Recovery of Potential Starter Cultures and Probiotics from Fermented Sorghum (Ting) Slurries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling, Isolation and Identification of LABs
2.2. Molecular Characterisation of Selected LABs
2.3. 16S rRNA and pheS Sequencing and Phylogenetic Analysis
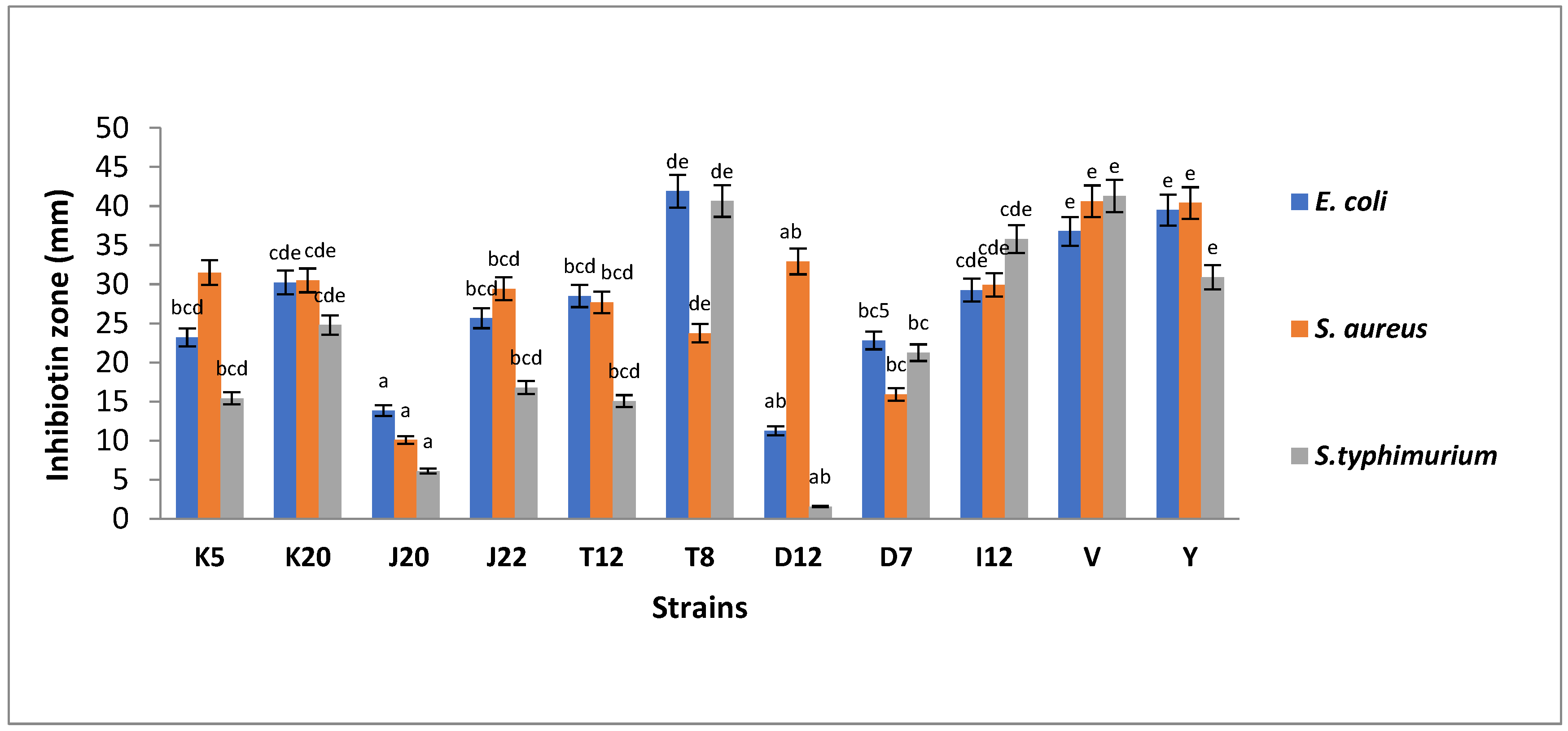
2.4. Antimicrobial Activity against Clinically Important Food Pathogens and Antibiotics
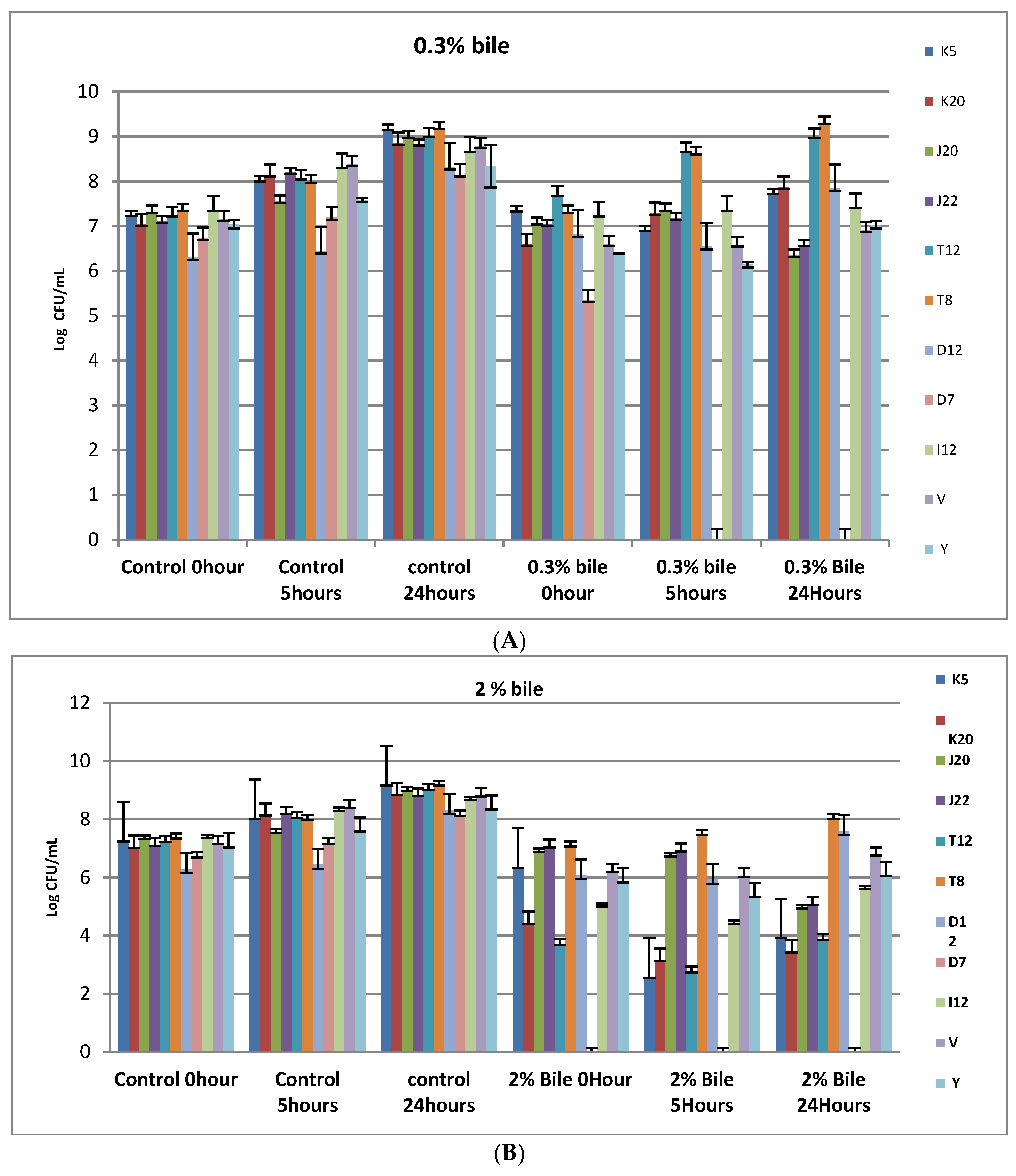
2.5. Bile Tolerance Test
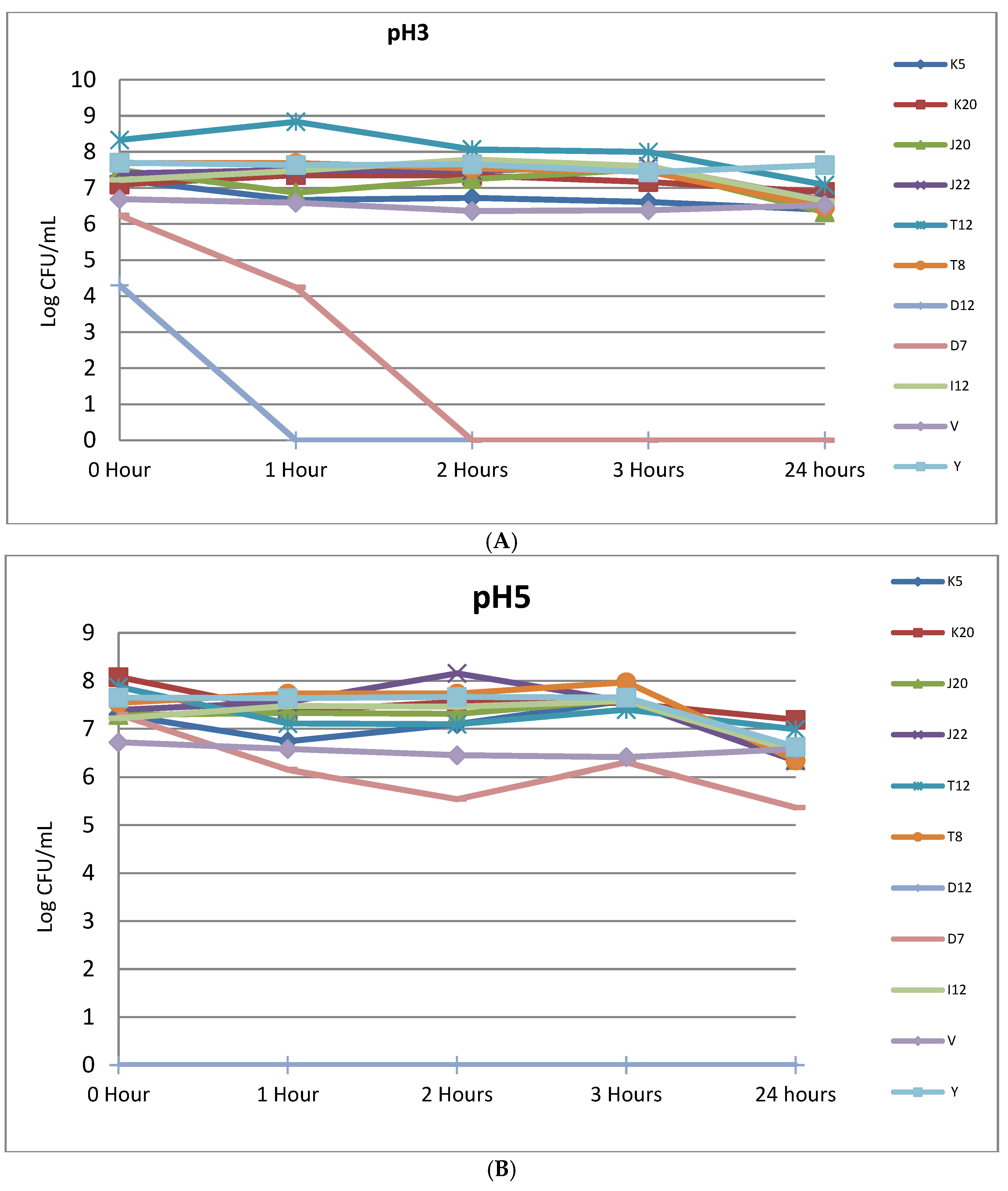
2.6. Tolerance to pH Fluctuation
2.7. Statistical Analysis
3. Results
3.1. Microbiological and Phenotypic Characteristics of Isolated LABs
3.2. Antibacterial Activity of the Selected LABs against Pathogenic Microorganisms
3.3. Antibiotic Susceptibility Profiles of Isolated LABs
3.4. Bile Tolerance Patterns of Isolated LABs
3.5. Acid Tolerance Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franz, C.M.A.P.; Huch, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African Fermented Foods and Probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyanzi, R.; Jooste, P.J. Cereal-Based Functional Foods. Probiotics 2012, 161–197. [Google Scholar] [CrossRef] [Green Version]
- Ashaolu, T.J. Safety and Quality of Bacterially Fermented Functional Foods and Beverages: A Mini Review. Food Qual. Saf. 2020, 4, 123–127. [Google Scholar] [CrossRef]
- Nyanzi, R. Identification and Properties of Potential Probiotic Bacteria for Application in Mageu; Tshwane University of Technology: Pretoria West, Pretoria, 2013. [Google Scholar]
- Ogola, J.R.O.; Kirina, K.O.O.T.K.; Droppers, B. Assessment of Experts’ Opinion on Irish Potato Farmers Perceptions about Climate Change and the Use of Climate Smart Agriculture Adaptation Strategies in Kenya. J. Agric. Econ. 2021, 7, 957–967. [Google Scholar]
- Sekwati-Monang, B.; Gänzle, M.G. Microbiological and Chemical Characterisation of Ting, a Sorghum-Based Sourdough Product from Botswana. Int. J. Food Microbiol. 2011, 150, 115–121. [Google Scholar] [CrossRef]
- Adebo, O.A. African Sorghum-Based Fermented Foods: Past, Current and Future Prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef]
- Mears, L.; Stocks, S.M.; Sin, G.; Gernaey, K.V. A Review of Control Strategies for Manipulating the Feed Rate in Fed-Batch Fermentation Processes. J. Biotechnol. 2017, 245, 34–46. [Google Scholar] [CrossRef]
- Wehrs, M.; de Beaumont-Felt, A.; Goranov, A.; Harrigan, P.; de Kok, S.; Lieder, S.; Vallandingham, J.; Tyner, K. You Get What You Screen for: On the Value of Fermentation Characterization in High-Throughput Strain Improvements in Industrial Settings. J. Ind. Microbiol. Biotechnol. Off. J. Soc. Ind. Microbiol. Biotechnol. 2020, 47, 913–927. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Badejo, A.A. Probiotic Potentials of Cereal-Based Beverages. Crit. Rev. Food Sci. Nutr. 2017, 57, 790–804. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus Plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [Green Version]
- Aka, S.; Dridi, B.; Bolotin, A.; Yapo, E.A.; Koussemon-Camara, M.; Bonfoh, B.; Renault, P. Characterization of Lactic Acid Bacteria Isolated from a Traditional Ivoirian Beer Process to Develop Starter Cultures for Safe Sorghum-Based Beverages. Int. J. Food Microbiol. 2020, 322, 108547. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F., III. Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Ekwanzala, M.D.; Budeli, P.; Dewar, J.B.; Kamika, I.; Momba, M.N.B. Draft Genome Sequences of Novel Sequence Type 3559 Carbapenem-Resistant Klebsiella Pneumoniae Isolates Recovered from the Environment. Microbiol. Resour. Announc. 2019, 8, e00518–e00519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the Probiotic Potential of Lactic Acid Bacteria from African Traditional Fermented Foods and Beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef] [Green Version]
- Sarao, L.K.; Arora, M. Probiotics, Prebiotics, and Microencapsulation: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Chauhan, A.; Singh, R. Probiotics in Aquaculture: A Promising Emerging Alternative Approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial Effects of Lactic Acid Bacteria on Human Beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef]
- Archer, A.C.; Halami, P.M. Fermented Foods, Microbiota and Human Health. In Mining of Microbial Wealth and MetaGenomics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 301–331. [Google Scholar]
- Madoroba, E.; Steenkamp, E.T.; Theron, J.; Scheirlinck, I.; Cloete, T.E.; Huys, G. Diversity and Dynamics of Bacterial Populations during Spontaneous Sorghum Fermentations Used to Produce Ting, a South African Food. Syst. Appl. Microbiol. 2011, 34, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Nyanzi, R.; Jooste, P.J.; Cameron, M.; Witthuhn, C. Comparison of RpoA and PheS Gene Sequencing to 16S RRNA Gene Sequencing in Identification and Phylogenetic Analysis of LAB from Probiotic Food Products and Supplements. Food Biotechnol. 2013, 27, 303–327. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional Foods and Nondairy Probiotic Food Development: Trends, Concepts, and Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, E.; Van Trijp, J.C.M.; Van Den Borne, J.; Zondervan, C. Successful Development of Satiety Enhancing Food Products: Towards a Multidisciplinary Agenda of Research Challenges. Crit. Rev. Food Sci. Nutr. 2012, 52, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Angmo, K.; Kumari, A.; Bhalla, T.C. Probiotic Characterization of Lactic Acid Bacteria Isolated from Fermented Foods and Beverage of Ladakh. LWT-Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Von Wright, A.; Axelsson, L. Lactic Acid Bacteria: An Introduction; CRC Press: Boca Raton, FL, USA, 2019; ISBN 0429057466. [Google Scholar]
- Khalid, K. An Overview of Lactic Acid Bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Okoronkwo, C.U. Isolation and Characterization of Lactic Acid Bacteria Involved in the Fermentation of Millet and Sorghum Sold in Nkwo-Achara Market, Abia State. IOSR J. Environ. Sci. Food Technol 2014, 8, 42–45. [Google Scholar]
- Catherine, A.-O.B.; Okechi, K.I. Evaluation of OGI (CORN CARAMEL) from Maize and Sorghum for Isolation and Characterisation of Lactic Acid Bacteria (LAB). Biochem. Mol. Biol. 2019, 4, 28. [Google Scholar] [CrossRef]
- Todorov, S.D.; de Melo Franco, B.D.G.; Tagg, J.R. Bacteriocins of Gram-Positive Bacteria Having Activity Spectra Extending beyond Closely-Related Species. Benef. Microbes 2019, 10, 315–328. [Google Scholar] [CrossRef]
- Adimpong, D.B.; Nielsen, D.S.; Sørensen, K.I.; Derkx, P.M.F.; Jespersen, L. Genotypic Characterization and Safety Assessment of Lactic Acid Bacteria from Indigenous African Fermented Food Products. BMC Microbiol. 2012, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Naser, B.; Bodinet, C.; Tegtmeier, M.; Lindequist, U. Thuja Occidentalis (Arbor Vitae): A Review of Its Pharmaceutical, Pharmacological and Clinical Properties. Evid. Based Complement. Altern. Med. 2005, 2, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Moodley, T.; Amonsou, E.O.; Kumar, S. Nutritional Quality and Acceptability of Buddleja Saligna Herbal Tea. J. Food Sci. Technol. 2015, 52, 7519–7524. [Google Scholar] [CrossRef]
- Lavefve, L.; Marasini, D.; Carbonero, F. Microbial Ecology of Fermented Vegetables and Non-Alcoholic Drinks and Current Knowledge on Their Impact on Human Health. Adv. Food Nutr. Res. 2019, 87, 147–185. [Google Scholar] [PubMed]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N. Antimicrobial Activity and Antibiotic Susceptibility of Lactobacillus and Bifidobacterium Spp. Intended for Use as Starter and Probiotic Cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, F.; Wu, T.; Tang, H.; Zhao, Z. The Acid, Bile Tolerance and Antimicrobial Property of Lactobacillus Acidophilus NIT. Food Control 2009, 20, 598–602. [Google Scholar] [CrossRef]
- Santos, A.C.D.M.; Santos, F.F.; Silva, R.M.; Gomes, T.A.T. Diversity of Hybrid-and Hetero-Pathogenic Escherichia Coli and Their Potential Implication in More Severe Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic Potential and Amylolytic Properties of Lactic Acid Bacteria Isolated from Chinese Fermented Cereal Foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chen, C.-H.; Lai, L.-S. Effect of Tapioca Starch/Decolorized Hsian-Tsao Leaf Gum-Based Active Coatings on the Qualities of Fresh-Cut Apples. Food Bioprocess Technol. 2013, 6, 2059–2069. [Google Scholar] [CrossRef]
- Yuksekdag, Z.N.; Aslim, B. Assessment of Potential Probiotic and Starter Properties of Pediococcus Spp. Isolated from Turkish-Type Fermented Sausages (Sucuk). J. Microbiol. Biotechnol. 2010, 20, 161–168. [Google Scholar] [CrossRef]
- Gao, G.; Gao, Q.; Bao, M.; Xu, J.; Li, X. Nitrogen Availability Modulates the Effects of Ocean Acidification on Biomass Yield and Food Quality of a Marine Crop Pyropia Yezoensis. Food Chem. 2019, 271, 623–629. [Google Scholar] [CrossRef]
- Quinto, E.J.; Jiménez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbés, T. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014, 5, 1765. [Google Scholar] [CrossRef] [Green Version]
- Dicks, L.M.T.; Dreyer, L.; Smith, C.; Van Staden, A.D. A Review: The Fate of Bacteriocins in the Human Gastro-Intestinal Tract: Do They Cross the Gut–Blood Barrier? Front. Microbiol. 2018, 9, 2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive Approaches for Assessing the Safety of Probiotic Bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Basavaraju, B.; Jamil, K. Identification and Characterization of Probiotics from New Sources. Int. J. Sci. Res. 2014, 3, 837–841. [Google Scholar]
- Samedi, L.; Charles, A.L. Isolation and Characterization of Potential Probiotic Lactobacilli from Leaves of Food Plants for Possible Additives in Pellet Feeding. Ann. Agric. Sci. 2019, 64, 55–62. [Google Scholar] [CrossRef]
- Jones, M. Feast: Why Humans Share Food; OUP: Oxford, UK, 2008; ISBN 0191623008. [Google Scholar]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile Resistance Mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.A.; Cronin, A.; Okotto-Okotto, J.; Yang, H.; Pedley, S.; Gundry, S.W. A Spatial Analysis of Pit Latrine Density and Groundwater Source Contamination. Environ. Monit. Assess. 2013, 185, 4261–4272. [Google Scholar] [CrossRef]
- Park, S.C.; Hwang, M.H.; Kim, Y.H.; Kim, J.C.; Song, J.C.; Lee, K.W.; Jeong, K.S.; Rhee, M.H.; Kim, K.S.; Kim, T.W. Comparison of PH and Bile Resistance of Lactobacillus Acidophilus Strains Isolated from Rat, Pig, Chicken, and Human Sources. World J. Microbiol. Biotechnol. 2006, 22, 35–37. [Google Scholar] [CrossRef]
- Schoster, A.; Kokotovic, B.; Permin, A.; Pedersen, P.D.; Dal Bello, F.; Guardabassi, L. Inávitro Inhibition of Clostridium Difficile and Clostridium Perfringens by Commercial Probiotic Strains. Anaerobe 2013, 20, 36–41. [Google Scholar] [CrossRef]
- Sahadeva, R.P.K.; Leong, S.F.; Chua, K.H.; Tan, C.H.; Chan, H.Y.; Tong, E.V.; Wong, S.Y.W.; Chan, H.K. Survival of Commercial Probiotic Strains to PH and Bile. Int. Food Res. J. 2011, 18, 1515–1522. [Google Scholar]
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the Acid Tolerance and the Technological Robustness of Probiotic Cultures for Fortification in Fruit Juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 279–284. [Google Scholar] [CrossRef]
| Name | Nucleotide Sequence | Fragment Size | References |
|---|---|---|---|
| Phes 21-F | 5′-CAYCCNGCHCGYGAYATGC-3′ | 411 bp | [23] |
| Phes-23-R | 5′-GGRTGRACCATVCCNGCHCC-3′ | ||
| 16S rRNA 27F 16S rRNA 1492R | 5′-AGAGTTTGATCMTGGCTCAG-3′ 5′-GGTTACCTTGTTACGACTT-3′ | 1466 bp | [23] |
| Scheme | No of Isolates | Isolate Codes | Morphology | Grams Reaction | Catalase Reaction | Oxidase Reaction |
|---|---|---|---|---|---|---|
| D—Coarse sorghum from North of Pretoria | 11 | D5, D13, D16, D18, D7, D19, D2, D3, D11, D12, D20 | Rods | + | − | − |
| K—Fine sorghum from Klipgat | 17 | K5, K20, K19, K18, K11, K10, K9, K8, K3 K1, K2, K17, K16, K12, K7, K6, K4 | Rods | + | − | − |
| I—coarse sorghum from Soshanguve | 15 | I6, I5, I7, I10, I21, I17, I16, I13, I1, I2, I15, I12, I11, I19, I20 | Rods | + | − | − |
| J—Coarse White sorghum from Pretoria | 9 | J21, J6, J14, J15, J16, J19, J7, J22, J20 | Rods | + | − | − |
| T—Fine Brown sorghum from Tembisa | 8 | T2, T5, T10, T12, T20 T6, T8, T16 | Rods | + | − | − |
| Strains | Initial Concentration | 1 h | 2 h | 3 h | 24 h |
|---|---|---|---|---|---|
| K5 | 7.06 ± 0.19 a | 0 | 0 | 0 | 0 |
| K20 | 7.13 ± 0.03 a | 4.97 ± 0.09 a | 0 | 0 | 0 |
| J20 | 1.72 ± 0.13 a | 0 | 0 | 0 | 0 |
| J22 | 7.14 ± 0.04 a | 0 | 0 | 0 | 0 |
| T12 | 7.84 ± 0.14 a | 0 | 0 | 0 | 0 |
| T8 | 7.54 ± 0.09 a | 0 | 0 | 0 | 0 |
| D12 | 6.3 ± 0.05 a | 0 | 0 | 0 | 0 |
| D7 | 6.8 ± 0.014 a | 0 | 0 | 0 | 0 |
| I12 | 7.3 ± 0.09 a | 5.97 ± 0.24 a | 0 | 0 | 0 |
| V | 6.21 ± 0.12 a | 0 | 0 | 0 | 0 |
| Y | 7.9 ± 0.16 a | 3.65 ± 0.38 a | 3.81 ± 0.15 a | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapoo, S.M.; Budeli, P.; Thaoge, M.L. Recovery of Potential Starter Cultures and Probiotics from Fermented Sorghum (Ting) Slurries. Microorganisms 2023, 11, 715. https://doi.org/10.3390/microorganisms11030715
Rapoo SM, Budeli P, Thaoge ML. Recovery of Potential Starter Cultures and Probiotics from Fermented Sorghum (Ting) Slurries. Microorganisms. 2023; 11(3):715. https://doi.org/10.3390/microorganisms11030715
Chicago/Turabian StyleRapoo, Seth Molamu, Phumudzo Budeli, and Mathoto Lydia Thaoge. 2023. "Recovery of Potential Starter Cultures and Probiotics from Fermented Sorghum (Ting) Slurries" Microorganisms 11, no. 3: 715. https://doi.org/10.3390/microorganisms11030715






