Sintering of Ti(C,N)-WC/Mo2C-(Ta,Nb)C-Co/Ni Cermets Investigated by CO and N2 Outgassing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Powders and Formulations
2.2. QMS Analysis
3. Results
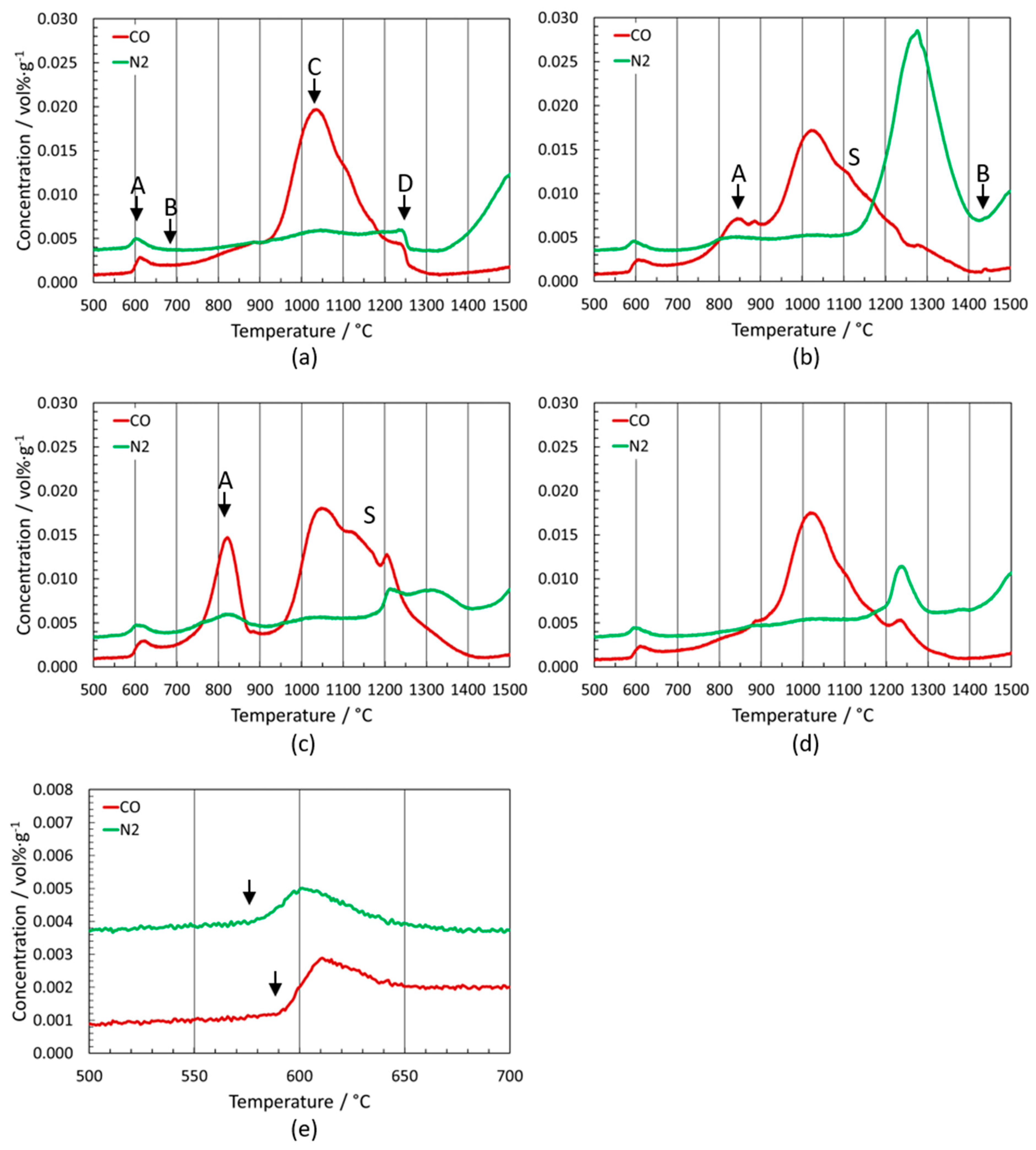
3.1. Model Alloys Ti(C,N)-Co/Ni with Additions
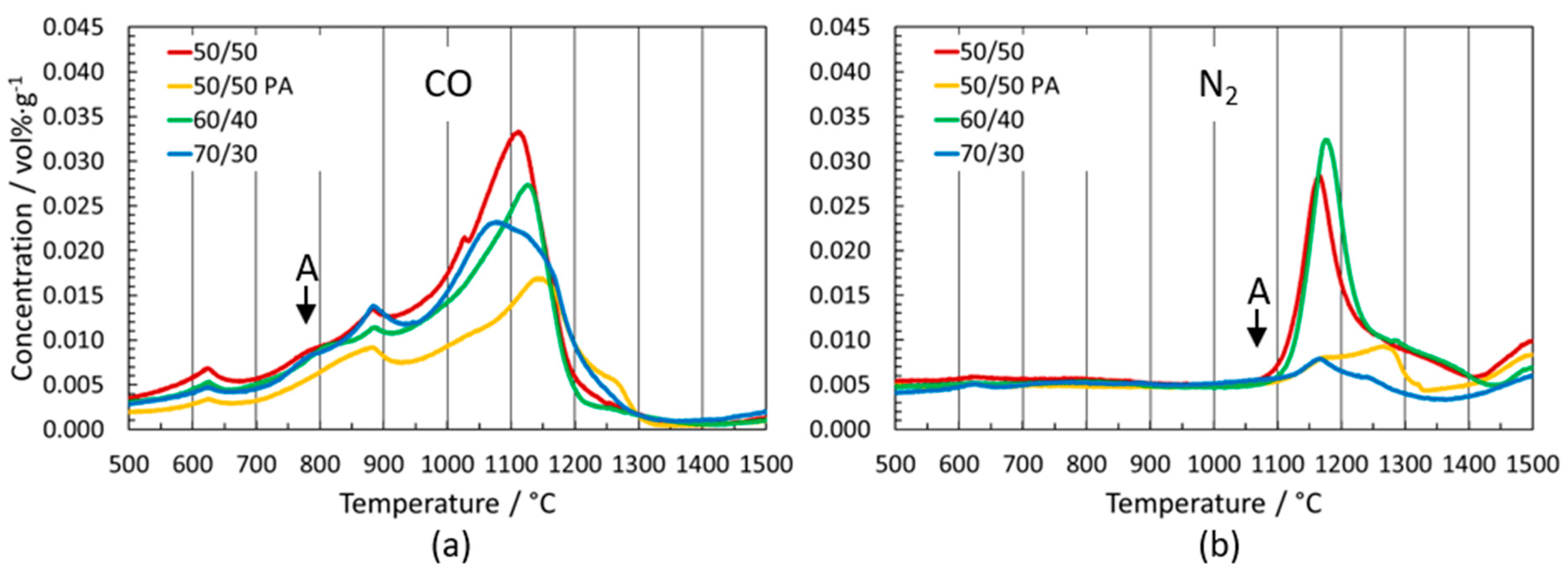
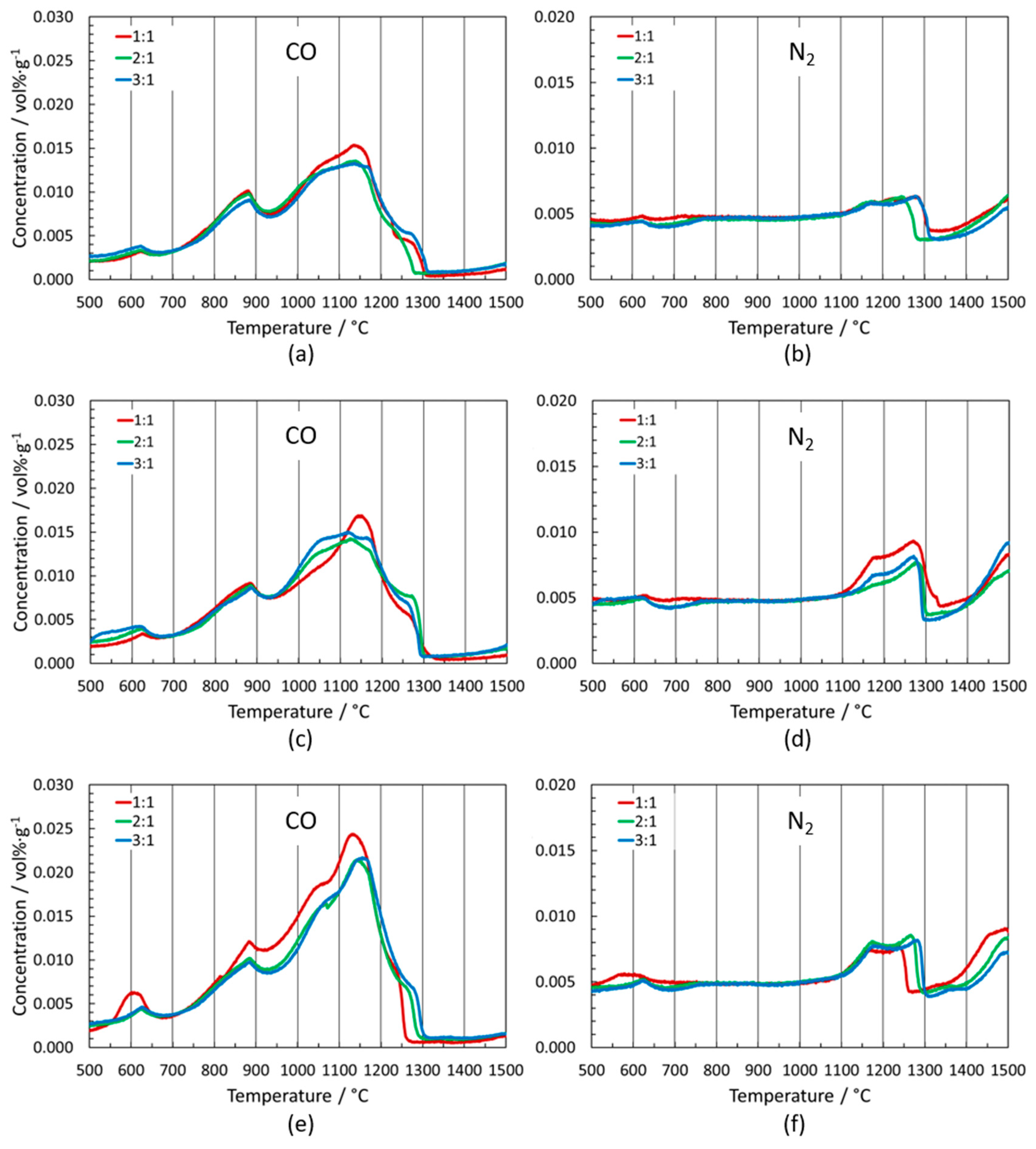
3.2. Outgassing of Cermets
3.2.1. Ti(C,N)-WC-(Ta,Nb)C-Co/Ni and Ti(C,N)-(Ti,W)C-(Ta,Nb)C-Co/Ni, (Co:Ni = 1:1)
3.2.2. Ti(C,N)-WC/Mo2C-(Ta,Nb)C-Co/Ni and Ti(C,N)-(Ti,W)C/(Ti,Mo)(C,N)-(Ta,Nb)C-Co/Ni, Co:Ni = 1:1
3.2.3. Ti(C,N)-Mo2C-(Ta,Nb)C-Co/Ni and Ti(C,N)-(Ti,Mo)(C,N)-(Ta,Nb)C-Co/Ni, Co:Ni = 1:1
3.2.4. Variation of Binder-Phase Composition
Grades with WC and/or Mo2C in the Starting Formulation
Grades with (Ti,W)C and/or (Ti,Mo)(C,N) in the Starting Formulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Pacher, O.; Schintlmeister, W.; Weirather, F. Der Einfluß definierter Sinteratmosphären auf den Kohlenstoffgehalt von Hartmetall. Planseeber. Pulvermet. 1978, 26, 244–251. [Google Scholar]
- Pacher, O.; Schintlmeister, W.; Raine, T. Influence of vacuum sintering furnace atmosphere on carbon content of hardmetal. Powder Metall. 1980, 4, 189–193. [Google Scholar] [CrossRef]
- Hack, R.; Kiefer, F.; Schuler, D.; Müller, N. Monitoring of vacuum sintering furnace atmosphere with a quadrupole mass spectrometer. Vacuum 1990, 41, 2171–2172. [Google Scholar] [CrossRef]
- Maurer, G. Monitoring of gas composition during sintering of cemented carbide and cermets using a mass spectrometry system. J. Hard Mater. 1992, 3, 235–245. [Google Scholar]
- Groschner, M. Metallurgical Reactions upon Sintering of Titanium Carbonitride Hardmetals. Doctoral Thesis, Vienna University of Technology, Vienna, Austria, 1992. (In German). [Google Scholar]
- Ettmayer, P.; Kolaska, H.; Lengauer, W.; Dreyer, K. Ti(C,N) Cermets—Metallurgy and Properties. Int. J. Refract. Met. Hard Mater. 1995, 13, 343–351. [Google Scholar] [CrossRef]
- Gestrich, T.; Leitner, G.; Jaenicke-Rößler, K. Gasanalyse beim Sintern von Hartmetall durch TA-Simulation. J. Therm. Anal. 1996, 47, 651–657. [Google Scholar] [CrossRef]
- Garcia, J.; Lengauer, W. Quantitative Mass Spectrometry of Decarburisation and Denitridation of Cemented Carbonitrides during Sintering. Microchim. Acta 2001, 136, 83–89. [Google Scholar] [CrossRef]
- Findenig, G.; Buchegger, C.; Lengauer, W.; Veitsch, C.; Demoly, A. Investigation of the main influencing parameters on the degassing behaviour of titanium carbonitrides using mass spectrometry. Int. J. Refract. Met. Hard Mater. 2017, 63, 38–46. [Google Scholar] [CrossRef]
- Schwarz, V.; Zivadinovic, I.; Lisnard, B.; Traxler, F.; Viala, R.; Lengauer, W. Optimised properties of Ti(C,N)-based cermets by variation of the W/Mo ratio. In European Congress and Exhibition on Powder Metallurgy. European PM Conference Proceedings; Hamburg (D), Session 45, USB Version; EPMA: Shrewsbury, UK, 2016; ISBN 978-1-899072-47-7. [Google Scholar]
- Gestrich, T.; Kaiser, A.; Pötschke, J.; Meinl, J.; Höhn, S. Thermal behaviour of cermets and hardmetals during debinding and sintering. Int. J. Refract. Met. Hard Mater. 2018, 73, 210–215. [Google Scholar] [CrossRef]
- Lengauer, W.; Scagnetto, F. Ti(C,N)-based cermets: Critical review of achievements and recent developments. Solid State Phenom. 2018, 274, 53–100. [Google Scholar] [CrossRef]
- Demoly, A.; Veitsch, C.; Lengauer, W.; Rabitsch, K. Cermets based on based on new submicron Ti(C,N) powder: Microstructural development during sintering and mechanical properties. In Advances in Sintering Science and Technology II; Kang, S.-J.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA.
- Lengauer, W.; Schwarz, V.; Scagnetto, F. Interdependency of hard phase and binder phase composition in Ti(C,N)-based cermets. In Proceedings of the WorldPM 2018, Part 5, Beijing, China, 16–20 September 2018; pp. 817–825. [Google Scholar]




| Set #1–12 Mixtures | [Mo]/([Mo] + [W]) | Employed Ti(C,N) Grades | Overall [C]/[N] | Co:Ni wt |
|---|---|---|---|---|
| Ti(C,N)-WC-(Ta,Nb)C | 0 0 0 | Ti(C0.5N0.5) Ti(C0.6N0.4)-A Ti(C0.7N0.3) | 1.4 1.7 3.0 | 1:1 1:1 1:1 |
| Ti(C,N)-(Ti,W)C-(Ta,Nb)C | 0 | C/N like Ti(C0.7N0.3) | 3.0 | 1:1 |
| Ti(C,N)-WC/Mo2C-(Ta,Nb)C | 0.6 0.6 0.6 | Ti(C0.5N0.5) Ti(C0.6N0.4)-A Ti(C0.7N0.3) | 1.4 1.7 3.0 | 1:1 1:1 1:1 |
| Ti(C,N)-(Ti,W)C/(Ti,Mo)(C,N)-(Ta,Nb)C | 0.6 | C/N like Ti(C0.5N0.5) | 3.0 | 1:1 |
| Ti(C,N)-WC/Mo2C-(Ta,Nb)C | 1 1 1 | Ti(C0.5N0.5) Ti(C0.6N0.4)-A Ti(C0.7N0.3) | 1.4 1.7 3.0 | 1:1 1:1 1:1 |
| Ti(C,N)-(Ti,Mo)(C,N)-(Ta,Nb)C | 1 | C/N like Ti(C0.5N0.5) | 1.4 | 1:1 |
| Set #2–9 Mixtures | Employed Ti(C,N) Grades | Overall [C]/[N] | Co:Ni wt |
|---|---|---|---|
| Ti(C,N)-WC/Mo2C-(Ta,Nb)C [Mo]/([Mo] + [W]) = 0, 0.6, 1 | Ti(C0.6N0.4)-A | 1.7 | 1:1 |
| Ti(C,N)-WC/Mo2C-(Ta,Nb)C [Mo]/([Mo] + [W]) = 0, 0.5, 1 | Ti(C0.6N0.4)-B | 1.7 | 2:1 |
| Ti(C,N)-WC/Mo2C-(Ta,Nb)C [Mo]/([Mo] + [W]) = 0, 0.5, 1 | Ti(C0.6N0.4)-B | 1.7 | 3:1 |
| Set #3–9 Mixtures | Employed Ti + W or Ti + Mo Grades | Overall [C]:/[N] | Co:Ni wt |
|---|---|---|---|
| Ti(C,N)-(Ti,W)C/(Ti,Mo)(C,N)-(Ta,Nb)C | (Ti,W)C (Ti,W)C + (Ti,Mo)(C,N) (Ti,Mo)(C,N) | 1.7 1.7 1.7 | 1:1, 2:1, 3:1 1:1, 2:1, 3:1 1:1, 2:1, 3:1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, V.; Scagnetto, F.; Lengauer, W. Sintering of Ti(C,N)-WC/Mo2C-(Ta,Nb)C-Co/Ni Cermets Investigated by CO and N2 Outgassing. Metals 2019, 9, 427. https://doi.org/10.3390/met9040427
Schwarz V, Scagnetto F, Lengauer W. Sintering of Ti(C,N)-WC/Mo2C-(Ta,Nb)C-Co/Ni Cermets Investigated by CO and N2 Outgassing. Metals. 2019; 9(4):427. https://doi.org/10.3390/met9040427
Chicago/Turabian StyleSchwarz, Viktoria, Fabio Scagnetto, and Walter Lengauer. 2019. "Sintering of Ti(C,N)-WC/Mo2C-(Ta,Nb)C-Co/Ni Cermets Investigated by CO and N2 Outgassing" Metals 9, no. 4: 427. https://doi.org/10.3390/met9040427






