Sulfate-Reducing Naphthalene Degraders Are Picky Eaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Site Description
2.3. Enrichment Cultivation
2.4. Oil Biodegradation
2.5. Chemical Analysis
2.6. Stable Isotope Probing (SIP) Microcosms
2.7. SIP Molecular Analysis
3. Results and Discussion
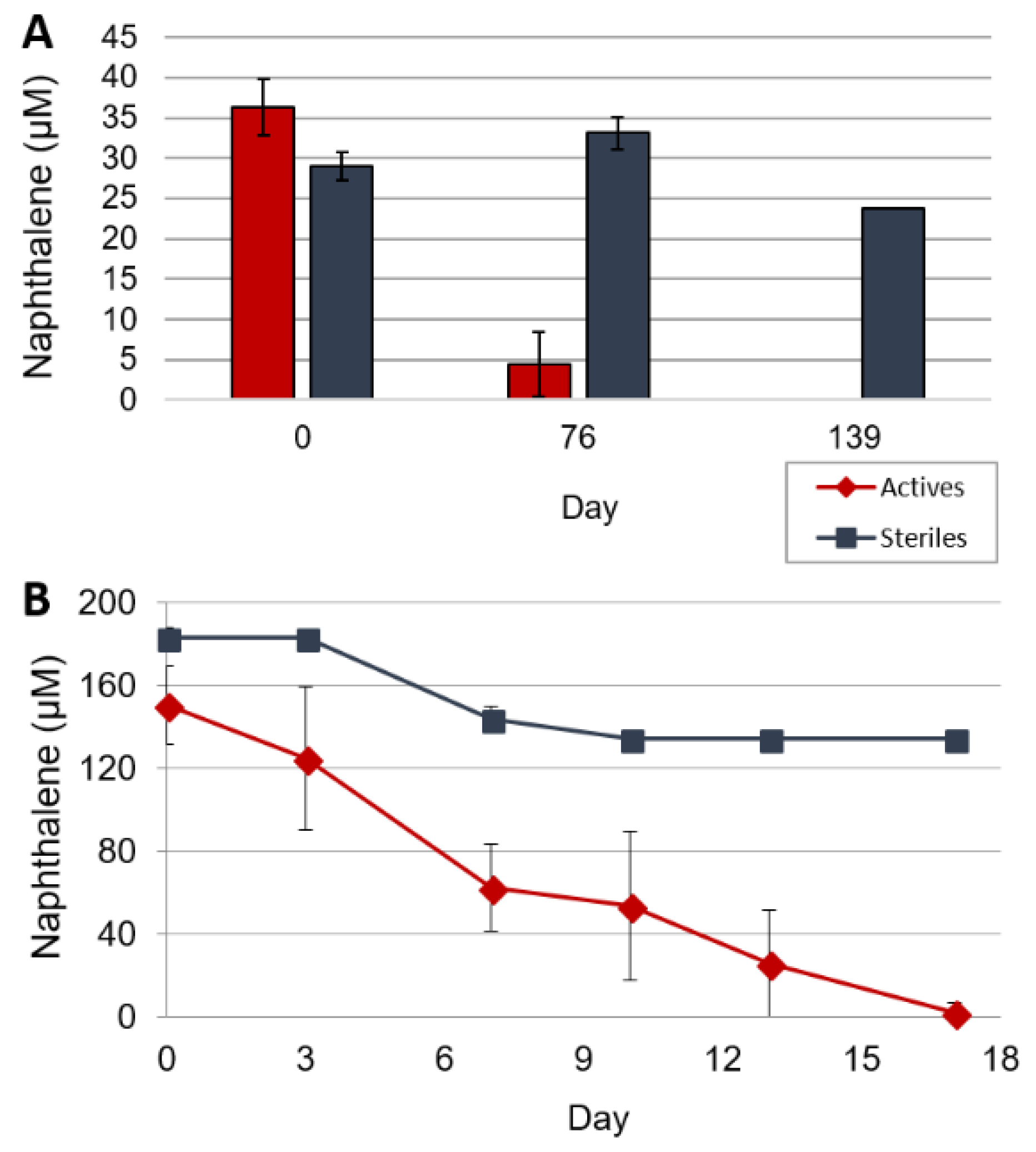
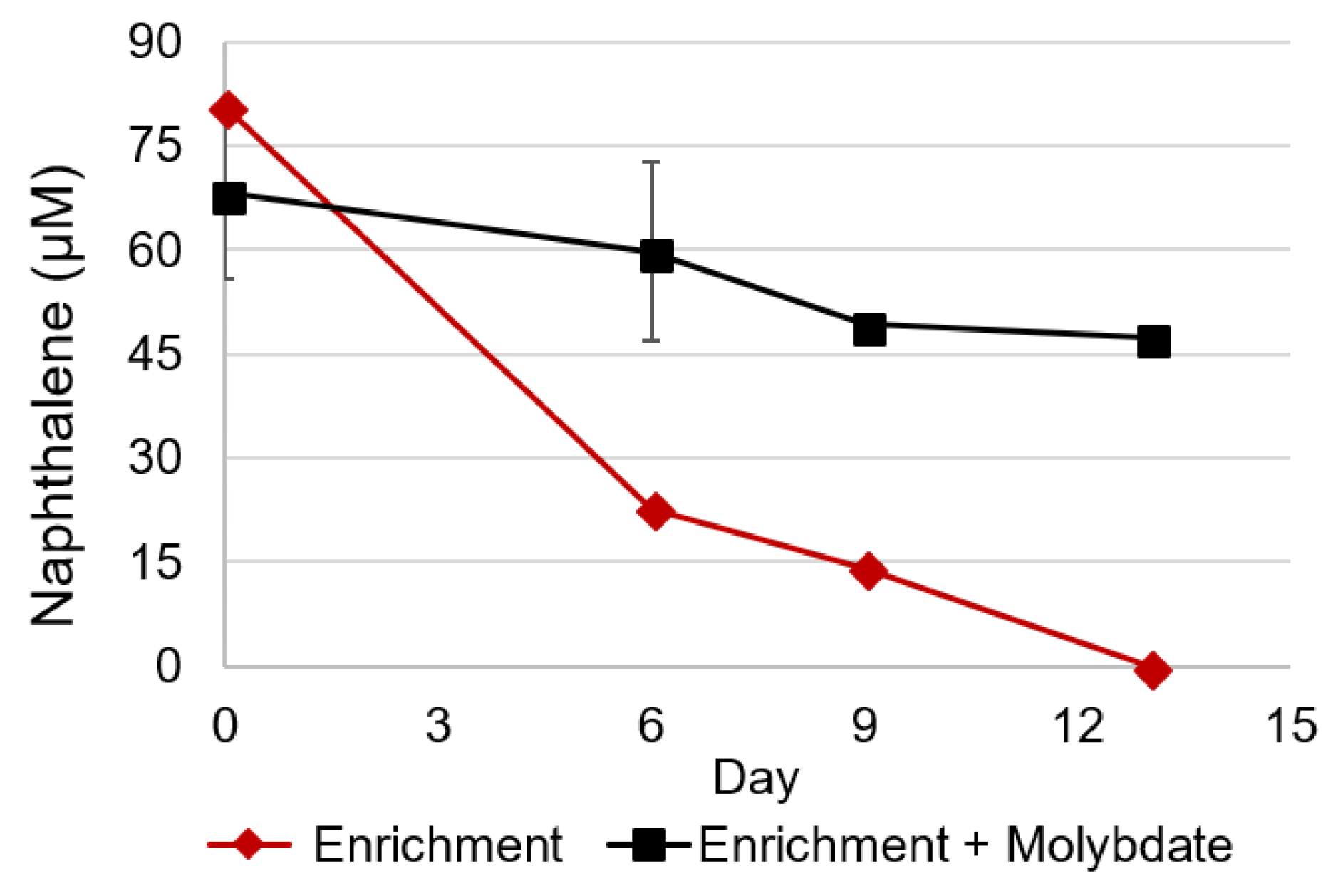
3.1. Naphthalene Degrading Enrichments
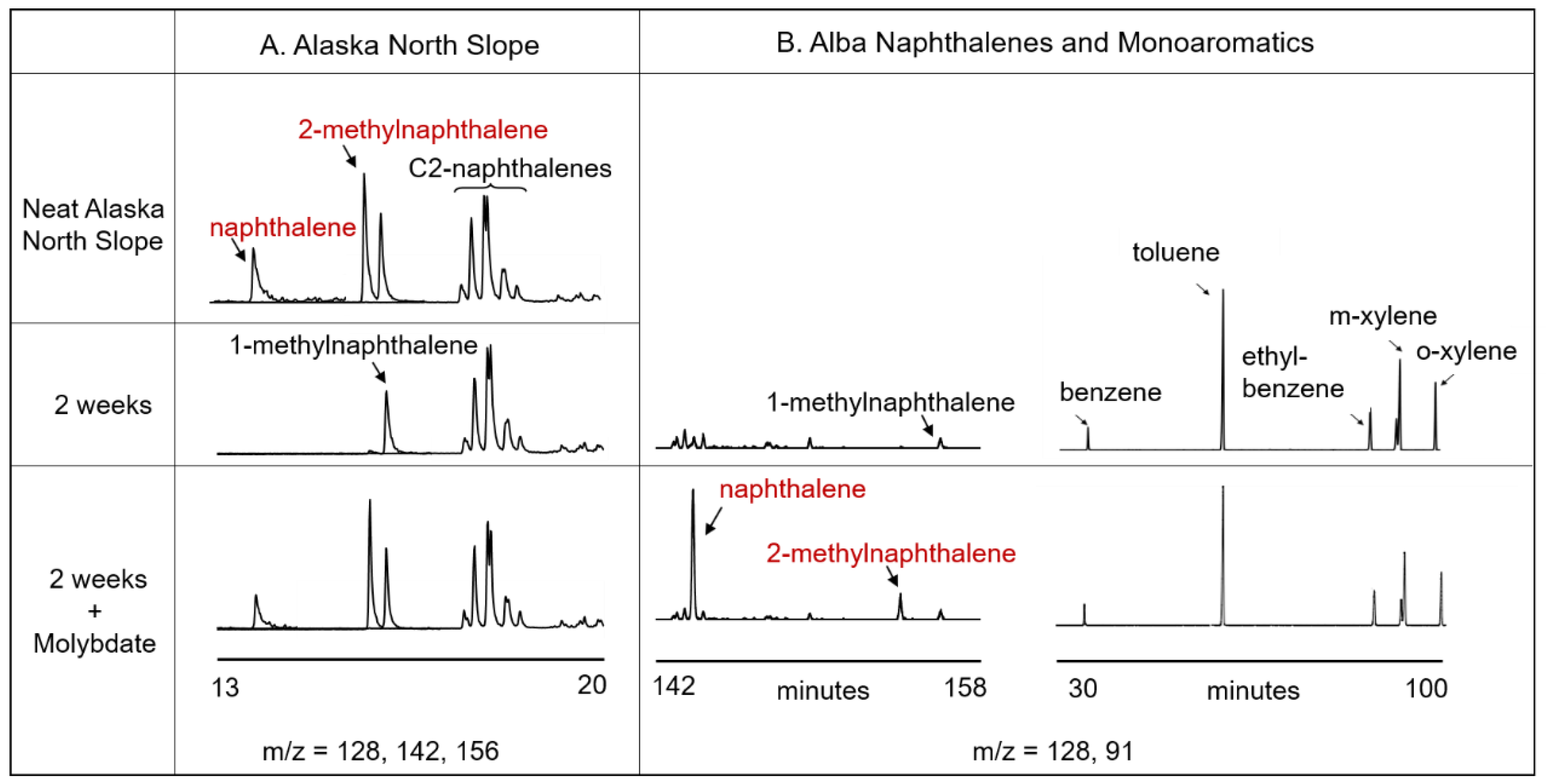
3.2. Crude Oil Biodegradation
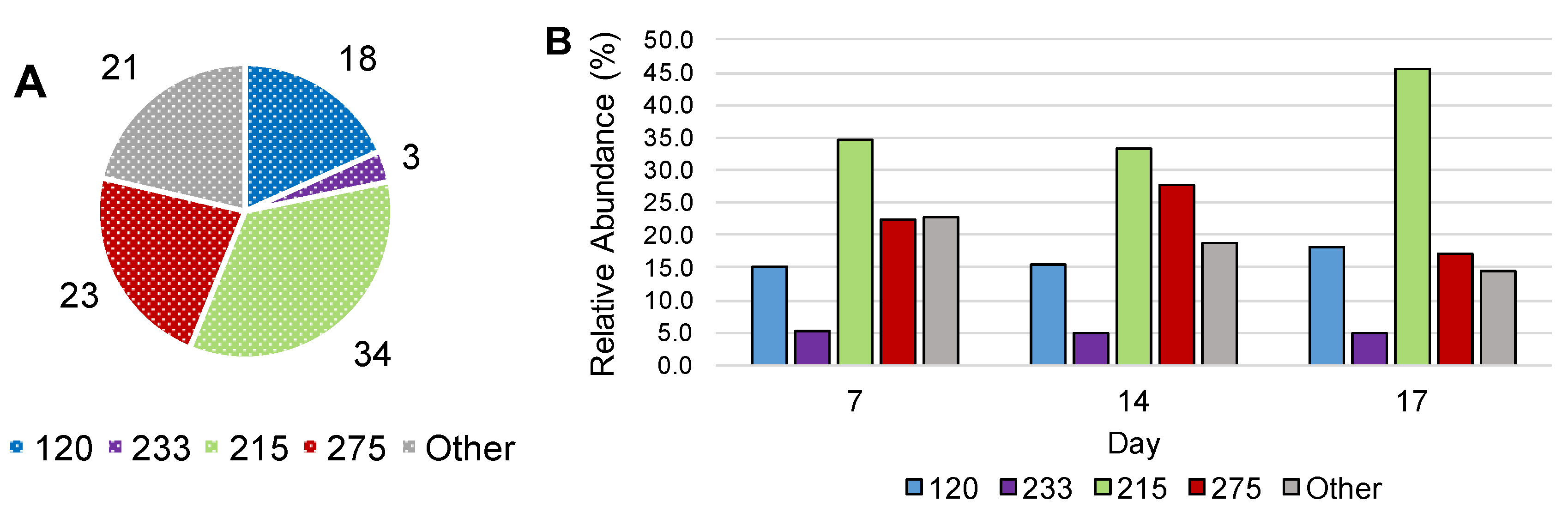
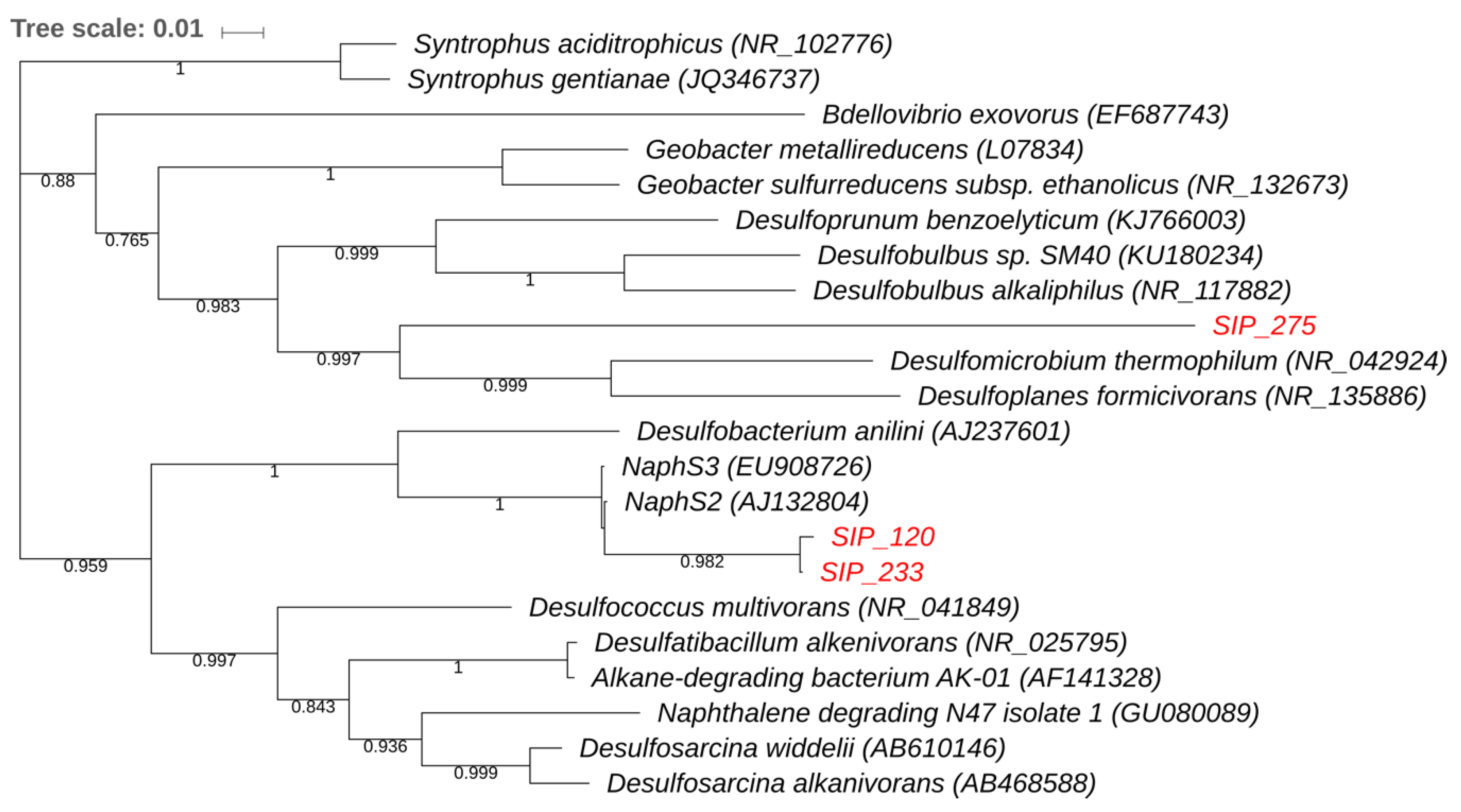
3.3. Stable Isotope Probing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gigliotti, C.L.; Totten, L.A.; Offenberg, J.H.; Dachs, J.; Reinfelder, J.R.; Nelson, E.D.; Glenn, T.R.; Eisenreich, S.J. Atmospheric concentrations and deposition of Polycyclic Aromatic Hydrocarbons to the Mid-Atlantic east coast region. Environ. Sci. Technol. 2005, 39, 5550–5559. [Google Scholar] [CrossRef] [PubMed]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Young, L.Y. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl. Environ. Microbiol. 1997, 63, 4759–4764. [Google Scholar] [PubMed]
- Galushko, A.; Minz, D.; Schink, B.; Widdel, F. Anaerobic degradation of naphthalene by a pure culture of a novel type of marine sulphate-reducing bacterium. Environ. Microbiol. 1999, 1, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Musat, F.; Galushko, A.; Jacob, J.; Widdel, F.; Kube, M.; Reinhardt, R.; Wilkes, H.; Schink, B.; Rabus, R. Anaerobic degradation of naphthalene and 2-methylnaphthalene by strains of marine sulfate-reducing bacteria. Environ. Microbiol. 2009, 11, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, E.; Michaelis, W.; Meckenstock, R.U. Anaerobic cometabolic conversion of benzothiophene by a sulfate-reducing enrichment culture and in a tar-oil-contaminated aquifer. Appl. Environ. Microbiol. 2001, 67, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Kümmel, S.; Herbst, F.-A.; Bahr, A.; Duarte, M.; Pieper, D.H.; Jehmlich, N.; Seifert, J.; von Bergen, M.; Bombach, P.; Richnow, H.H.; et al. Anaerobic naphthalene degradation by sulfate-reducing Desulfobacteraceae from various anoxic aquifers. FEMS Microbiol. Ecol. 2015, 91, 3. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.R.; Zhang, X.; Phelps, C.; Young, L.Y. Anaerobic mineralization of stable-isotope-labeled 2-methylnaphthalene. Appl. Environ. Microbiol. 2001, 67, 4353–4357. [Google Scholar] [CrossRef] [PubMed]
- Kennish, M.J.; Bricker, S.B.; Dennison, W.C.; Glibert, P.M.; Livingston, R.J.; Moore, K.A.; Noble, R.T.; Paerl, H.W.; Ramstack, J.M.; Seitzinger, S.; et al. Barnegat Bay–Little Egg Harbor Estuary: Case study of a highly eutrophic coastal bay system. Ecol. Appl. 2007, 17, S3–S6. [Google Scholar] [CrossRef]
- Vane, C.H.; Harrison, I.; Kim, A.W.; Moss-Hayes, V.; Vickers, B.P.; Horton, B.P. Status of organic pollutants in surface sediments of Barnegat Bay-Little Egg Harbor Estuary, New Jersey, USA. Mar. Pollut. Bull. 2008, 56, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, T.J.; Armstrong, T.N.; Thelen, J.B.; Ludwig, D.F.; Firstenberg, C.E. Characterization of chemical contamination in shallow-water estuarine habitats of an industrialized river. Part 1: Organic Compounds. Soil Sediment Contam. Int. J. 2005, 14, 13–33. [Google Scholar] [CrossRef]
- Rustic, G.T. Polycyclic Hydrocarbon Contamination in and around the Arthur Kill, Staten Island, New York. Master’s Thesis, Rutgers University, New Brunswick, NJ, USA, 2011. [Google Scholar]
- Christiansen, C.; Leipe, T.; Witt, G.; Christoffersen, P.L.; Lund-Hansen, L.C. Selected elements, PCBs, and PAHs in sediments of the North Sea—Baltic Sea transition zone: Sources and transport as derived from the distribution pattern. Geogr. Tidsskr. Dan. J. Geogr. 2009, 109, 81–94. [Google Scholar] [CrossRef]
- Jacquot, F.; Dréau, Y.L.; Doumenq, P.; Munoz, D.; Guiliano, M.; Imbert, G.; Mille, G. The origins of hydrocarbons trapped in the Lake of Berre sediments. Chemosphere 1999, 39, 1407–1419. [Google Scholar] [CrossRef]
- Phelps, C.D.; Kazumi, J.; Young, L.Y. Anaerobic degradation of benzene in BTX mixtures dependent on sulfate reduction. FEMS Microbiol. Lett. 1996, 145, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; Parkerton, T.F.; Lee, C. The primary aerobic biodegradation of gasoline hydrocarbons. Environ. Sci. Technol. 2007, 41, 3316–3321. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.M.; Young, L.Y.; McGuinness, L.M.; Kerkhof, L.J. Detection of 2,4,6-trinitrotoluene-utilizing anaerobic bacteria by 15N and 13C incorporation. Appl. Environ. Microbiol. 2010, 76, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.M.; Salganik, M.; Vega, L.; Pickering, K.D.; Kerkhof, L.J. Replicability of bacterial communities in denitrifying bioreactors as measured by PCR/T-RFLP analysis. Environ. Sci. Technol. 2006, 40, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Mouttaki, H. Anaerobic degradation of non-substituted aromatic hydrocarbons. Curr. Opin. Biotechnol. 2011, 22, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Townsend, G.T.; Prince, R.C.; Suflita, J.M. Anaerobic oxidation of crude oil hydrocarbons by the resident microorganisms of a contaminated anoxic aquifer. Environ. Sci. Technol. 2003, 37, 5213–5218. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, E.; Materna, A.; Safinowski, M.; Kappler, A.; Richnow, H.H.; Michaelis, W.; Meckenstock, R.U. Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl. Environ. Microbiol. 2000, 66, 5329–5333. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, R.J., Jr.; Young, N.D.; Butler, J.E.; Chin, K.-J.; Hixson, K.K.; Mouser, P.; Lipton, M.S.; DeBoy, R.; Methé, B.A. Genome sequence of the Deltaproteobacterial strain Naphs2 and analysis of differential gene expression during anaerobic growth on naphthalene. PLoS ONE 2010, 5, e14072. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.E.; Gieg, L.M.; Parisi, V.A.; Tanner, R.S.; Tringe, S.G.; Bristow, J.; Suflita, J.M. Biocorrosive thermophilic microbial communities in Alaskan North Slope oil facilities. Environ. Sci. Technol. 2009, 43, 7977–7984. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, B.-L.; Mbadinga, S.M.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Functional genes (dsr) approach reveals similar sulphidogenic prokaryotes diversity but different structure in saline waters from corroding high temperature petroleum reservoirs. Appl. Microbiol. Biotechnol. 2014, 98, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.; McGuinness, L.; Phelps, C.; Young, L.Y.; Kerkhof, L.J. 13C-Carrier DNA shortens the incubation time needed to detect benzoate-utilizing denitrifying bacteria by stable-isotope probing. Appl. Environ. Microbiol. 2005, 71, 5192–5196. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.T.; Overmann, J.; Lince, M.T.; Manske, A.K.; Lang, A.S.; Blankenship, R.E.; Dover, C.L.V.; Martinson, T.A.; Plumley, F.G. An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc. Natl. Acad. Sci. USA 2005, 102, 9306–9310. [Google Scholar] [CrossRef] [PubMed]
- Manske, A.K.; Glaeser, J.; Kuypers, M.M.M.; Overmann, J. Physiology and phylogeny of green sulfur bacteria forming a monospecific phototrophic assemblage at a depth of 100 meters in the Black Sea. Appl. Environ. Microbiol. 2005, 71, 8049–8060. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Zuluaga, D.A.; Timmers, P.H.A.; Plugge, C.M.; Stams, A.J.M.; Buisman, C.J.N.; Weijma, J. Thiosulphate conversion in a methane and acetate fed membrane bioreactor. Environ. Sci. Pollut. Res. Int. 2016, 23, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- So, C.M.; Young, L.Y. Initial reactions in anaerobic alkane degradation by a sulfate reducer, strain AK-01. Appl. Environ. Microbiol. 1999, 65, 5532–5540. [Google Scholar] [PubMed]
- Porter, A.W.; Young, L.Y. The bamA gene for anaerobic ring fission is widely distributed in the environment. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- So, C.M.; Phelps, C.D.; Young, L.Y. Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3. Appl. Environ. Microbiol. 2003, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Aeckersberg, F.; Bak, F.; Widdel, F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 1991, 156, 5–14. [Google Scholar] [CrossRef]

| Water System | Reported PAH Range (μg/kg) | Evidence of Naphthalene Degradation | Oil Contamination? |
|---|---|---|---|
| Barnegat Bay, NJ [10] | Σ18PAH = 37–1696 | Enriched in this study | − |
| Mullica River, NJ [11] | Σ25PAH = 436–1380 | Least disturbed river in North East USA, naphthalene degradation not evaluated | − |
| Arthur Kill, NY/NJ [3,12] | Σ26PAH = 3192–11484 | 1st anaerobic naphthalene degradation enrichment | + |
| North Sea, Germany/Denmark [5,13] | Σ22PAH = 849–3769 | NaphS3 | + |
| Etang de Berre, France [4,14] | Σ16PAH = 1595–3359 | NaphS2 | + |
| Predicted SO42− Loss (mM) | Observed SO42− Loss (mM) | |
|---|---|---|
| Active Cultures | 3.0 | 3.0 (±1.2) |
| Sterile Cultures | 0 | 0 (±0) |
| C10H8 + 6 H2SO4 → 10 CO2 + 6 H2S + 4 H2O | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfson, S.J.; Porter, A.W.; Kerkhof, L.J.; McGuinness, L.M.; Prince, R.C.; Young, L.Y. Sulfate-Reducing Naphthalene Degraders Are Picky Eaters. Microorganisms 2018, 6, 59. https://doi.org/10.3390/microorganisms6030059
Wolfson SJ, Porter AW, Kerkhof LJ, McGuinness LM, Prince RC, Young LY. Sulfate-Reducing Naphthalene Degraders Are Picky Eaters. Microorganisms. 2018; 6(3):59. https://doi.org/10.3390/microorganisms6030059
Chicago/Turabian StyleWolfson, Sarah J., Abigail W. Porter, Lee J. Kerkhof, Lora M. McGuinness, Roger C. Prince, and Lily Y. Young. 2018. "Sulfate-Reducing Naphthalene Degraders Are Picky Eaters" Microorganisms 6, no. 3: 59. https://doi.org/10.3390/microorganisms6030059
APA StyleWolfson, S. J., Porter, A. W., Kerkhof, L. J., McGuinness, L. M., Prince, R. C., & Young, L. Y. (2018). Sulfate-Reducing Naphthalene Degraders Are Picky Eaters. Microorganisms, 6(3), 59. https://doi.org/10.3390/microorganisms6030059






