Detection of Old and New World Relapsing Fever Borreliae in Ornithodoros Ticks Collected from Warthog Burrows in Zambia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
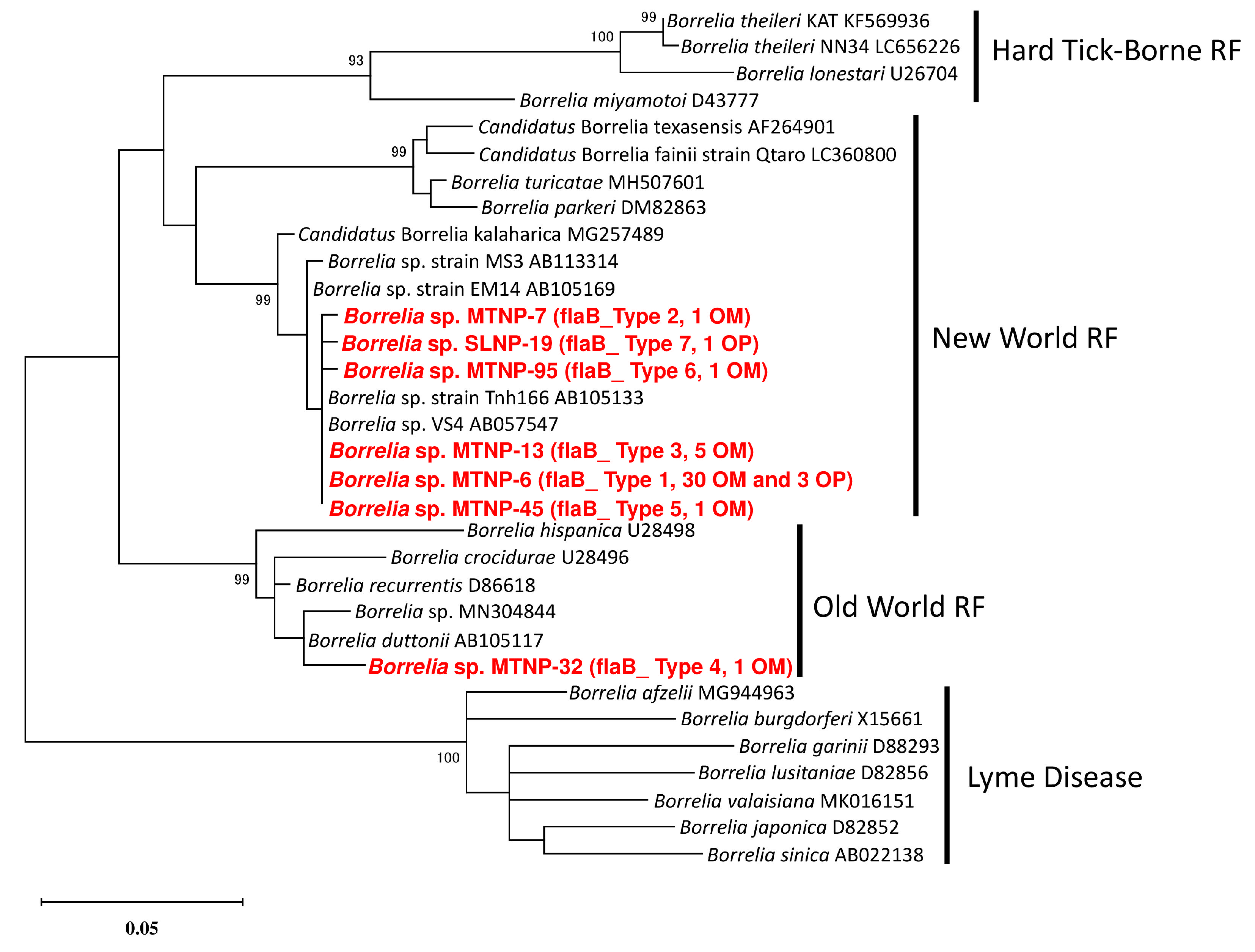
3.1. Screening of Borrelia with flaB Nested-PCR
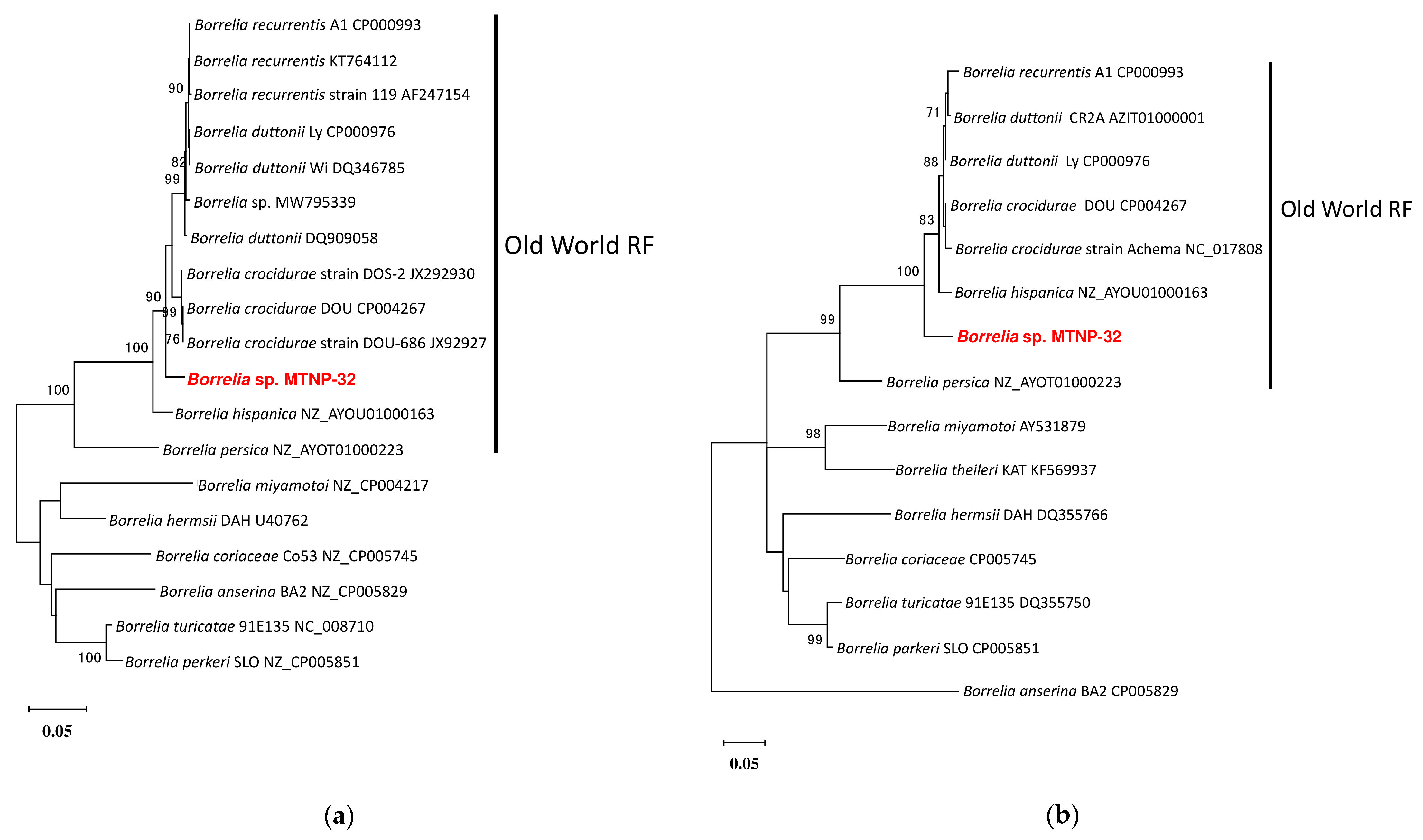
3.2. Analysis of 16S Ribosomal DNA
3.3. Analysis of glpQ and hpt Genes
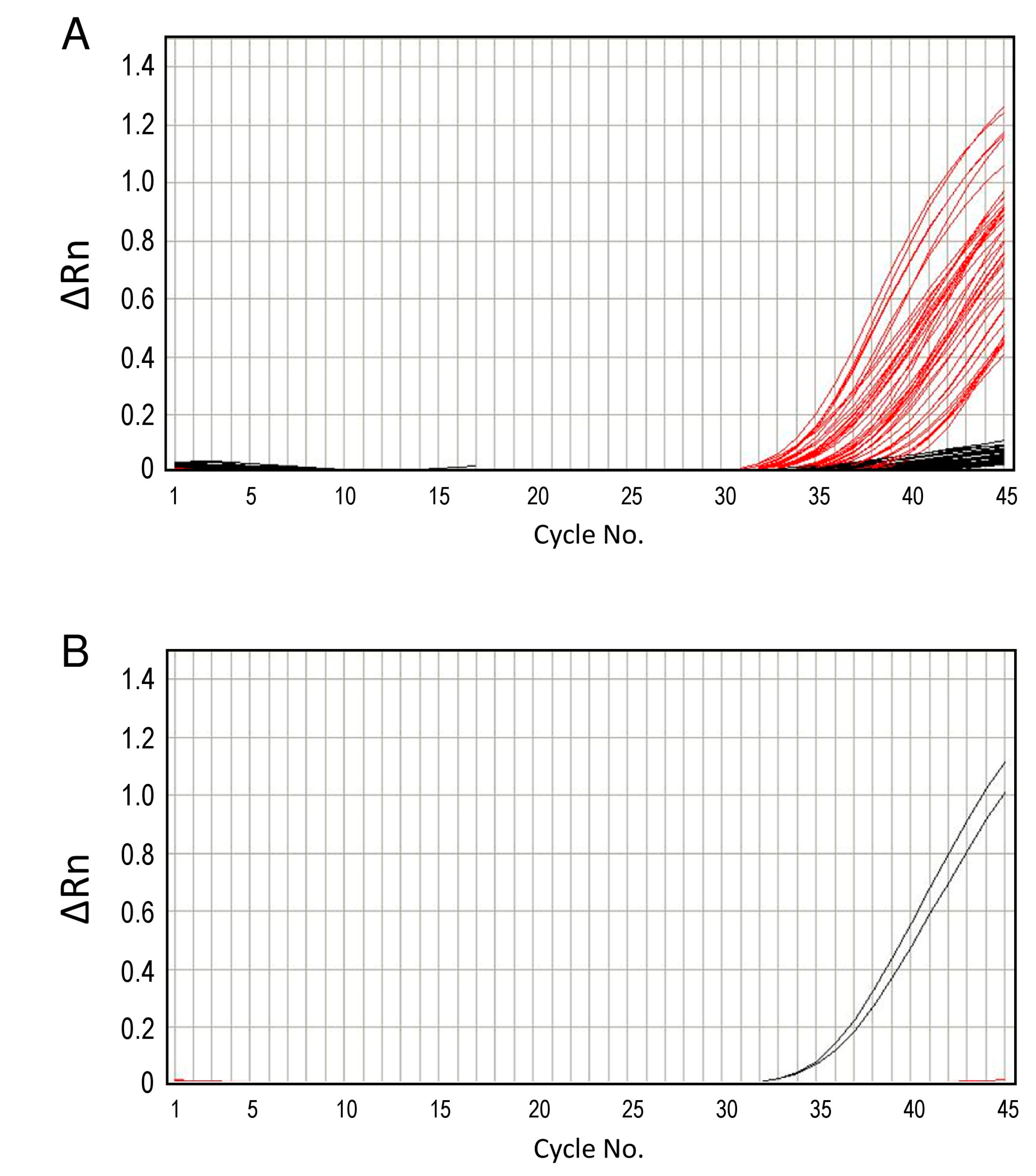
3.4. Real-Time PCR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margos, G.; Fingerle, V.; Cutler, S.; Gofton, A.; Stevenson, B.; Estrada-Peña, A. Controversies in bacterial taxonomy: The example of the genus Borrelia. Ticks Tick Borne Dis. 2020, 11, 101335. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Goka, K.; Une, Y.; Shimada, Y.; Fujita, H.; Shiino, T.; Watanabe, H.; Kawabata, H. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ. Microbiol. 2010, 12, 134–146. [Google Scholar] [CrossRef]
- Takano, A.; Fujita, H.; Kadosaka, T.; Konnai, S.; Tajima, T.; Watanabe, H.; Ohnishi, M.; Kawabata, H. Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with Lyme disease and relapsing fever Borrelia spp. Environ. Microbiol. Rep. 2011, 3, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.-M.; Gillett, A.; Ryan, U.; Irwin, P.; Oskam, C. Molecular characterization of ‘Candidatus Borrelia tachyglossi’ (family Spirochaetaceae) in echidna ticks, Bothriocroton concolor. Int. J. Syst. Evol. Microbiol. 2017, 67, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, A.G. Relapsing fever. In Tick-Borne Diseases of Humans; Jesse, L., Goodman, D.T.D., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 268–291. [Google Scholar]
- Trevisan, G.; Cinco, M.; Trevisini, S.; Di Meo, N.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 2: Borrelia Relapsing Fever Group and Unclassified Borrelia. Biology 2021, 10, 1117. [Google Scholar] [CrossRef]
- Schwan, T.G.; Anderson, J.M.; Lopez, J.E.; Fischer, R.J.; Raffel, S.J.; McCoy, B.N.; Safronetz, D.; Sogoba, N.; Maïga, O.; Traoré, S.F. Endemic Foci of the Tick-Borne Relapsing Fever Spirochete Borrelia crocidurae in Mali, West Africa, and the Potential for Human Infection. PLoS Neg. Trop. Dis. 2012, 6, e1924. [Google Scholar] [CrossRef]
- Mediannikov, O.; Socolovschi, C.; Bassene, H.; Diatta, G.; Ratmanov, P.; Fenollar, F.; Sokhna, C.; Raoult, D. Borrelia crocidurae Infection in Acutely Febrile Patients, Senegal. Emerg. Infect. Dis. 2014, 20, 1335–1338. [Google Scholar] [CrossRef]
- Sarih, M.H.; Garnier, M.; Boudebouch, N.; Bouattour, A.; Rihani, A.; Hassar, M.; Gern, L.; Postic, D.; Cornet, M. Borrelia hispanica Relapsing Fever, Morocco. Emerg. Infect. Dis. 2009, 15, 1626–1629. [Google Scholar] [CrossRef]
- Cutler, S.J.; Bonilla, E.M.; Singh, R.J. Population Structure of East African Relapsing Fever Borrelia spp. Emerg. Infect. Dis. 2010, 16, 1076–1080. [Google Scholar] [CrossRef]
- Qiu, Y.; Nakao, R.; Hang’ombe, B.M.; Sato, K.; Kajihara, M.; Kanchela, S.; Changula, K.; Eto, Y.; Ndebe, J.; Sasaki, M.; et al. Human Borreliosis Caused by a New World Relapsing Fever Borrelia-like Organism in the Old World. Clin. Infect. Dis. 2019, 69, 107–112. [Google Scholar] [CrossRef]
- Qiu, Y.; Simuunza, M.; Kajihara, M.; Chambaro, H.; Harima, H.; Eto, Y.; Simulundu, E.; Squarre, D.; Torii, S.; Takada, A.; et al. Screening of tick-borne pathogens in argasid ticks in Zambia: Expansion of the geographic distribution of Rickettsia lusitaniae and Rickettsia hoogstraalii and detection of putative novel Anaplasma species. Ticks Tick Borne Dis. 2021, 12, 101720. [Google Scholar] [CrossRef]
- Dupont, H.T.; La Scola, B.; Williams, R.; Raoult, D. A focus of tick-borne relapsing fever in southern Zaire. Clin. Infect. Dis. 1997, 25, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kahlig, P.; Paris, D.H.; Neumayr, A. Louse-borne relapsing fever—A systematic review and analysis of the literature: Part 1—Epidemiology and diagnostic aspects. PLoS Negl. Trop. Dis. 2021, 15, e0008564. [Google Scholar] [CrossRef] [PubMed]
- Nordstrand, A.; Bunikis, I.; Larsson, C.; Tsogbe, K.; Schwan, T.G.; Nilsson, M.; Bergström, S. Tickborne Relapsing Fever Diagnosis Obscured by Malaria, Togo. Emerg. Infect. Dis. 2007, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Fish, D.; Hoen, A.G.; Tsao, J.I.; Diuk-Wasser, M.A.; Bunikis, J.; Travinsky, B. Niche Partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the Same Tick Vector and Mammalian Reservoir Species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambaro, H.M.; Sasaki, M.; Sinkala, Y.; Gonzalez, G.; Squarre, D.; Fandamu, P.; Lubaba, C.; Mataa, L.; Shawa, M.; Mwape, K.E.; et al. Evidence for exposure of asymptomatic domestic pigs to African swine fever virus during an inter-epidemic period in Zambia. Transbound Emerg. Dis. 2020, 67, 2741–2752. [Google Scholar] [CrossRef]
- Chitanga, S.; Chambaro, H.M.; Moonga, L.C.; Hayashida, K.; Yamagishi, J.; Muleya, W.; Changula, K.; Mubemba, B.; Simbotwe, M.; Squarre, D.; et al. Rickettsia lusitaniae in Ornithodoros Porcinus Ticks, Zambia. Pathogens 2021, 10, 1306. [Google Scholar] [CrossRef]
- Roux, V.; Raoult, D. Body Lice as Tools for Diagnosis and Surveillance of Reemerging Diseases. J. Clin. Microbiol. 1999, 37, 596–599. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.; Anda, P.; Escudero, R.; Larsson, C.; Bergstrom, S.; Benach, J.L. Phylogenetic analysis of a virulent Borrelia species isolated from patients with relapsing fever. J. Clin. Microbiol. 2010, 48, 2484–2489. [Google Scholar] [CrossRef] [Green Version]
- McCoy, B.N.; Maiga, O.; Schwan, T.G. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick Borne Dis. 2014, 5, 401–403. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Takano, A.; Toyomane, K.; Konnai, S.; Ohashi, K.; Nakao, M.; Ito, T.; Andoh, M.; Maeda, K.; Watarai, M.; Sato, K.; et al. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS ONE 2014, 9, e104532. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Takano, A.; Taylor, K.; Sashika, M.; Shimozuru, M.; Konnai, S.; Kawabata, H.; Tsubota, T. A relapsing fever group Borrelia sp. similar to Borrelia lonestari found among wild sika deer (Cervus nippon yesoensis) and Haemaphysalis spp. ticks in Hokkaido, Japan. Ticks Tick Borne Dis. 2014, 5, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Talbert, A.; Nyange, A.; Molteni, F. Spraying tick-infested houses with lambda-cyhalothrin reduces the incidence of tick-borne relapsing fever in children under five years old. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.J.; Coulter, J.B. Tick-borne relapsing fever in central Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 852–856. [Google Scholar] [CrossRef]
- Fukunaga, M.; Ushijima, Y.; Aoki, L.Y.; Talbert, A. Detection of Borrelia duttonii, a tick-borne relapsing fever agent in central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector Borne Zoonotic Dis. 2001, 1, 331–338. [Google Scholar] [CrossRef]
- Kisinza, W.N.; McCall, P.J.; Mitani, H.; Talbert, A.; Fukunaga, M. A newly identified tick-borne Borrelia species and relapsing fever in Tanzania. Lancet 2003, 362, 1283–1284. [Google Scholar] [CrossRef]
- Mitani, H.; Talbert, A.; Fukunaga, M. New World relapsing fever Borrelia found in Ornithodoros porcinus ticks in central Tanzania. Microbiol. Immunol. 2004, 48, 501–505. [Google Scholar] [CrossRef]
- Fingerle, V.; Pritsch, M.; Wächtler, M.; Margos, G.; Ruske, S.; Jung, J.; Löscher, T.; Wendtner, C.; Wieser, A. “Candidatus Borrelia kalaharica” Detected from a Febrile Traveller Returning to Germany from Vacation in Southern Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004559. [Google Scholar] [CrossRef]
- Stete, K.; Rieg, S.; Margos, G.; Häcker, G.; Wagner, D.; Kern, W.V.; Fingerle, V. Case Report and Genetic Sequence Analysis of Candidatus Borrelia kalaharica, Southern Africa. Emerg. Infect. Dis. 2018, 24, 1659–1664. [Google Scholar] [CrossRef]
- Cutler, S.J.; Idris, J.M.; Ahmed, A.O.; Elelu, N. Ornithodoros savignyi, the Tick Vector of “Candidatus Borrelia kalaharica” in Nigeria. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.-J.; Liu, J.-W.; Wen, H.-L.; Li, Z.-M.; Lei, S.-C.; Qin, X.-R.; Zhou, C.-M.; Yu, H.; Xiao, X.; Yu, X.-J. Pathogenic New World Relapsing Fever Borrelia in a Myotis Bat, Eastern China, 2015. Emerg. Infect. Dis. 2020, 26, 3083–3085. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-M.; Xiao, X.; Zhou, C.-M.; Liu, J.-X.; Gu, X.-L.; Fang, L.-Z.; Liu, B.-Y.; Wang, L.-R.; Yu, X.-J.; Han, H.-J. Human-pathogenic relapsing fever Borrelia found in bats from Central China phylogenetically clustered together with relapsing fever borreliae reported in the New World. PLoS Negl. Trop. Dis. 2021, 15, e0009113. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Squarre, D.; Nakamura, Y.; Lau, A.C.C.; Moonga, L.C.; Kawai, N.; Ohnuma, A.; Hayashida, K.; Nakao, R.; Yamagishi, J.; et al. Evidence of Borrelia theileri in Wild and Domestic Animals in the Kafue Ecosystem of Zambia. Microorganisms 2021, 9, 2405. [Google Scholar] [CrossRef] [PubMed]
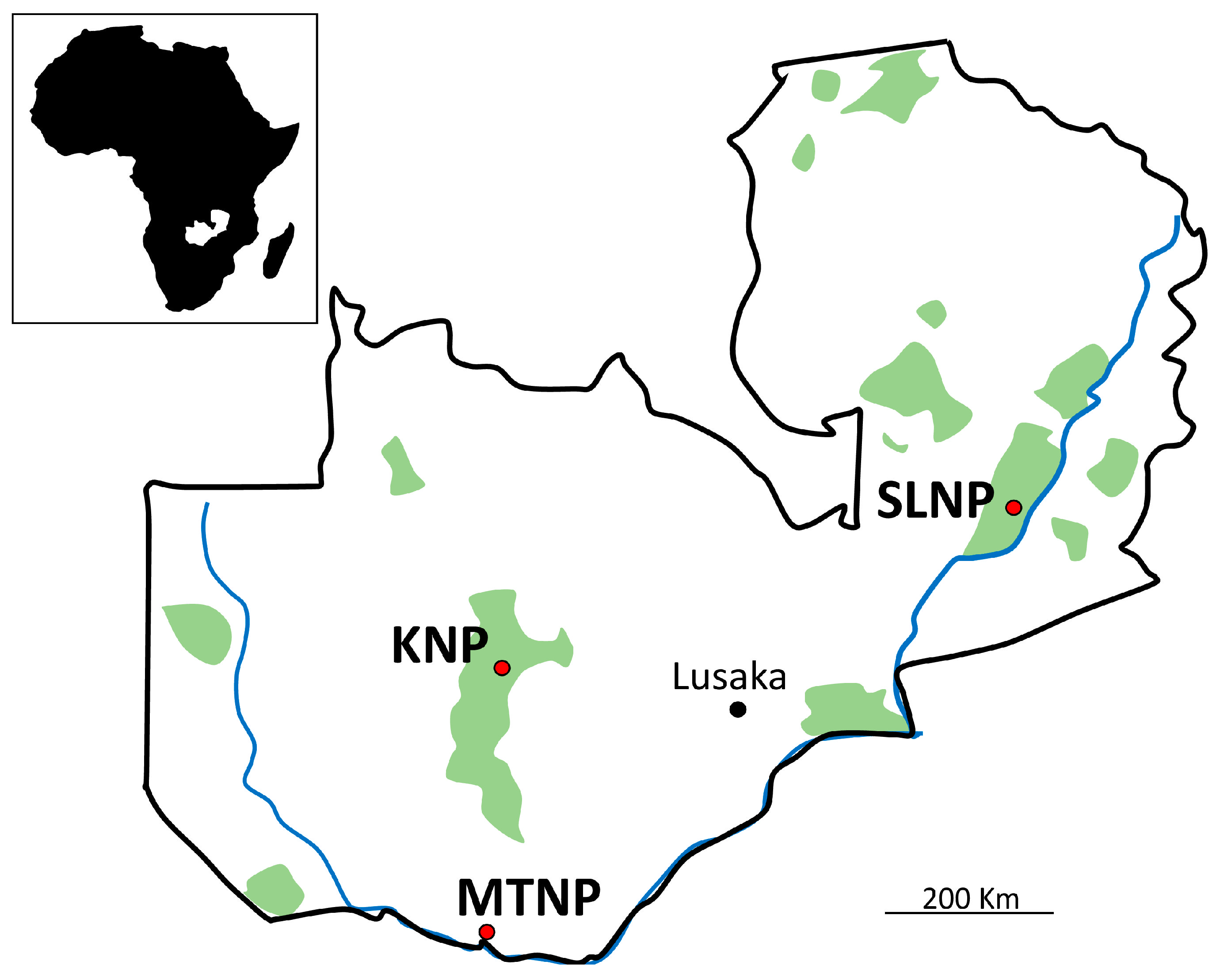

| Tick Species | Sampling Location | No. Tick Collected | No. Positive (Tested Pools) | Pool Prevalence |
|---|---|---|---|---|
| Ornithodoros moubata | Mosi-oa-Tunya National Park | 724 | 39 (124) | 31.6% |
| Ornithodoros porcinus | Kafue National Park | 75 | 0 (24) | NA |
| Ornithodoros porcinus | South Luangwa National Park | 87 | 4 (34) | 11.8% |
| Total | 886 | 43 (182) | 23.6% |
| Variants | Sample IDs | Identity | Species |
|---|---|---|---|
| flaB_Type 1 | MTNP-6, MTNP-8, MTNP-9, MTNP-10, MTNP-14, MTNP-20, MTNP-22, MTNP-24, MTNP-28, MTNP-29, MTNP-30, MTNP-31, MTNP-33, MTNP-34, MTNP-36, MTNP-37, MTNP-40, MTNP-41, MTNP-42, MTNP-43, MTNP-44, MTNP-49, MTNP-80, MTNP-87, MTNP-88, MTNP-93, MTNP-117, MTNP-118, MTNP-120, MTNP-122, SLNP-13, SLNP-27, SLNP-34 | 100% (294/294 bp) | Borrelia sp. VS4 (AB057547) * |
| flaB_Type 2 | MTNP-7 | 99.7% (293/294 bp) | Borrelia sp. VS4 (AB057547) * |
| flaB_Type 3 | MTNP-13, MTNP-89, MTNP-96, MTNP-100, MTNP-103 | 99.7% (293/294 bp) | Borrelia sp. VS4 (AB057547) * |
| flaB_Type 4 | MTNP-32 | 92.6% (275/297 bp) | B. duttonii (AB105117) |
| flaB_Type 5 | MTNP-45 | 93.9% (276/294 bp) | Borrelia sp. VS4 (AB057547) * |
| flaB_Type 6 | MTNP-95 | 99.3% (292/294 bp) | Borrelia sp. VS4 (AB057547) * |
| flaB_Type 7 | SLNP-19 | 99.7% (293/294 bp) | Borrelia sp. VS4 (AB057547) * |
| Variants | Sample IDs | Identity | Species |
|---|---|---|---|
| 16S_Type 1 | MTNP-7, MTNP-8, MTNP-9, MTNP-13, MTNP-14, MTNP-22, MTNP-24, MTNP-29, MTNP-31, MTNP-33, MTNP-41, MTNP-42, MTNP-43, MTNP-45, MTNP-80, MTNP-88, MTNP-100, MTNP-122, SLNP-13, SLNP-27, SLNP-34 | 100% (1348/1348 bp) | Borrelia sp. VS4 (AB113315) * |
| 16S_Type 2 | MTNP-10 | 99.9% (1347/1348 bp) | Borrelia sp. VS4 (AB113315) * |
| 16S_Type 3 | MTNP-28, MTNP-37 | 99.7% (1344/1348 bp) | Borrelia sp. VS4 (AB113315) * |
| 16S_Type 4 | MTNP-30, MTNP-36 | 99.9% (1347/1348 bp) | Borrelia sp. VS4 (AB113315) * |
| 16S_Type 5 | MTNP-32 | 99.8% (1352/1355 bp) | B. duttonii strain Ly (CP000976) and B. crocidurae strain Achema (CP003426). |
| 16S_Type 6 | MTNP-34 | 99.8% (1345/1348 bp) | Borrelia sp. VS4 (AB113315) * |
| 16S_Type 7 | SLNP-19 | 99.9% (1347/1348 bp) | Borrelia sp. VS4 (AB113315) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Chambaro, H.M.; Sato, K.; Squarre, D.; Simulundu, E.; Kajihara, M.; Changula, K.; Simbotwe, M.; Harima, H.; Ndebe, J.; et al. Detection of Old and New World Relapsing Fever Borreliae in Ornithodoros Ticks Collected from Warthog Burrows in Zambia. Microorganisms 2023, 11, 200. https://doi.org/10.3390/microorganisms11010200
Qiu Y, Chambaro HM, Sato K, Squarre D, Simulundu E, Kajihara M, Changula K, Simbotwe M, Harima H, Ndebe J, et al. Detection of Old and New World Relapsing Fever Borreliae in Ornithodoros Ticks Collected from Warthog Burrows in Zambia. Microorganisms. 2023; 11(1):200. https://doi.org/10.3390/microorganisms11010200
Chicago/Turabian StyleQiu, Yongjin, Herman M. Chambaro, Kozue Sato, David Squarre, Edgar Simulundu, Masahiro Kajihara, Katendi Changula, Manyando Simbotwe, Hayato Harima, Joseph Ndebe, and et al. 2023. "Detection of Old and New World Relapsing Fever Borreliae in Ornithodoros Ticks Collected from Warthog Burrows in Zambia" Microorganisms 11, no. 1: 200. https://doi.org/10.3390/microorganisms11010200








