Transcriptome and Metabolome Analyses of Thitarodes xiaojinensis in Response to Ophiocordyceps sinensis Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fungi
2.2. Experimental Insects
2.3. Collection of Larvae Samples
2.4. Quantitative Analysis of TAGs
2.5. RNA Extraction, Library Preparation and RNA-Sequencing
2.6. Gene Expression Profiling and Data Analysis
2.7. Metabolite Detection
2.8. Differential Metabolite Analysis
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3. Results
3.1. The Host Body Weight and TAG Concentration Increased in the Fat Body after Ophiocordyceps sinensis Infection
3.2. Transcriptome Profiling
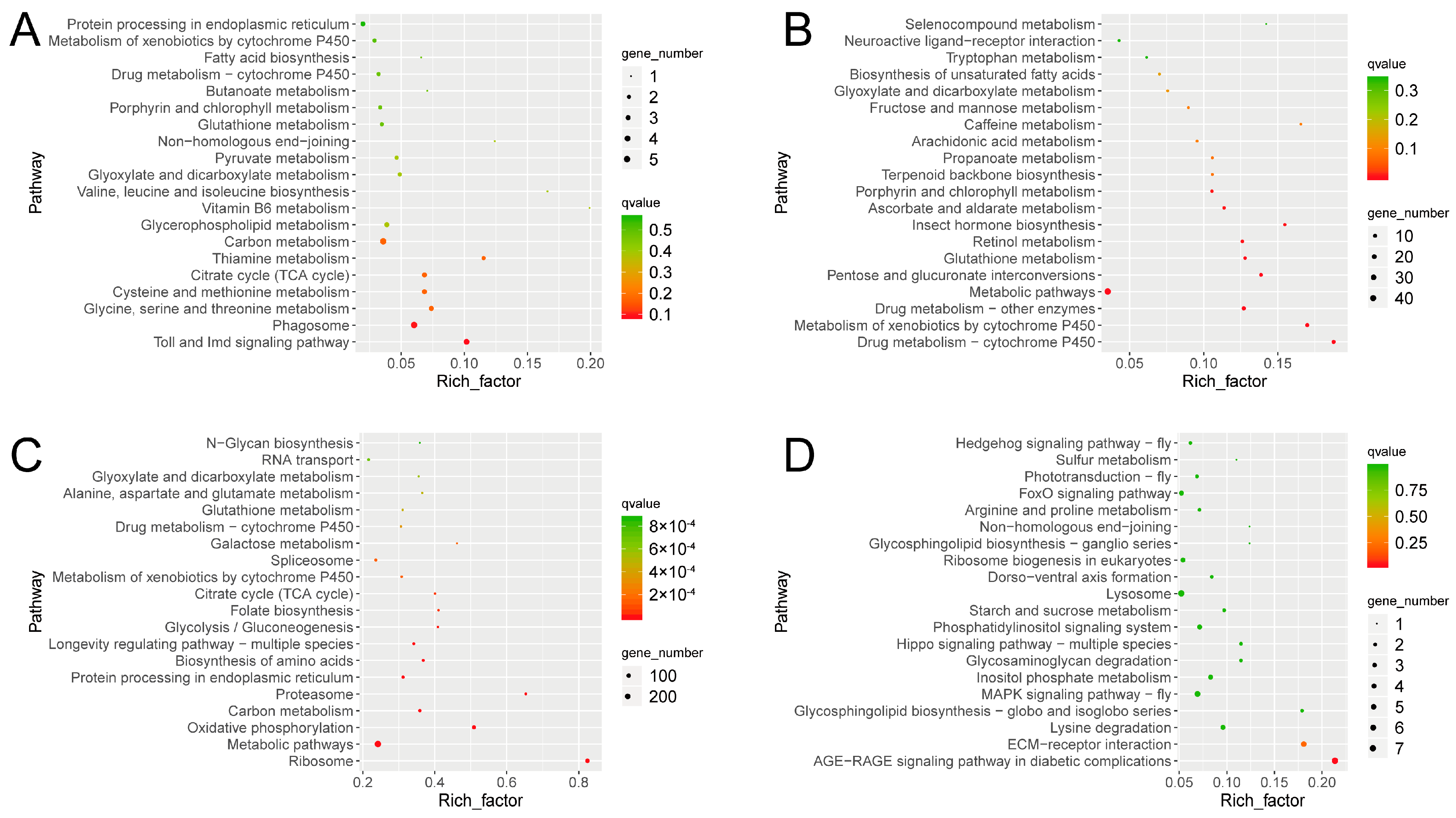
3.3. Gene Responses to Ophiocordyceps sinensis Infection
3.4. Metabolome Profiling
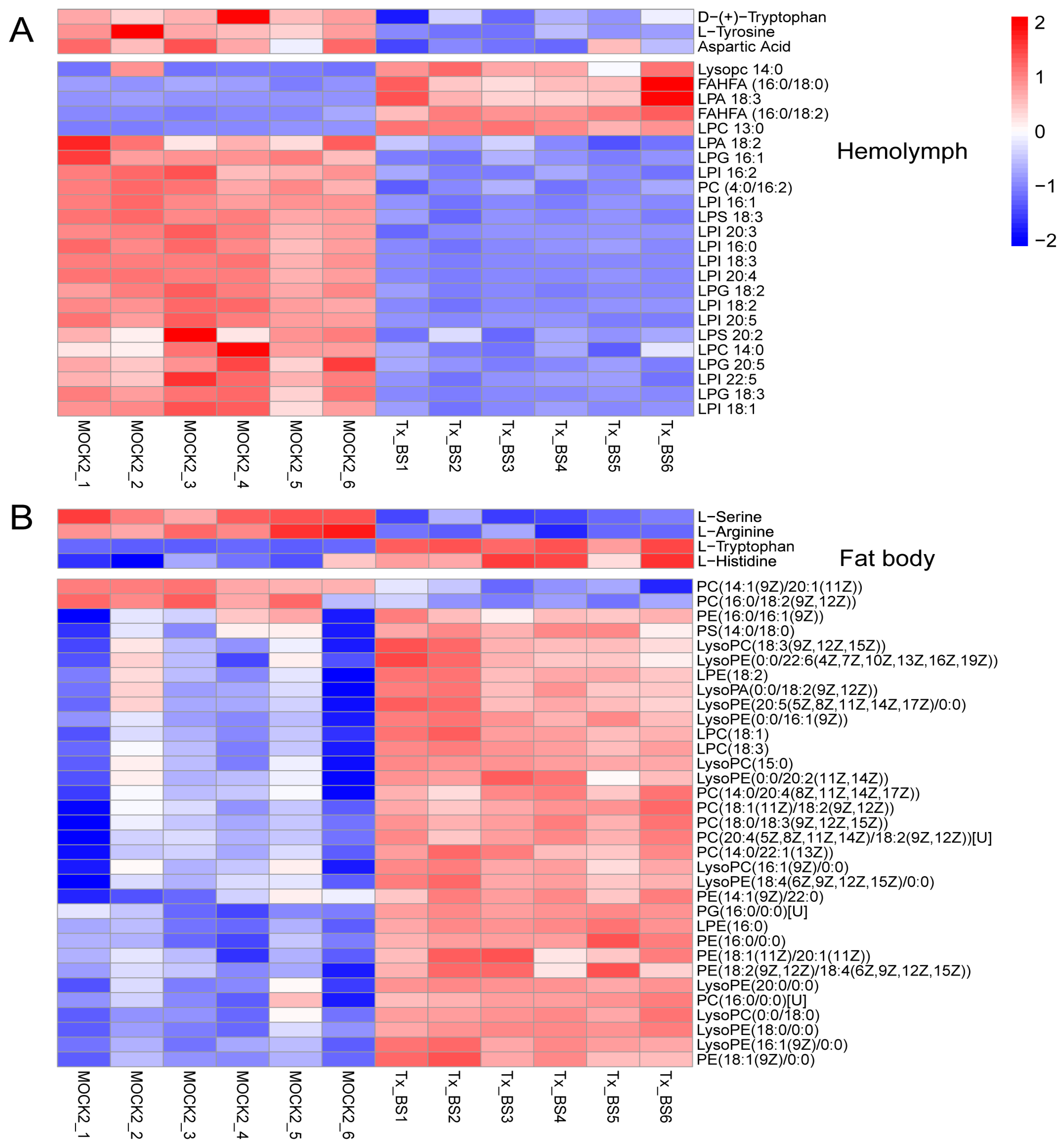
3.5. Thitarodes Xiaojinensis Metabolite Profiles Were Altered after Ophiocordyceps sinensis Infection
3.6. Thitarodes Xiaojinensis Lipid Metabolism Was Affected by Ophiocordyceps sinensis Infection
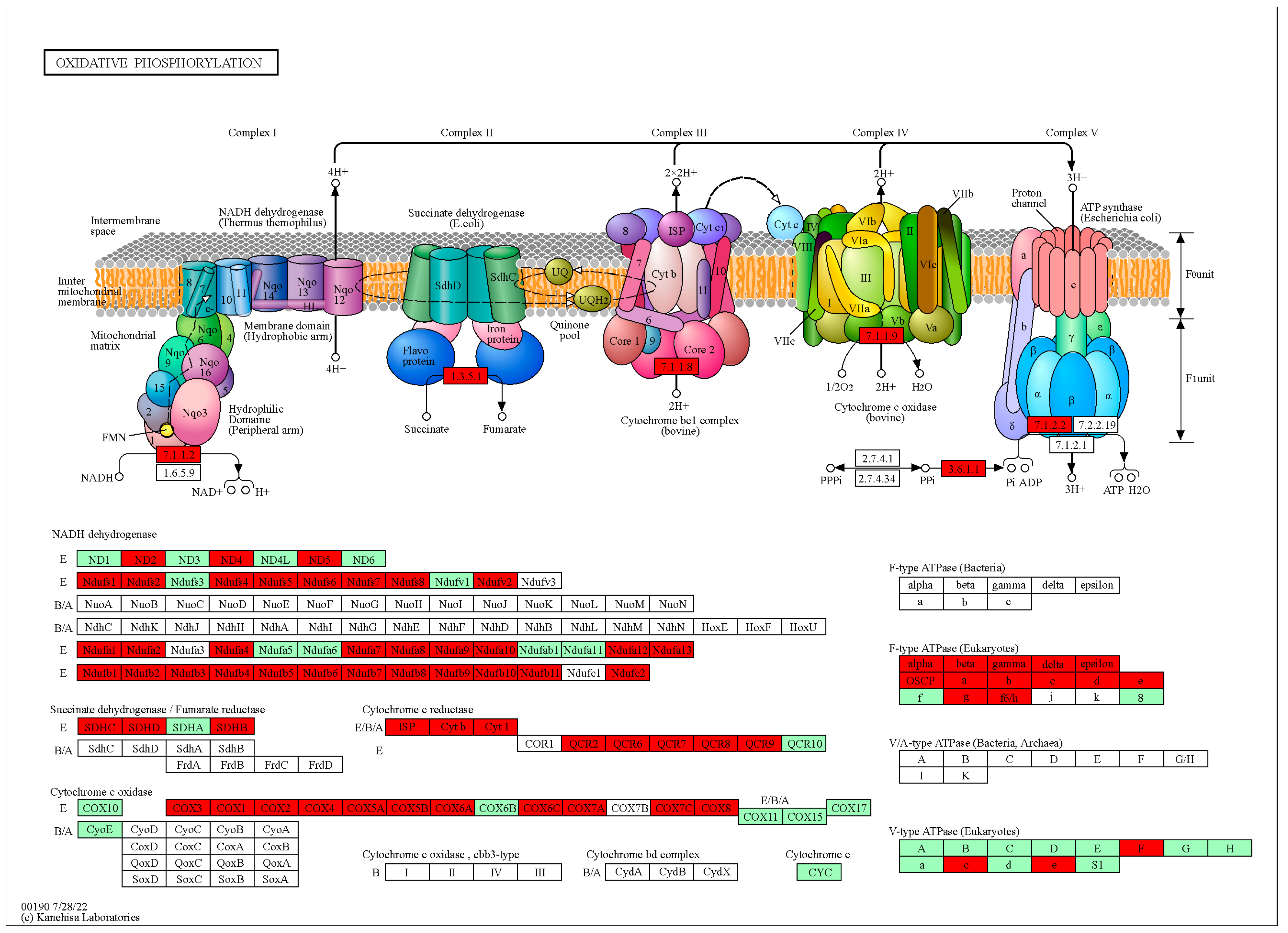
3.7. Thitarodes Xiaojinensis Oxidative Phosphorylation Pathway Was Affected by Ophiocordyceps sinensis Infection
3.8. The Immune Response Effectors Were Upregulated in Tx-BP but Barely Detected in Tx-BS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, G.; Gu, D.X.; Liu, X. A new species of Hepialus (Lepidoptera, Hepialidae) from China. Acta Zootaxonomica Sin. 2007, 32, 473–476. [Google Scholar]
- Zou, Z.; Liu, X.; Zhang, G. Revision of taxonomic system of the genus Hepialus (Lepidoptera, Hepialidae) currently adopted in China. J. Hunan Univ. Sci. Technol. 2010, 25, 114–120. [Google Scholar]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y. Host insect species of Ophiocordyceps sinensis: A review. Zookeys 2011, 127, 43–59. [Google Scholar]
- Liu, Y.; Wang, J.; Wang, W.; Zhang, H.; Zhang, X.; Han, C. The chemical constituents and pharmacological actions of Cordyceps sinensis. J. Evid.-Based Integr. Med. 2015, 2015, 575063. [Google Scholar]
- Holliday, J.; Cleaver, M. Medicinal value of the caterpillar fungi species of the genus Cordyceps (Fr.) Link (Ascomycetes). A Review. Int. J. Med. Mushrooms 2008, 10, 219–234. [Google Scholar] [CrossRef]
- Wu, P.; Qin, Q.; Zhang, J.; Zhang, H.; Li, X.; Wang, H.; Meng, Q. The invasion process of the entomopathogenic fungus Ophiocordyceps sinensis into the larvae of ghost moths (Thitarodes xiaojinensis) using a GFP-labeled strain. Front. Microbiol. 2022, 13, 974323. [Google Scholar] [CrossRef]
- Li, M.; Meng, Q.; Zhang, H.; Ni, R.; Zhou, G.; Zhao, Y.; Wu, P.; Shu, R.; Qin, Q.; Zhang, J. Vegetative development and host immune interaction of Ophiocordyceps sinensis within the hemocoel of the ghost moth larva, Thitarodes xiaojinensis. J. Invertebr. Pathol. 2020, 170, e107331. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, P.; Li, M.; Shu, R.; Zhou, G.; Zhang, J.; Zhang, H.; Jiang, H.; Qin, Q.; Zou, Z. Distinct responses of Thitarodes xiaojinensis beta-1,3-Glucan recognition protein-1 and immulectin-8 to Ophiocordyceps sinensis and Cordyceps militaris infection. J. Immun. 2021, 207, 200–209. [Google Scholar] [CrossRef]
- Ribeiro, C.; Brehélin, M. Insect haemocytes: What type of cell is that? J. Insect Physiol. 2006, 52, 417–429. [Google Scholar] [CrossRef]
- Wu, G.; Yi, Y. Haemocoel injection of PirA(1)B(1) to Galleria mellonella larvae leads to disruption of the haemocyte immune functions. Sci. Rep. 2016, 6, 34996. [Google Scholar] [CrossRef]
- Vilmos, P.; Kurucz, É. Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef]
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. BioControl. 2010, 55, 89–102. [Google Scholar] [CrossRef]
- Thomas, M.B.; Read, A.F. Can fungal biopesticides control malaria? Nat. Rev. Microbiol. 2007, 5, 377–383. [Google Scholar] [CrossRef]
- Xia, Y.; Clarkson, J.M.; Charnley, A.K. Acid phosphatases of Metarhizium anisopliae during infection of the tobacco hornworm Manduca sexta. Arch. Microbiol. 2001, 176, 427–434. [Google Scholar] [CrossRef]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae enzymes and toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, F.; Gao, Q.; Shang, Y.; Wang, C. Metabolomics reveals insect metabolic responses associated with fungal infection. Anal. Bioanal. Chem. 2015, 407, 4815–4821. [Google Scholar] [CrossRef]
- Meng, Q.; Yu, H.; Zhang, H.; Zhu, W.; Wang, M.; Zhang, J.; Zhou, G.; Li, X.; Qin, Q.; Hu, S.; et al. Transcriptomic insight into the immune defenses in the ghost moth, Hepialus xiaojinensis, during an Ophiocordyceps sinensis fungal infection. Insect Biochem. Mol. Biol. 2015, 64, 1–15. [Google Scholar] [CrossRef]
- Golebiowski, M.; Urbanek, A.; Pietrzak, A.; Naczk, A.M.; Bojke, A.; Tkaczuk, C.; Stepnowski, P. Effects of the entomopathogenic fungus Metarhizium flavoviride on the fat body lipid composition of Zophobas morio larvae (Coleoptera: Tenebrionidae). Sci. Nat. 2020, 107, 7. [Google Scholar] [CrossRef]
- Seyoum, E.; Bateman, R.P.; Charnley, A.K. The effect of Metarhizium anisopliae var acridum on haemolymph energy reserves and flight capability in the desert locust, Schistocerca gregaria. J. Appl. Entomol. 2002, 126, 119–124. [Google Scholar] [CrossRef]
- Allmann, S.; Mazet, M.; Ziebart, N.; Bouyssou, G.; Fouillen, L.; Dupuy, J.W.; Bonneu, M.; Moreau, P.; Bringaud, F.; Boshart, M. Triacylglycerol storage in lipid droplets in procyclic Trypanosoma brucei. PLoS ONE 2014, 9, e114628. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Daum, G. The life cycle of neutral lipids: Synthesis, storage and degradation. Cell. Mol. Life Sci. 2006, 63, 1355–1369. [Google Scholar] [CrossRef]
- Ogg, C.L.; Meinke, L.J.; Howard, R.W.; Stanley-Samuelson, D.W. Phospholipid and triacylglycerol fatty acid compositions of five species of Diabrotica (insecta: Coleoptera: Chrysomelidae). Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 105, 69–77. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Hollywood, K.; Brison, D.R.; Goodacre, R. Metabolomics: Current technologies and future trends. Proteomics 2006, 6, 4716–4723. [Google Scholar] [CrossRef]
- Hirai, M.Y.; Klein, M.; Fujikawa, Y.; Yano, M.; Goodenowe, D.B.; Yamazaki, Y.; Kanaya, S.; Nakamura, Y.; Kitayama, M.; Suzuki, H.; et al. Elucidation of gene-to-gene and metabolite-to-gene networks in arabidopsis by integration of metabolomics and transcriptomics. J. Biol. Chem. 2005, 280, 25590–25595. [Google Scholar] [CrossRef]
- Blow, F.; Douglas, A.E. The hemolymph microbiome of insects. J. Insect Physiol. 2019, 115, 33–39. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Pass, G. The insect circulatory system: Structure, function, and evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Li, M.; Meng, Q.; Zhang, H.; Shu, R.; Zhao, Y.; Wu, P.; Li, X.; Zhou, G.; Qin, Q.; Zhang, J. Changes in transcriptomic and metabolomic profiles of morphotypes of Ophiocordyceps sinensis within the hemocoel of its host larvae, Thitarodes xiaojinensis. BMC Genomics 2020, 21, 789. [Google Scholar] [CrossRef]
- David, M.; Hercules, T.L.S. Chemiluminescent determination of serum glycerol and triglycerides. Anal. Chem. 1978, 50, 22–25. [Google Scholar]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B: Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Vatanparast, M.; Ahmed, S.; Sajjadian, S.M.; Kim, Y. A prophylactic role of a secretory PLA2 of Spodoptera exigua against entomopathogens. Dev. Comp. Immunol. 2019, 95, 108–117. [Google Scholar] [CrossRef]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid receptors: Signaling and biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar] [CrossRef]
- Atella, G.C.; Shahabuddin, M. Differential partitioning of maternal fatty acid and phospholipid in neonate mosquito larvae. J. Exp. Biol. 2002, 205, 3623–3630. [Google Scholar] [CrossRef]
- Piotto, S.; Trapani, A.; Bianchino, E.; Ibarguren, M.; Lopez, D.J.; Busquets, X.; Concilio, S. The effect of hydroxylated fatty acid-containing phospholipids in the remodeling of lipid membranes. Biochim. Biophys. Acta, Biomembr. 2014, 1838, 1509–1517. [Google Scholar] [CrossRef]
- Wilson, D.F.; Vinogradov, S.A. Mitochondrial cytochrome c oxidase: Mechanism of action and role in regulating oxidative phosphorylation. J. Appl. Physiol. 2014, 117, 1431–1439. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Z.; Wang, J.M.; Xing, L.S.; Xiong, G.H.; Zou, Z. CTL14, a recognition receptor induced in late stage larvae, modulates anti-fungal immunity in cotton bollworm Helicoverpa armigera. Dev. Comp. Immunol. 2018, 84, 142–152. [Google Scholar] [CrossRef]
- Xiong, G.; Xing, L.; Lin, Z.; Saha, T.; Wang, C.; Jiang, H.; Zou, Z. High throughput profiling of the cotton bollworm Helicoverpa armigera immunotranscriptome during the fungal and bacterial infections. BMC Genomics 2015, 16, 321. [Google Scholar] [CrossRef]
- Huang, C.; Xu, R.; Liégeois, S.; Chen, D.; Li, Z.; Ferrandon, D. Differential requirements for mediator complex subunits in Drosophila melanogaster host defense against fungal and bacterial pathogens. Front. Immunol. 2020, 11, 478958. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Y.; Shen, D.; Hong, F.; Wang, G.; An, C. Serine proteases SP1 and SP13 mediate the melanization response of Asian corn borer, Ostrinia furnacalis, against entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol. 2015, 128, 64–72. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, C.; Lu, Z.-Q.; Kanost, M.R. β-1,3-Glucan recognition protein-2 (βGRP-2) from Manduca sexta: An acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem. Mol. Biol. 2004, 34, 89–100. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar]
- Williams, B.A. Unique physiology of host-parasite interactions in microsporidia infections. Cell. Microbiol. 2009, 11, 1551–1560. [Google Scholar] [CrossRef]
- Turkish, A.R.; Sturley, S.L. The genetics of neutral lipid biosynthesis: An evolutionary perspective. Am. J. Physiol. 2009, 297, E19–E27. [Google Scholar] [CrossRef]



| Gene Name | TPM | Log2 (Fold Change) | ||||
|---|---|---|---|---|---|---|
| MOCK1 | Tx-BP | MOCK2 | Tx-BS | Tx-BP | Tx-BS | |
| DEF | 729.75 | 18,663.43 | 1807.60 | 1097.30 | 4.68 | −0.72 |
| Att3 | 86.30 | 92.32 | 577.28 | 157.19 | 0.09 | −1.88 |
| Att4 | 521.38 | 4824.39 | 894.29 | 332.45 | 3.21 | −1.43 |
| Att5 | 10.40 | 27.39 | 10.82 | 9.40 | 1.40 | −0.20 |
| Cec1 | 2.63 | 4.30 | 8.37 | 0.81 | 0.71 | −3.37 |
| Cec2 | 4.22 | 69.44 | 23.61 | 7.38 | 4.04 | −1.68 |
| Cec3 | 0.95 | 8.30 | 1.07 | 0.33 | 3.13 | −1.70 |
| Cec5 | 5.36 | 65.61 | 19.32 | 8.73 | 3.61 | −1.15 |
| Gloverin | 174.37 | 5204.20 | 475.21 | 609.07 | 4.90 | 0.36 |
| Lys1 | 11,787.02 | 22,424.07 | 11,140.33 | 20,480.92 | 0.93 | 0.88 |
| Lys2 | 79.76 | 418.33 | 365.93 | 139.23 | 2.39 | −1.39 |
| Lys3 | 5.13 | 294.97 | 11.15 | 5.06 | 5.85 | −1.14 |
| Lys5 | 57.02 | 119.74 | 35.47 | 140.93 | 1.07 | 1.99 |
| Lys6 | 24.71 | 7.46 | 14.08 | 55.34 | −1.73 | 1.98 |
| LLP1 | 378.07 | 550.47 | 878.01 | 392.43 | 0.54 | −1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, J.; Qin, Q.; Zhang, H.; Li, X.; Wang, H.; Meng, Q. Transcriptome and Metabolome Analyses of Thitarodes xiaojinensis in Response to Ophiocordyceps sinensis Infection. Microorganisms 2023, 11, 2361. https://doi.org/10.3390/microorganisms11092361
Li M, Zhang J, Qin Q, Zhang H, Li X, Wang H, Meng Q. Transcriptome and Metabolome Analyses of Thitarodes xiaojinensis in Response to Ophiocordyceps sinensis Infection. Microorganisms. 2023; 11(9):2361. https://doi.org/10.3390/microorganisms11092361
Chicago/Turabian StyleLi, Miaomiao, Jihong Zhang, Qilian Qin, Huan Zhang, Xuan Li, Hongtuo Wang, and Qian Meng. 2023. "Transcriptome and Metabolome Analyses of Thitarodes xiaojinensis in Response to Ophiocordyceps sinensis Infection" Microorganisms 11, no. 9: 2361. https://doi.org/10.3390/microorganisms11092361





