Directed Evolution of Saccharomyces cerevisiae for Increased Selenium Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Plasmid Construction
2.2. Media and Growth Conditions
2.3. Measurement of Selenium Content
2.4. Growth Rates
2.5. Wet-to-Dry Weight Conversion
2.6. Statistical Analyses
3. Results
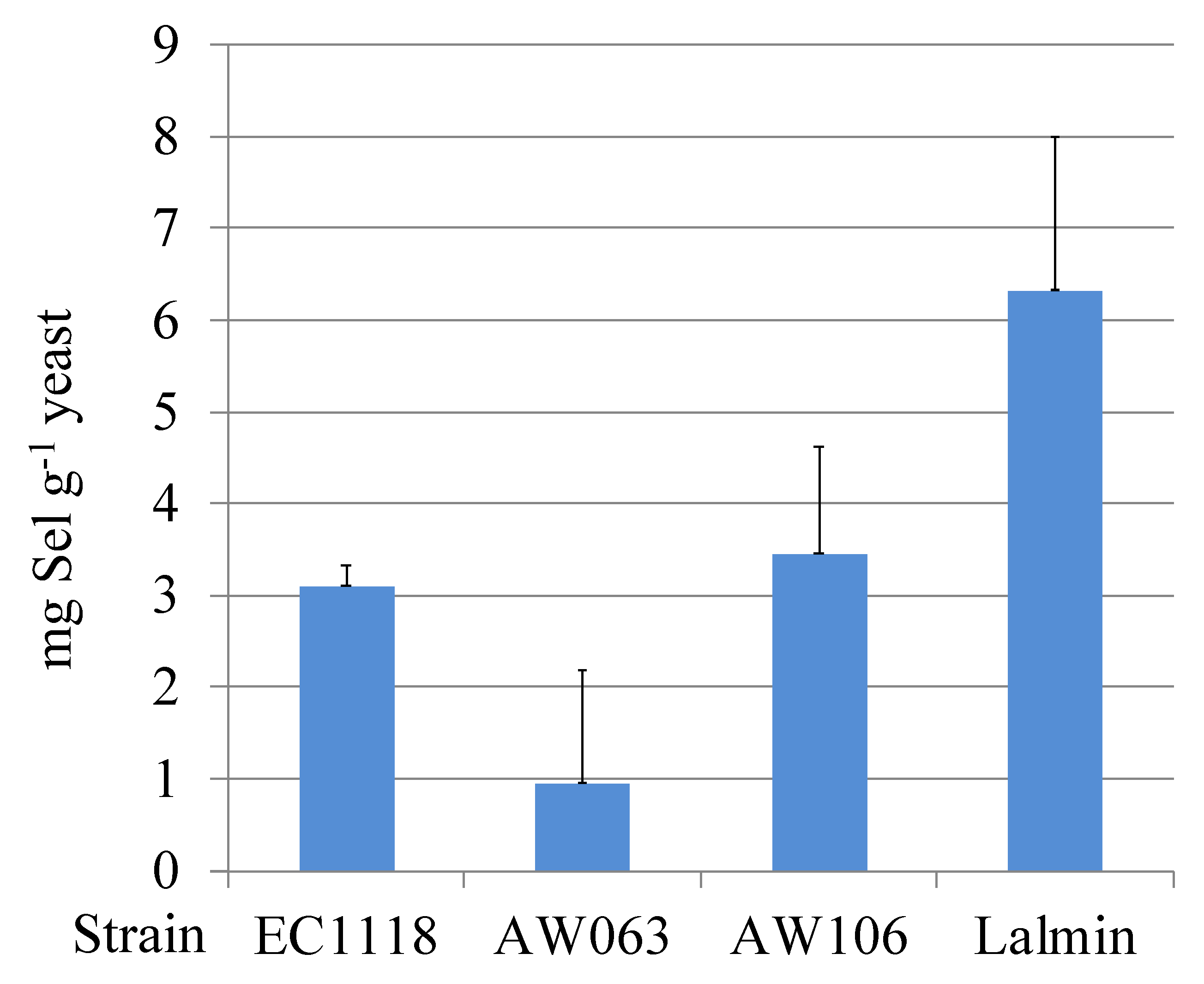
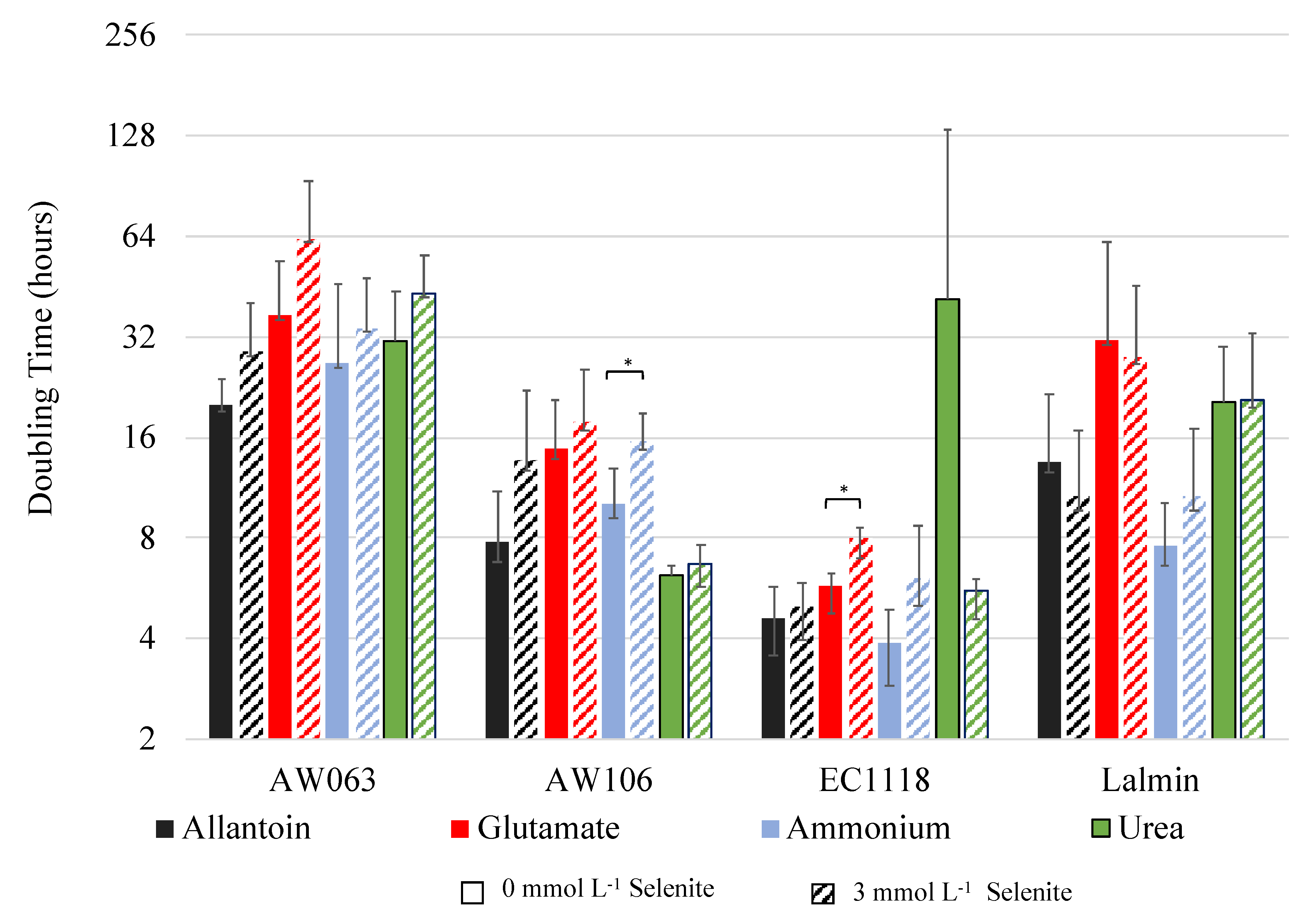
3.1. Correlations of Selenite Resistance and Selenium Accumulation
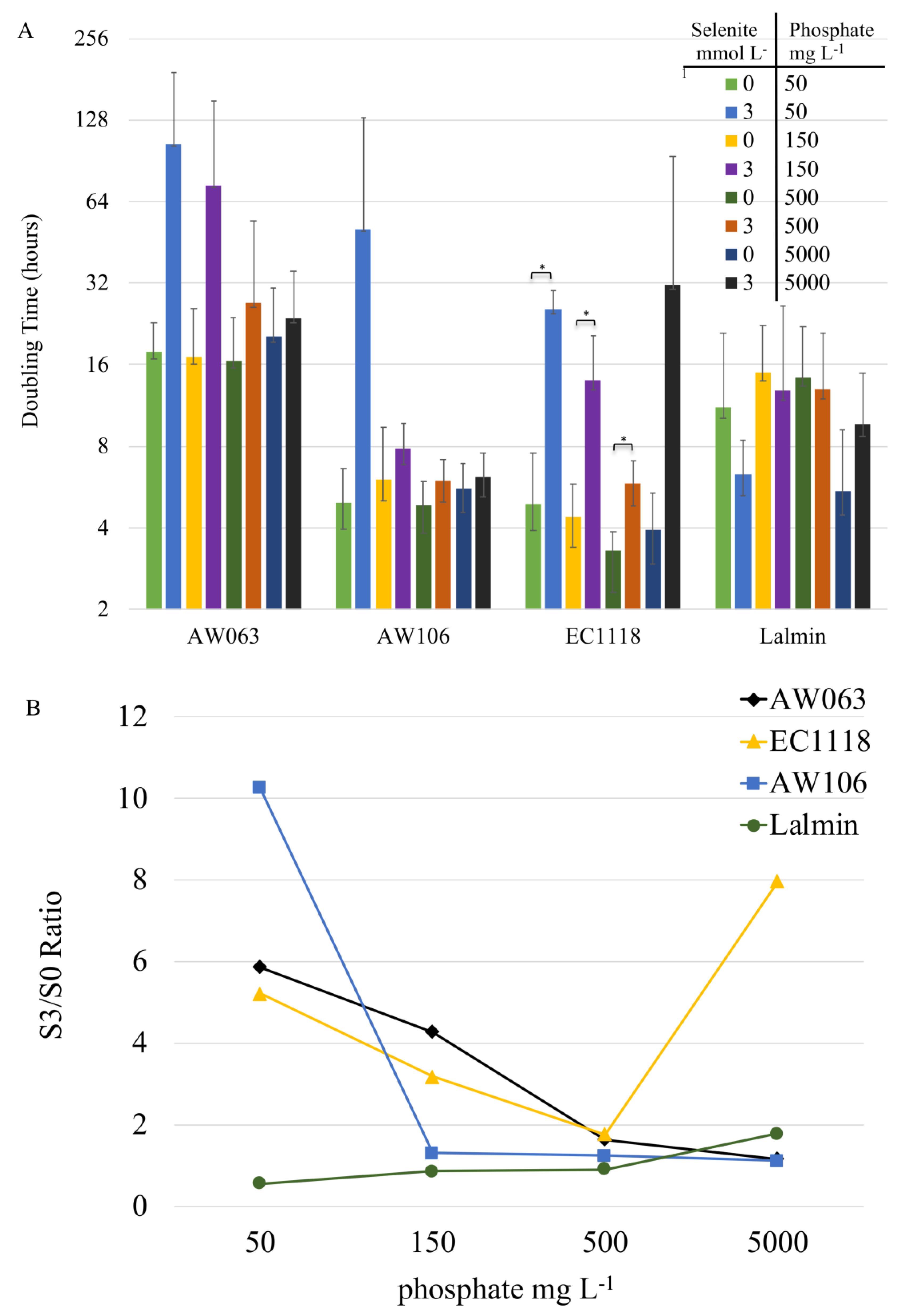
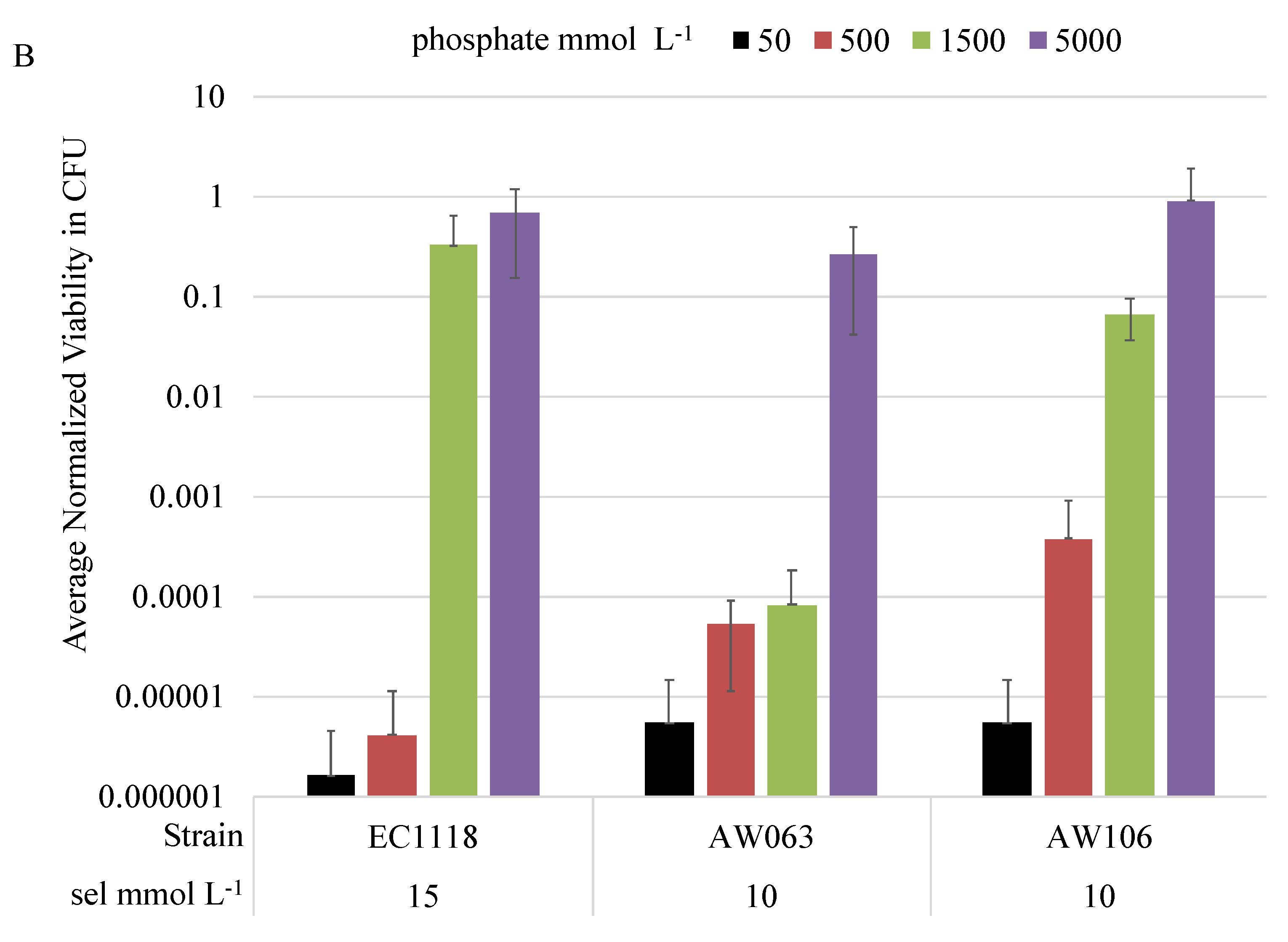
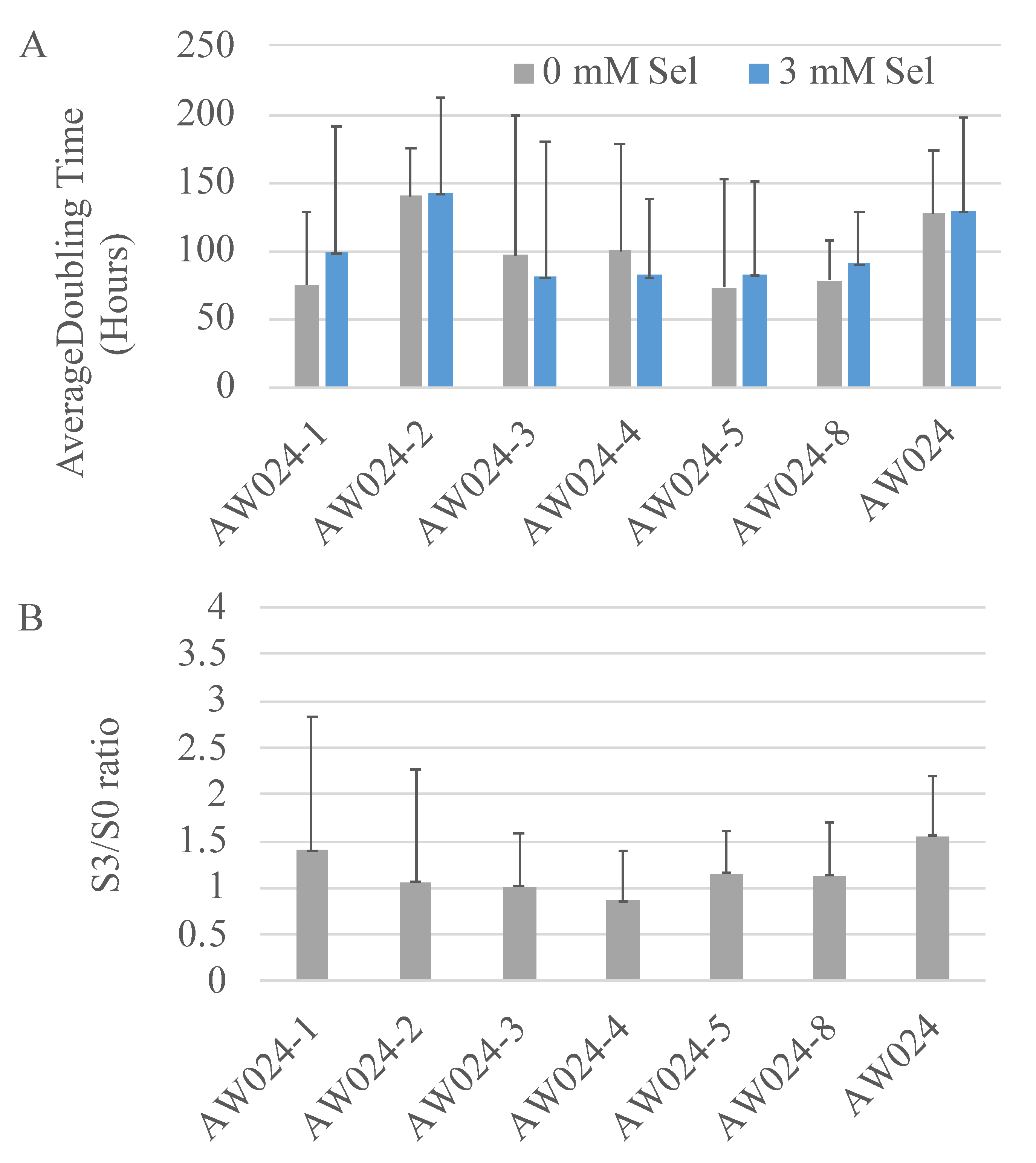
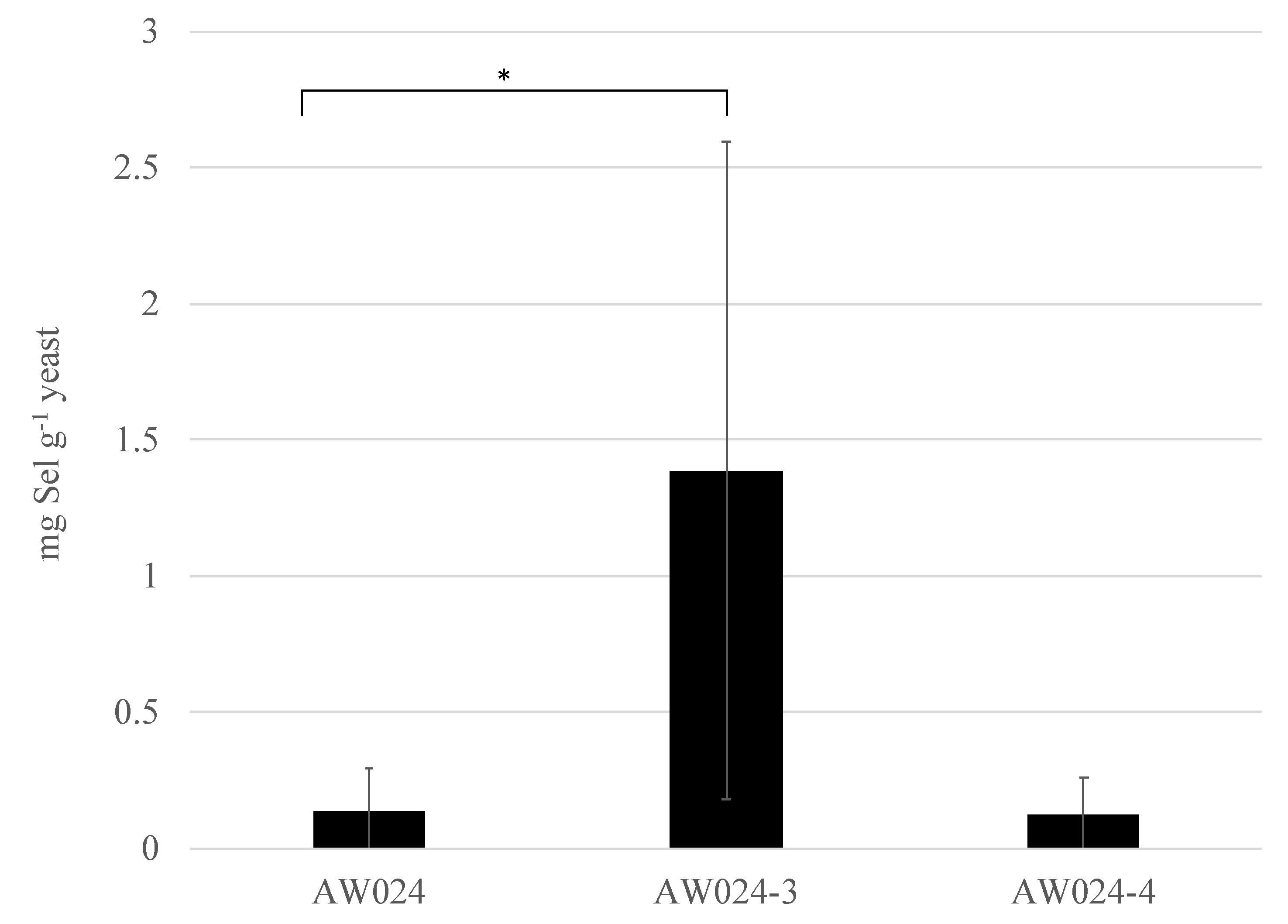
3.2. Selection for Increased Selenite Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rossignol, T.; Dulau, L.; Julien, A.; Blondin, B. Genome-wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast 2003, 20, 1369–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallemand Bio Ingredients. Available online: http://www.bio-lallemand.com/our-brands/lalmin/ (accessed on 1 January 2017).
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mapelli, V.; Hillestrøm, P.R.; Patil, K.; Larsen, E.H.; Olsson, L. The interplay between sulphur and selenium metabolism influences the intracellular redox balance in Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Lazard, M.; Blanquet, S.; Fisicaro, P.; Labarraque, G.; Plateau, P. Uptake of Selenite by Saccharomyces cerevisiae Involves the High and Low Affinity Orthophosphate Transporters. J. Biol. Chem. 2010, 285, 32029–32037. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.; Pometto, A.L.; Cox, D.J. Enhanced organically bound selenium yeast production by fed-batch fermentation. J. Agric. Food Chem. 1999, 47, 2496–2500. [Google Scholar] [CrossRef] [PubMed]
- Ponce de León, C.A.; Bayón, M.M.; Paquin, C.; Caruso, J.A. Selenium incorporation into Saccharomyces cerevisiae cells: A study of different incorporation methods. J. Appl. Microbiol. 2002, 92, 602–610. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.R.; Rosen, B.P.; Liu, Z. Jen1p: A high affinity selenite transporter in yeast. Mol. Biol. Cell 2010, 21, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Bartosz, G. A role for yeast glutaredoxin genes in selenite-mediated oxidative stress. Fungal Genet. Biol. 2008, 45, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, A.Z.; Koshland, D.E. A simple colony-formation assay in liquid medium, termed “tadpoling”, provides a sensitive measure of Saccharomyces cerevisiae culture viability. Yeast 2013, 30, 501–509. [Google Scholar] [CrossRef] [PubMed]


| Name | Genotype | Source |
|---|---|---|
| EC1118 | MATa/MATα wild-type | Lalvin |
| AW063 | MATα ∆ade1 ∆pha2 ∆arg8 ∆lys2 ∆gal7 | This study |
| AW106 | MATα ∆gal2 ∆mal2 | This study |
| Lalmin® SE2000 | wild-type | Lallemand Co. |
| AW024 | MATα ura | This study |
| AW024-1 | MATα ura | This study |
| AW024-2 | MATα ura | This study |
| AW024-3 | MATα ura | This study |
| AW024-4 | MATα ura | This study |
| AW024-5 | MATα ura | This study |
| AW024-8 | MATα ura | This study |
| Carbon | Strain | Slope 1 | Selenium Accumulation 2 | r-Value 3 | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Ethanol | AW063 | 2033 | 0.95 | –0.66 | 1.23 | 0.34 |
| AW106 | 2990 | 3.45 | ||||
| EC1118 | 894 | 3.10 | ||||
| Lalmin | −206 | 6.32 | ||||
| Galactose | AW063 | 765 | 0.95 | –0.67 | 1.29 | 0.33 |
| AW106 | 140 | 3.45 | ||||
| EC1118 | 1391 | 3.10 | ||||
| Lalmin | −318 | 6.32 | ||||
| Glucose | AW063 | 718 | 0.95 | –0.80 | 1.87 | 0.20 |
| AW106 | 99 | 3.45 | ||||
| EC1118 | 69 | 3.10 | ||||
| Lalmin | 35 | 6.32 | ||||
| Glycerol | AW063 | 1421 | 0.95 | –0.99 | 8.69 | 0.013 |
| AW106 | 668 | 3.45 | ||||
| EC1118 | 932 | 3.10 | ||||
| Lalmin | 152 | 6.32 | ||||
| Lactate | AW063 | 1400 | 0.95 | –0.64 | 1.19 | 0.36 |
| AW106 | 2191 | 3.45 | ||||
| EC1118 | 2558 | 3.10 | ||||
| Lalmin | −427 | 6.32 |
| Nitrogen | Strain | S3/S0 1 | Selenium Accumulation 2 | r-Value 3 | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Allantoin | AW063 | 1.44 | 0.95 | 0.62 | 1.11 | 0.38 |
| AW106 | 1.77 | 3.45 | ||||
| EC1118 | 1.08 | 3.10 | ||||
| Lalmin | 0.79 | 6.32 | ||||
| Glutamate | AW063 | 1.70 | 0.95 | 0.98 | 7.63 | 0.017 |
| AW106 | 1.19 | 3.45 | ||||
| EC1118 | 1.38 | 3.10 | ||||
| Lalmin | 0.88 | 6.32 | ||||
| Ammonium | AW063 | 1.27 | 0.95 | 0.31 | 0.46 | 0.69 |
| AW106 | 1.54 | 3.45 | ||||
| EC1118 | 1.55 | 3.10 | ||||
| Lalmin | 1.40 | 6.32 | ||||
| Urea | AW063 | 1.39 | 0.95 | 0.18 | 0.25 | 0.82 |
| AW106 | 1.08 | 3.45 | ||||
| EC1118 | 0.14 | 3.10 | ||||
| Lalmin | 1.01 | 6.32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshinaga, M.; How, S.; Blanco, D.; Murdoch, I.S.; Grudny, M.; Powers, S.L.; Molina, N.; Rosen, B.P.; Welch, A.Z. Directed Evolution of Saccharomyces cerevisiae for Increased Selenium Accumulation. Microorganisms 2018, 6, 81. https://doi.org/10.3390/microorganisms6030081
Yoshinaga M, How S, Blanco D, Murdoch IS, Grudny M, Powers SL, Molina N, Rosen BP, Welch AZ. Directed Evolution of Saccharomyces cerevisiae for Increased Selenium Accumulation. Microorganisms. 2018; 6(3):81. https://doi.org/10.3390/microorganisms6030081
Chicago/Turabian StyleYoshinaga, Masafumi, Stephanie How, Damien Blanco, Ian S. Murdoch, Matteo Grudny, Samantha L. Powers, Nelson Molina, Barry P. Rosen, and Aaron Z. Welch. 2018. "Directed Evolution of Saccharomyces cerevisiae for Increased Selenium Accumulation" Microorganisms 6, no. 3: 81. https://doi.org/10.3390/microorganisms6030081