Growth Performance and Recovery of Nosocomial Aspergillus spp. in Blood Culture Bottles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nosocomial Isolates and Reference Control Strain
2.2. Inoculum Adjustment
2.3. Experimental Strategy
2.4. Sterility Compendial Method
2.5. Automated Blood Culture Methods
2.6. Subculture
2.7. Parameters under Investigation
2.8. Specificity
2.9. Detection Limit
2.10. Ruggedness and Repeatability
2.11. Technical Cross-Contamination
2.12. Comparative Data Analysis
3. Results
3.1. Specificity
3.2. Detection Limit
3.3. Ruggedness and Repeatability
3.4. Technical Cross-Contaminations
3.5. Comparative Data Analisys
3.5.1. Comparative Analysis of the Growth Promotion Test (GPT) for the Three Systems
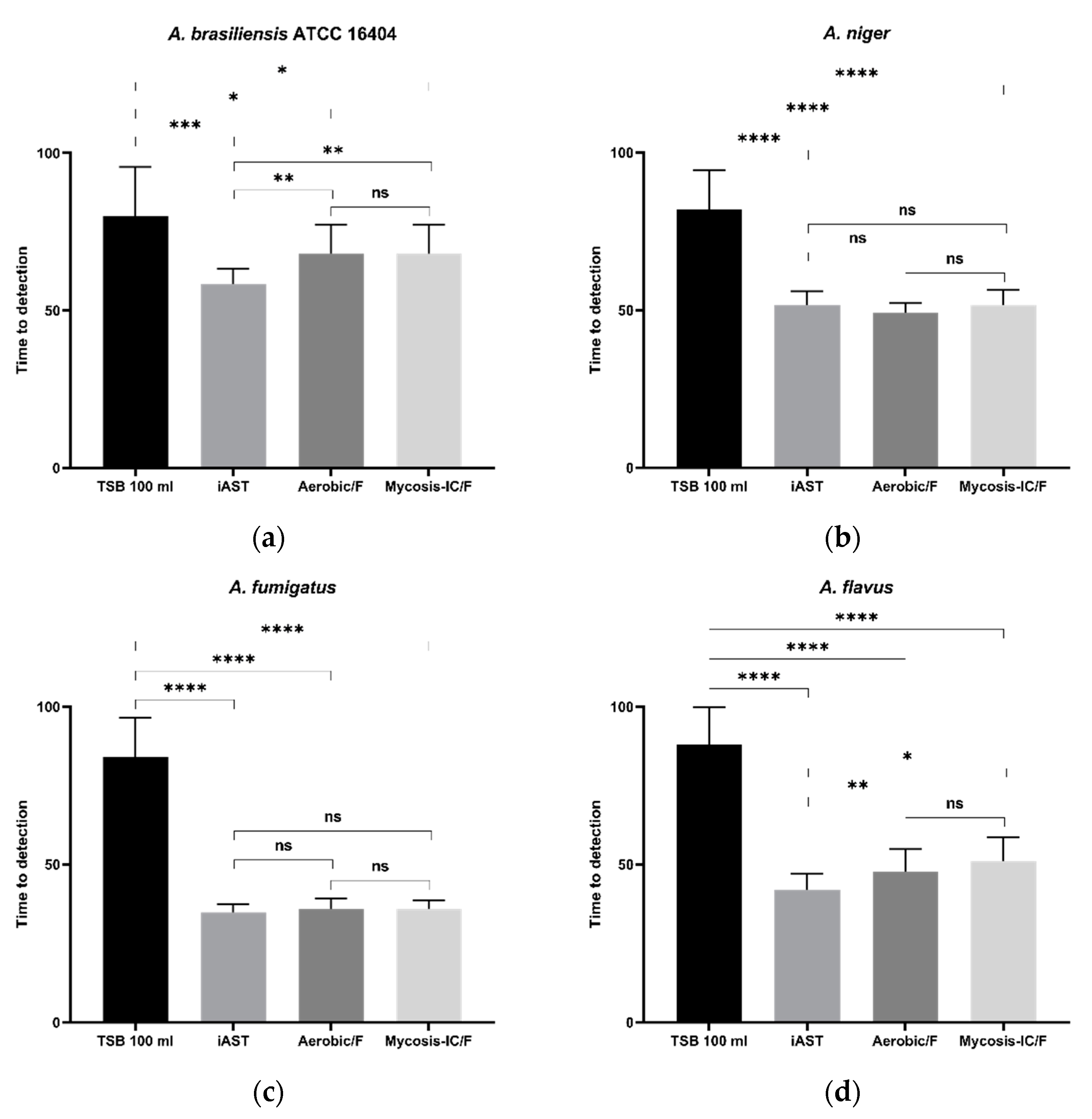
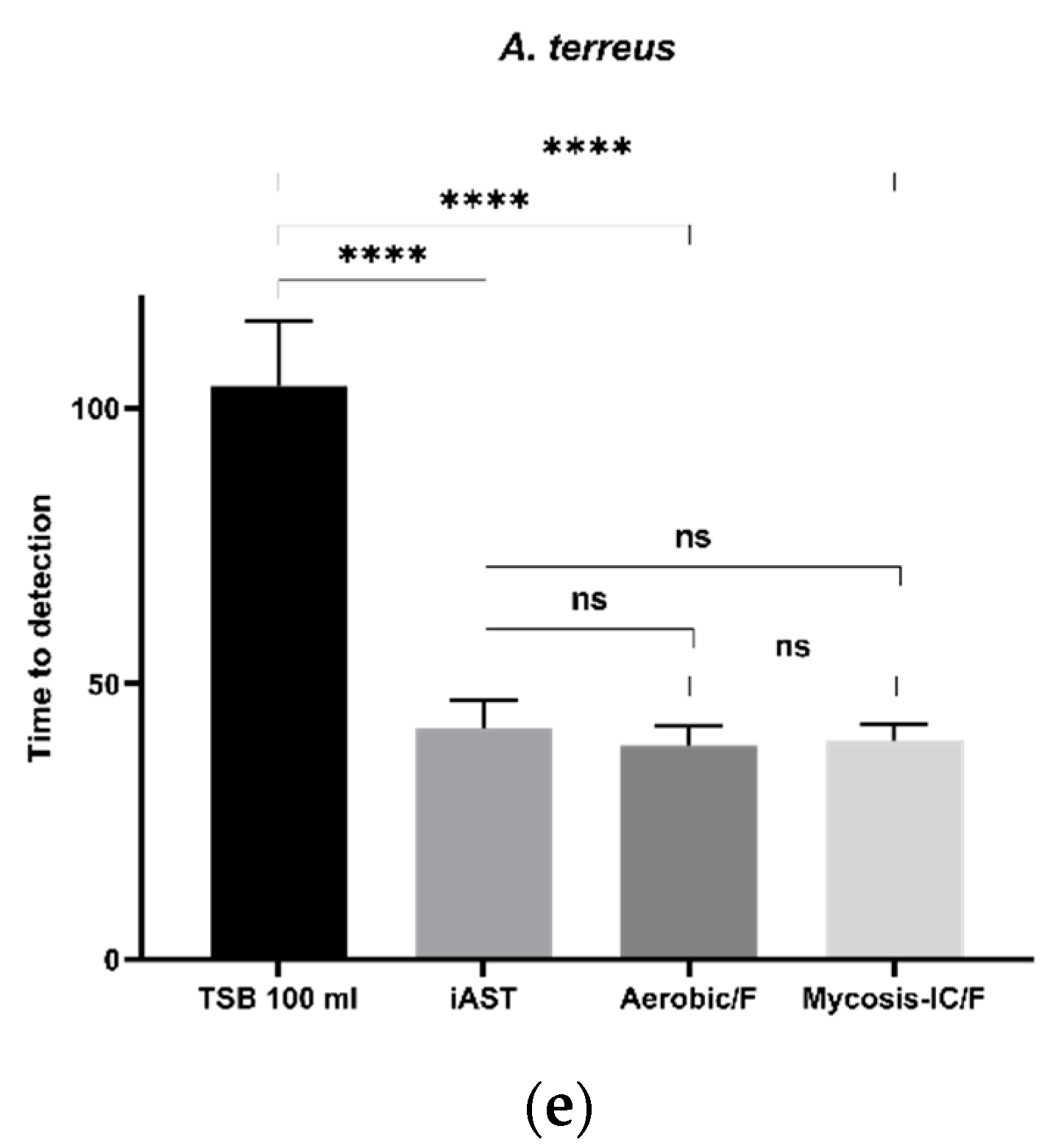
3.5.2. Time to Detection (TTD)
3.6. Recovery of Aspergillus spp. on Solid Culture Medium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. North Am. 2021, 35, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Gresnigt, M.S.; Lecompte, T.; Bibert, S.; Manuel, O.; Joosten, L.A.B.; Rüeger, S.; Berger, C.; Boggian, K.; Cusini, A.; et al. IL1B and DEFB1 Polymorphisms Increase Susceptibility to Invasive Mold Infection After Solid-Organ Transplantation. J. Infect. Dis. 2015, 211, 1646–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, B.D. Diagnosis of Fungal Infection: New Technologies for the Mycology Laboratory. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2002, 4 (Suppl. S3), 32–37. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Peghin, M.; Cervera, C.; Gudiol, C.; Ruiz-Camps, I.; Moreno, A.; Royo-Cebrecos, C.; Roselló, E.; De La Bellacasa, J.P.; Ayats, J.; et al. Causes of Death in a Contemporary Cohort of Patients with Invasive Aspergillosis. PLoS ONE 2015, 10, e0120370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and Sites of Involvement of Invasive Fungal Infections in Patients with Haematological Malignancies: A 20-Year Autopsy Study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Dullius Almeida, I.; Schmalfuss, T.; Röhsig, L.M.; Zubaran Goldani, L. Autologous Transplant: Microbial Contamination of Hematopoietic Stem Cell Products. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2012, 16, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Gain, P.; Thuret, G.; Chiquet, C.; Vautrin, A.C.; Carricajo, A.; Acquart, S.; Maugery, J.; Aubert, G. Use of a Pair of Blood Culture Bottles for Sterility Testing of Corneal Organ Culture Media. Br. J. Ophthalmol. 2001, 85, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Murray, L.; McGowan, N.; Fleming, J.; Bailey, L. Use of the BacT/Alert System for Rapid Detection of Microbial Contamination in a Pilot Study Using Pancreatic Islet Cell Products. J. Clin. Microbiol. 2014, 52, 3769–3771. [Google Scholar] [CrossRef] [Green Version]
- Putnam, N.E.; Lau, A.F. Comprehensive Study Identifies a Sensitive, Low-Risk, Closed-System Model for Detection of Fungal Contaminants in Cell and Gene Therapy Products. J. Clin. Microbiol. 2021, 59, e0135721. [Google Scholar] [CrossRef]
- Jenks, J.D.; Salzer, H.J.F.; Hoenigl, M. Improving the Rates of Aspergillus Detection: An Update on Current Diagnostic Strategies. Expert Rev. Anti-Infect. Ther. 2019, 17, 39–50. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Rosa, C.; Araujo, R.; Rodrigues, A.G.; Pinto-de-Sousa, M.I.; Pina-Vaz, C. Detection of Aspergillus Species in BACTEC Blood Cultures. J. Med. Microbiol. 2011, 60, 1467–1471. [Google Scholar] [CrossRef] [Green Version]
- European Commission Home Page. Available online: http://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 10 October 2022).
- Khuu, H.M.; Patel, N.; Carter, C.S.; Murray, P.R.; Read, E.J. Sterility Testing of Cell Therapy Products: Parallel Comparison of Automated Methods with a CFR-Compliant Method. Transfusion 2006, 46, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Khuu, H.M.; Stock, F.; McGann, M.; Carter, C.S.; Atkins, J.W.; Murray, P.R.; Read, E.J. Comparison of Automated Culture Systems with a CFR/USP-Compliant Method for Sterility Testing of Cell-Therapy Products. Cytotherapy 2004, 6, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Lysák, D.; Holubová, M.; Bergerová, T.; Vávrová, M.; Cangemi, G.C.; Ciccocioppo, R.; Kruzliak, P.; Jindra, P. Validation of Shortened 2-Day Sterility Testing of Mesenchymal Stem Cell-Based Therapeutic Preparation on an Automated Culture System. Cell Tissue Bank. 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Ramirez-Arcos, S.; Kou, Y.; Yang, L.; Perkins, H.; Taha, M.; Halpenny, M.; Elmoazzen, H. Validation of Sterility Testing of Cord Blood: Challenges and Results. Transfusion 2015, 55, 1985–1992. [Google Scholar] [CrossRef]
- Akel, S.; Lorenz, J.; Regan, D. Sterility Testing of Minimally Manipulated Cord Blood Products: Validation of Growth-Based Automated Culture Systems. Transfusion 2013, 53, 3251–3261. [Google Scholar] [CrossRef]
- Mastronardi, C.; Yang, L.; Halpenny, M.; Toye, B.; Ramírez-Arcos, S. Evaluation of the Sterility Testing Process of Hematopoietic Stem Cells at Canadian Blood Services. Transfusion 2012, 52, 1778–1784. [Google Scholar] [CrossRef]
- Pasqua, S.; Vitale, G.; Pasquariello, A.; Douradinha, B.; Tuzzolino, F.; Cardinale, F.; Cusimano, C.; Di Bartolo, C.; Conaldi, P.G.; D’Apolito, D. Use of 27G Needles Improves Sensitivity and Performance of ATCC Anaerobe Reference Microorganism Detection in BacT/Alert System. Mol. Ther.-Methods Clin. Dev. 2021, 20, 542–550. [Google Scholar] [CrossRef]
- ICH Q2(R2) Validation of Analytical Procedures|European Medicines Agency. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures (accessed on 31 August 2022).
- <1223> Validation of Alternative Microbiological Methods. Available online: https://www.drugfuture.com/pharmacopoeia/usp32/pub/data/v32270/usp32nf27s0_c1223.html (accessed on 31 August 2022).
- Binder, U.; Lass-Flörl, C. Epidemiology of Invasive Fungal Infections in the Mediterranean Area. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e20110016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, M.; Lass-Flörl, C. Changing Epidemiology of Systemic Fungal Infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008, 14 (Suppl. S4), 5–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoch, D.A.; Ludlam, H.A.; Brown, N.M. Invasive Fungal Infections: A Review of Epidemiology and Management Options. J. Med. Microbiol. 2006, 55, 809–818. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Bernal-Martinez, L.; Buitrago, M.J.; Castelli, M.V.; Gomez-Lopez, A.; Zaragoza, O.; Rodriguez-Tudela, J.L. Update on the Epidemiology and Diagnosis of Invasive Fungal Infection. Int. J. Antimicrob. Agents 2008, 32 (Suppl. S2), S143–S147. [Google Scholar] [CrossRef]
- Lee, L.D.; Hachem, R.Y.; Berkheiser, M.; Hackett, B.; Jiang, Y.; Raad, I.I. Hospital Environment and Invasive Aspergillosis in Patients with Hematologic Malignancy. Am. J. Infect. Control 2012, 40, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.D.; Berkheiser, M.; Jiang, Y.; Hackett, B.; Hachem, R.Y.; Chemaly, R.F.; Raad, I.I. Risk of Bioaerosol Contamination with Aspergillus Species before and after Cleaning in Rooms Filtered with High-Efficiency Particulate Air Filters That House Patients with Hematologic Malignancy. Infect. Control. Hosp. Epidemiol. 2007, 28, 1066–1070. [Google Scholar] [CrossRef]
- Doll, M.; Stevens, M.; Bearman, G. Environmental Cleaning and Disinfection of Patient Areas. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2018, 67, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Pappas, E.; Simmons, A.; Fox, J.; Donskey, C.J.; Deshpande, A. Environmental Cleaning and Disinfection of Hospital Rooms: A Nationwide Survey. Am. J. Infect. Control 2021, 49, 34–39. [Google Scholar] [CrossRef]
- Meyer, J.; Nippak, P.; Cumming, A. An Evaluation of Cleaning Practices at a Teaching Hospital. Am. J. Infect. Control 2021, 49, 40–43. [Google Scholar] [CrossRef]
- Di Martino, G.; Pasqua, S.; Douradinha, B.; Monaco, F.; Di Bartolo, C.; Conaldi, P.G.; D’Apolito, D. Efficacy of Three Commercial Disinfectants in Reducing Microbial Surfaces’ Contaminations of Pharmaceuticals Hospital Facilities. Int. J. Environ. Res. Public Health 2021, 18, 779. [Google Scholar] [CrossRef]
- Barton, R.C. Laboratory Diagnosis of Invasive Aspergillosis: From Diagnosis to Prediction of Outcome. Scientifica 2013, 2013, 1–29. [Google Scholar] [CrossRef] [PubMed]
| Clinical Isolates | Reference Strain |
|---|---|
| Aspergillus fumigatus | Aspergillus brasiliensis ATCC 16404 |
| Aspergillus flavus Aspergillus niger Aspergillus terreus |
| Aspergillus | Bottle Media | Recovery | Recovery TTD (h) ± SD |
|---|---|---|---|
| A. brasiliensis (ATCC 16404) | TSB | 12/12 | 80 ± 15.6 |
| iAST | 12/12 | 58.4 ± 4,8 | |
| Aerobic/F | 12/12 | 68 ± 9.3 | |
| Mycosis-IC/F | 12/12 | 68 ± 9.3 | |
| A. niger | TSB | 12/12 | 79.5 ± 11.3 |
| iAST | 12/12 | 51.6 ± 4.4 | |
| Aerobic/F | 12/12 | 49.2 ± 3.1 | |
| Mycosis-IC/F | 12/12 | 51.6 ± 4.7 | |
| A. fumigatus | TSB | 12/12 | 84 ± 12.5 |
| iAST | 12/12 | 34.8 ± 2.6 | |
| Aerobic/F | 12/12 | 35.8 ± 3.5 | |
| Mycosis-IC/F | 12/12 | 35.8 ± 2.8 | |
| A. flavus | TSB | 12/12 | 87.2 ± 12.1 |
| iAST | 12/12 | 41.54 ± 4.8 | |
| Aerobic/F | 12/12 | 48.9 ± 6 | |
| Mycosis-IC/F | 12/12 | 52.6 ± 5.4 | |
| A. terrus | TSB | 12/12 | 104 ± 11.8 |
| iAST | 12/12 | 42 ± 4.9 | |
| Aerobic/F | 12/12 | 38.3 ± 3.7 | |
| Mycosis-IC/F | 12/12 | 39.6 ± 3.2 |
| Range of Total CFU Inoculated | 25–50 | 5–10 | 2–5 | 1–2 | 0–1 | |
|---|---|---|---|---|---|---|
| Aspergillus | Bottle Media | Recovery | Recovery | Recovery | Recovery | Recovery |
| A. brasiliensis (ATCC 16404) | TSB | 12/12 | 12/12 | 11/12 | 9/12 | 2/12 |
| iAST | 12/12 | 12/12 | 12/12 | 9/12 | 0/12 | |
| Aerobic/F | 12/12 | 12/12 | 10/12 | 10/12 | 1/12 | |
| Mycosis-IC/F | 12/12 | 12/12 | 12/12 | 9/12 | 1/12 | |
| A. niger | TSB | 12/12 | 12/12 | 12/12 | 10/12 | 1/12 |
| iAST | 12/12 | 12/12 | 12/12 | 9/12 | 0/12 | |
| Aerobic/F | 12/12 | 12/12 | 11/12 | 10/12 | 3/12 | |
| Mycosis-IC/F | 12/12 | 12/12 | 10/12 | 8/12 | 0/12 | |
| A. fumigatus | TSB | 12/12 | 12/12 | 12/12 | 9/12 | 0/12 |
| iAST | 12/12 | 12/12 | 11/12 | 10/12 | 2/12 | |
| Aerobic/F | 12/12 | 12/12 | 11/12 | 9/12 | 0/12 | |
| Mycosis-IC/F | 12/12 | 12/12 | 11/12 | 10/12 | 1/12 | |
| A. flavus | TSB | 12/12 | 12/12 | 11/12 | 9/12 | 2/12 |
| iAST | 12/12 | 12/12 | 10/12 | 9/12 | 0/12 | |
| Aerobic/F | 12/12 | 12/12 | 12/12 | 9/12 | 1/12 | |
| Mycosis-IC/F | 12/12 | 12/12 | 10/12 | 10/12 | 1/12 | |
| A. terrus | TSB | 12/12 | 12/12 | 10/12 | 9/12 | 2/12 |
| iAST | 12/12 | 12/12 | 10/12 | 10/12 | 0/12 | |
| Aerobic/F | 12/12 | 12/12 | 11/12 | 9/12 | 1/12 | |
| Mycosis-IC/F | 12/12 | 12/12 | 10/12 | 10/12 | 1/12 | |
| Range of Total CFU Inoculated | 25–50 | ||||
|---|---|---|---|---|---|
| Aspergillus | Bottle Media | Positive Detection in Liquid Medium | Recovery from Solid Medium | ||
| First Procedure 1 | Second Procedure 2 | Third Procedure 3 | |||
| A. brasiliensis (ATCC 16404) | TSB | 12/12 | 1/12 | 2/12 | 12/12 |
| iAST | 12/12 | 0/12 | 2/12 | 12/12 | |
| Aerobic/F | 12/12 | 1/12 | 4/12 | 12/12 | |
| Mycosis-IC/F | 12/12 | 0/12 | 3/12 | 12/12 | |
| A. niger | TSB | 12/12 | 1/12 | 3/12 | 12/12 |
| iAST | 12/12 | 0/12 | 3/12 | 12/12 | |
| Aerobic/F | 12/12 | 2/12 | 2/12 | 12/12 | |
| Mycosis-IC/F | 12/12 | 0/12 | 3/12 | 12/12 | |
| A. fumigatus | TSB | 12/12 | 1/12 | 3/12 | 12/12 |
| iAST | 12/12 | 1/12 | 2/12 | 12/12 | |
| Aerobic/F | 12/12 | 0/12 | 4/12 | 12/12 | |
| Mycosis-IC/F | 12/12 | 1/12 | 3/12 | 12/12 | |
| A. flavus | TSB | 12/12 | 2/12 | 3/12 | 12/12 |
| iAST | 12/12 | 0/12 | 2/12 | 12/12 | |
| Aerobic/F | 12/12 | 0/12 | 2/12 | 12/12 | |
| Mycosis-IC/F | 12/12 | 1/12 | 3/12 | 12/12 | |
| A. terrus | TSB | 12/12 | 1/12 | 3/12 | 12/12 |
| iAST | 12/12 | 1/12 | 2/12 | 12/12 | |
| Aerobic/F | 12/12 | 0/12 | 2/12 | 12/12 | |
| Mycosis-IC/F | 12/12 | 0/12 | 2/12 | 12/12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqua, S.; Monaco, F.; Cardinale, F.; Bonelli, S.; Conaldi, P.G.; D’Apolito, D. Growth Performance and Recovery of Nosocomial Aspergillus spp. in Blood Culture Bottles. Microorganisms 2022, 10, 2026. https://doi.org/10.3390/microorganisms10102026
Pasqua S, Monaco F, Cardinale F, Bonelli S, Conaldi PG, D’Apolito D. Growth Performance and Recovery of Nosocomial Aspergillus spp. in Blood Culture Bottles. Microorganisms. 2022; 10(10):2026. https://doi.org/10.3390/microorganisms10102026
Chicago/Turabian StylePasqua, Salvatore, Francesco Monaco, Francesca Cardinale, Simone Bonelli, Pier Giulio Conaldi, and Danilo D’Apolito. 2022. "Growth Performance and Recovery of Nosocomial Aspergillus spp. in Blood Culture Bottles" Microorganisms 10, no. 10: 2026. https://doi.org/10.3390/microorganisms10102026
APA StylePasqua, S., Monaco, F., Cardinale, F., Bonelli, S., Conaldi, P. G., & D’Apolito, D. (2022). Growth Performance and Recovery of Nosocomial Aspergillus spp. in Blood Culture Bottles. Microorganisms, 10(10), 2026. https://doi.org/10.3390/microorganisms10102026







