induced changes within photosystem II protects Microcoleus sp. in biological desert sand crusts against excess light. PLoS ONE 2010, 5, e11000. [Google Scholar] [CrossRef]
- Raanan, H.; Oren, N.; Treves, H.; Berkowicz, S.M.; Hagemann, M.; Pade, N.; Keren, N.; Kaplan, A. Simulated soil crust conditions in a chamber system provide new insights on cyanobacterial acclimation to desiccation. Environ. Microbiol. 2016, 18, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Oren, N.; Raanan, H.; Murik, O.; Keren, N.; Kaplan, A. Dawn illumination prepares desert cyanobacteria for dehydration. Curr. Biol. 2017, 27, R1056–R1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Deng, S.; Liu, J.; Ye, C.; Zhou, X.; Chen, L. Cell damage caused by ultraviolet B radiation in the desert cyanobacterium Phormidium tenue and its recovery process. Ecotoxicol. Environ. Saf. 2017, 144, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, J.; Rettberg, P.; Cockell, C.S. Aggregated cell masses provide protection against space extremes and a microhabitat for hitchhiking co-inhabitants. Astrobiology 2019, 19, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, L.; Xu, H.; Zhu, Z.; Gao, X. Flexibility–rigidity coordination of the dense exopolysaccharide matrix in terrestrial cyanobacteria acclimated to periodic desiccation. Appl Environ. Microbiol. 2017, 83, e01619-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Zhou, Q.; Zhang, X.; Wei, J. Comparative transcriptome analysis of the lichen-forming fungus Endocarpon pusillum elucidates its drought adaptation mechanisms. Sci. China Life Sci. 2015, 58, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Murik, O.; Oren, N.; Shotland, Y.; Raanan, H.; Treves, H.; Kedem, I.; Keren, N.; Hagemann, M.; Pade, N.; Kaplan, A. What distinguishes cyanobacteria able to revive after desiccation from those that cannot: The genome aspect. Environ. Microbiol. 2017, 19, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Nejidat, A. Nitrification and occurrence of salt-tolerant nitrifying bacteria in the Negev desert soils. FEMS Microbiol. Ecol. 2005, 52, 21–29. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity is a key determinant for soil microbial communities in a desert ecosystem. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef]
- Hallmann, C.; Stannek, L.; Fritzlar, D.; Hause-Reitner, D.; Friedl, T.; Hoppert, M. Molecular diversity of phototrophic biofilms on building stone. FEMS Microbiol. Ecol. 2013, 84, 355–372. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F.; Krakova, L.; Pangallo, D.; Bruno, L. Effects of biocide treatments on the biofilm community in Domitilla’s catacombs in Rome. Sci. Total Environ. 2016, 572, 252–262. [Google Scholar] [CrossRef]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; Philippis, R.D. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef]
- Adessi, A.; Cruz de Carvalho, R.; De Philippis, R.; Branquinho, C.; Marques da Silva, J. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Koźlecki, T. Silver nanoparticles as a control agent against facades coated by aerial algae—a model study of Apatococcus lobatus (green algae). PLoS ONE 2017, 12, e0183276. [Google Scholar] [CrossRef]
- Gambino, M.; Ali Ahmed, M.A.; Villa, F.; Cappitelli, F. Zinc oxide nanoparticles hinder fungal biofilm development in an ancient Egyptian tomb. Int. Biodeterior. Biodegrad. 2017, 122, 92–99. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Miller, A.Z.; Martin-Sanchez, P.M.; Hernandez-Marine, M. Uncovering the origin of the black stains in Lascaux Cave in France. Environ. Microbiol. 2012, 14, 3220–3323. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm–based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Miller, A.Z.; Laiz, L.; Gonzalez, J.M.; Dionísio, A.; Macedo, M.F.; Saiz-Jimenez, C. Reproducing stone monument photosynthetic–based colonization under laboratory conditions. Sci. Total Environ. 2008, 405, 278–285. [Google Scholar] [CrossRef]
- Miller, A.Z.; Liaz, L.; Dionisio, A.; Macedo, M.F.; Saiz-Jimenez, C. Growth of phototrophic biofilms from limestone monuments under laboratory conditions. Int. Biodeterior. Biodegrad. 2009, 63, 860–867. [Google Scholar] [CrossRef]
- Vázquez-Nion, D.; Rodríguez-Castro, J.; López-Rodríguez, M.C.; Fernández-Silva, I.; Prieto, B. Subaerial biofilms on granitic historic buildings: Microbial diversity and development of phototrophic multi–species cultures. Biofouling 2016, 32, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Noack-Schönmann, S.; Bus, T.; Banasiak, R.; Knabe, N.; Broughton, W.J.; Den Dulk–Ras, H.; Hooykaas, P.J.; Gorbushina, A.A. Genetic transformation of Knufia petricola A95–a model organism for biofilm-material interactions. AMB Express 2014, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Nai, C.; Wong, H.Y.; Pannenbecker, A.; Broughton, W.J.; Benoit, I.; de Vries, R.P.; Gueidan, C.; Gorbushina, A.A. Nutritional physiology of a rock–inhabiting, model microcolonial fungus from an ancestral lineage of the Chaetothyriales (Ascomycetes). Fungal Genet. Biol. 2013, 56, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, F.; Bandow, N.; Bouchez, J.; von Blanckenburg, F.; Gorbushina, A.A. Microbial colonization of bare rocks: Laboratory biofilm enhances mineral weathering. Procedia Earth Planet 2014, 10, 123–129. [Google Scholar] [CrossRef]
- Seiffert, F.; Bandow, N.; Kalbe, U.; Milke, R.; Gorbushina, A.A. Laboratory tools to quantify biogenic dissolution of rocks and minerals: A model rock biofilm growing in percolation columns. Front. Earth Sci. 2016, 4, 31. [Google Scholar] [CrossRef]
- Grinberg, M.; Orevi, T.; Kashtan, N. Bacterial surface colonization, preferential attachment and fitness under periodic stress. PLoS Comput. Biol. 2019, 15, e1006815. [Google Scholar] [CrossRef] [PubMed]
- Chertov, O.; Gorbushina, A.A.; Deventer, B. A model for microcolonial fungi growth on rock surfaces. Ecol. Modell 2004, 177, 415–426. [Google Scholar] [CrossRef]
- Porada, P.; Lenton, T.; Pohl, A.; Weber, B.; Mander, L.; Donnadieu, Y.; Beer, C.; Pöschl, U.; Kleidon, A. High potential for weathering and climate effects of non–vascular vegetation in the Late Ordovician. Nat. Commun. 2016, 7, 12113. [Google Scholar] [CrossRef]
- Porada, P.; Pöschl, U.; Kleidon, A.; Beer, C.; Weber, B. Estimating global nitrous oxide emissions by lichens and bryophytes with a process–based productivity model. Biogeosciences 2017, 14, 1593–1602. [Google Scholar] [CrossRef]
- Porada, P.; Weber, B.; Elbert, W.; Pöschl, U.; Kleidon, A. Estimating global carbon uptake by lichens and bryophytes with a process–based model. Biogeosciences 2013, 10, 6989–7033. [Google Scholar] [CrossRef]
- Porada, P.; Weber, B.; Elbert, W.; Pöschl, U.; Kleidon, A. Estimating impacts of lichens and bryophytes on global biogeochemical cycles. Global Biogeochem. Cy 2014, 28, 71–85. [Google Scholar] [CrossRef]

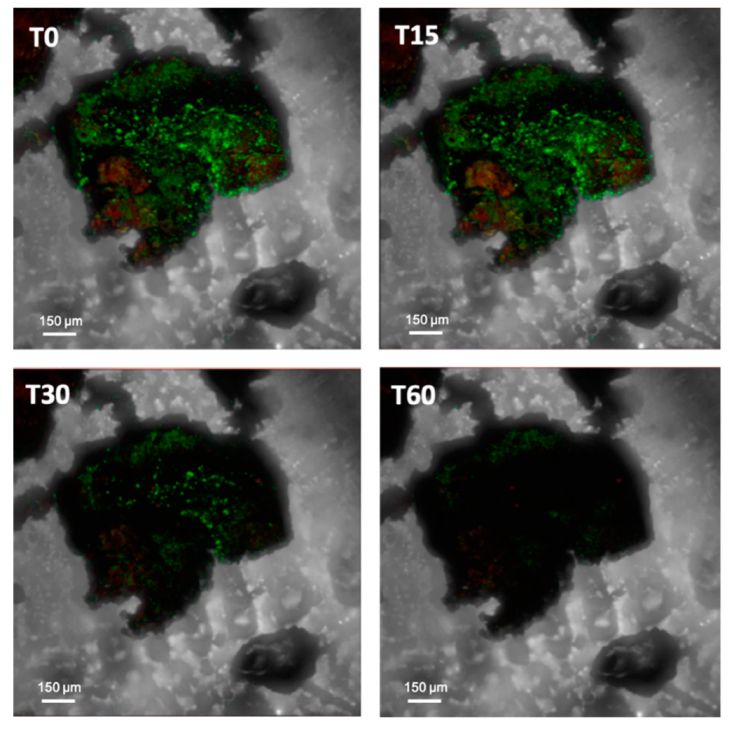
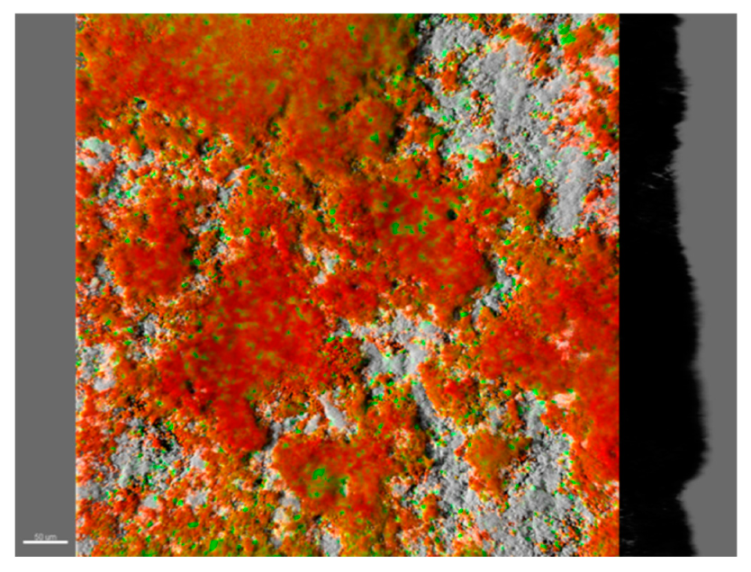
| Core Microbiome (Metagenomic Studies) | Main Functional Traits | Biologically-Driven Processes | Mechanisms of Drought Resistance | |
|---|---|---|---|---|
| Biological Soil Crusts | Bacteria: Cyanobacteria, Actinobacteria, Acidobacteria, Alpha-proteobacteria, and Bacteroidetes Fungi: Ascomycota, Basidiomycota, and Chytridiomycota Archaea: Crenarchaeota [16,17,18,19,20,21] | Functional genes associated with C degradation and N cycling. [20] | Modulating C and N heterogeneity and cycling. Increasing the capture of nutrient-rich dust. Modulating, surface albedo, water fluxes and erosion. Influencing soil fertility and plant establishment patterns. [22,23,24] | EPSs act as a repository for water and stabilize desiccation-tolerant enzymes and molecules. Activation of a non-radioactive cyclic electron transfer route during photosinthesis to minimize oxidative damage. Synthesis and degradation of osmolytes are used to balance the changing water potential. [23,24,25] |
| Desert Rocks | Bacteria: Actinobacteria, Cyanobacteria, Proteobacteria, and Chloroflexi Fungi: Ascomycota Archaea: Crenarchaeota [26,27,28] | Transition metal-related molecular functions such as manganese ion binding and iron ion binding. [29,30] | Modulating C and N heterogeneity and cycling. Clogging the surface rock pores through secretion of extracellular polymeric substances (EPSs), lowering evaporation and slowing salt crystallization. [31] | EPSs act as a repository for water and stabilize desiccation-tolerant enzymes and molecules. Synthesis of heat-shock proteins and chaperons. Production of antioxidant enzymes, DNA damage repair systems, and UV-absorbing pigments. Dormant cells. [23,32] |
| Stone Heritage | Bacteria: Cyanobacteria, Actinobacteria Proteobacteria, Bacteroidetes, Acidobacteria, and Chloroflexi Fungi: Ascomycota Archaea: Euryarchaeota and Crenarchaeota [33,34,35,36] | Functional genes associated with C, N and S cycling autotrophic carbon fixation and mineral transformation processes. [10,11] | Modulating C and N heterogeneity and cycling. Weakening of the mineral lattice through wetting and drying cycles and sub-sequent expansion and contraction of the EPS matrix. Dissolving minerals through the excretion of H+, CO2, organic and inorganic acids, siderophores and other metabolites. Mediating the formation of minerals. Regulating water fluxes in the stone. Increasing hydrophobicity of the surface. Incorporation of mineral grains into the biofilm. [14] | EPSs act as a repository for water and stabilize desiccation-tolerant enzymes and molecules. Synthesis of antioxidant. Synthesis and degradation of osmolytes to balance the changing water potential. Dormant cells. [23,32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, F.; Cappitelli, F. The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments. Microorganisms 2019, 7, 380. https://doi.org/10.3390/microorganisms7100380
Villa F, Cappitelli F. The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments. Microorganisms. 2019; 7(10):380. https://doi.org/10.3390/microorganisms7100380
Chicago/Turabian StyleVilla, Federica, and Francesca Cappitelli. 2019. "The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments" Microorganisms 7, no. 10: 380. https://doi.org/10.3390/microorganisms7100380
APA StyleVilla, F., & Cappitelli, F. (2019). The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments. Microorganisms, 7(10), 380. https://doi.org/10.3390/microorganisms7100380







