Multiscale Comparison Study of Void Closure Law and Mechanism in the Bimetal Roll-Bonding Process
Abstract
:1. Introduction
2. Closure Process of Interface Voids
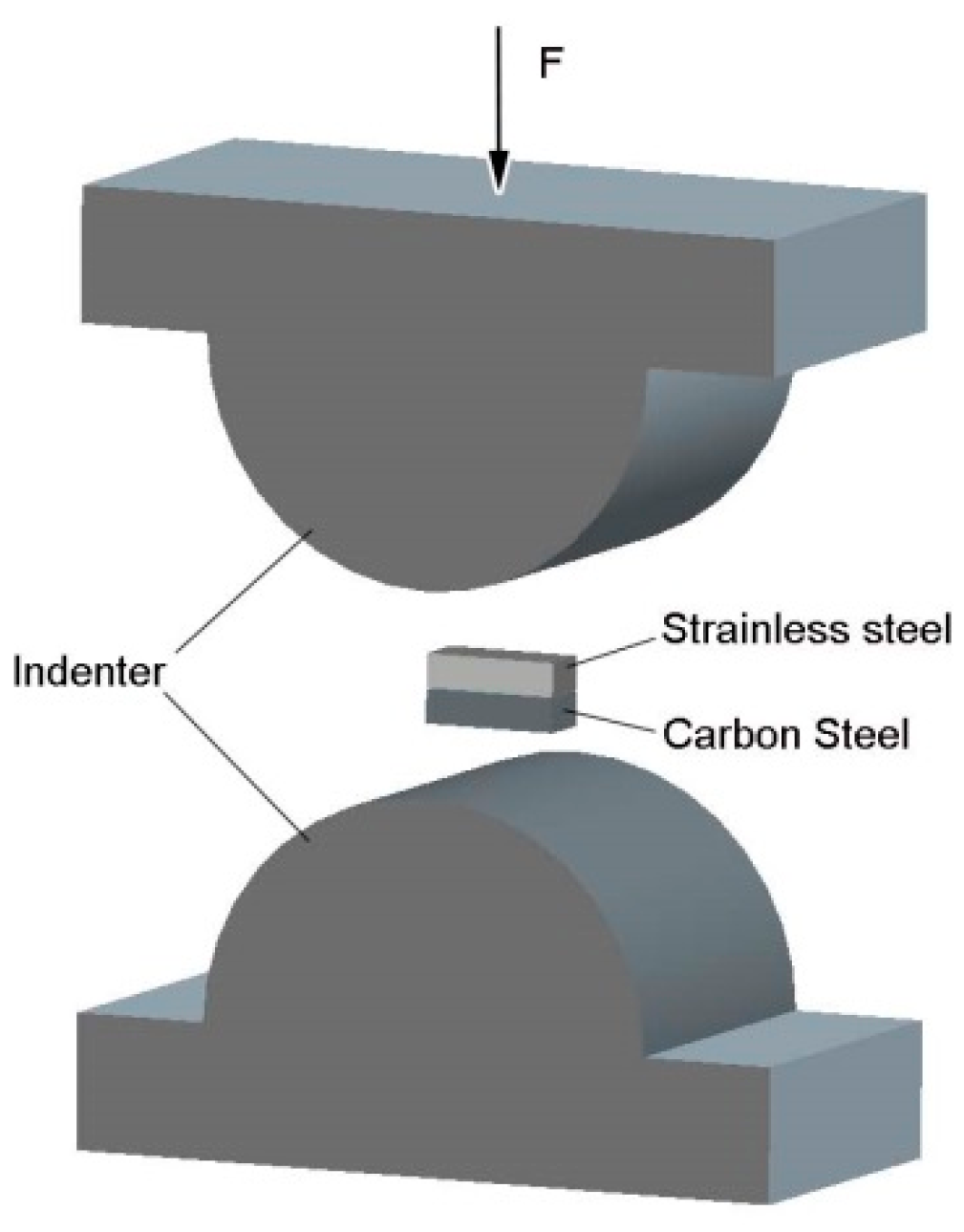
2.1. Experimental Methods
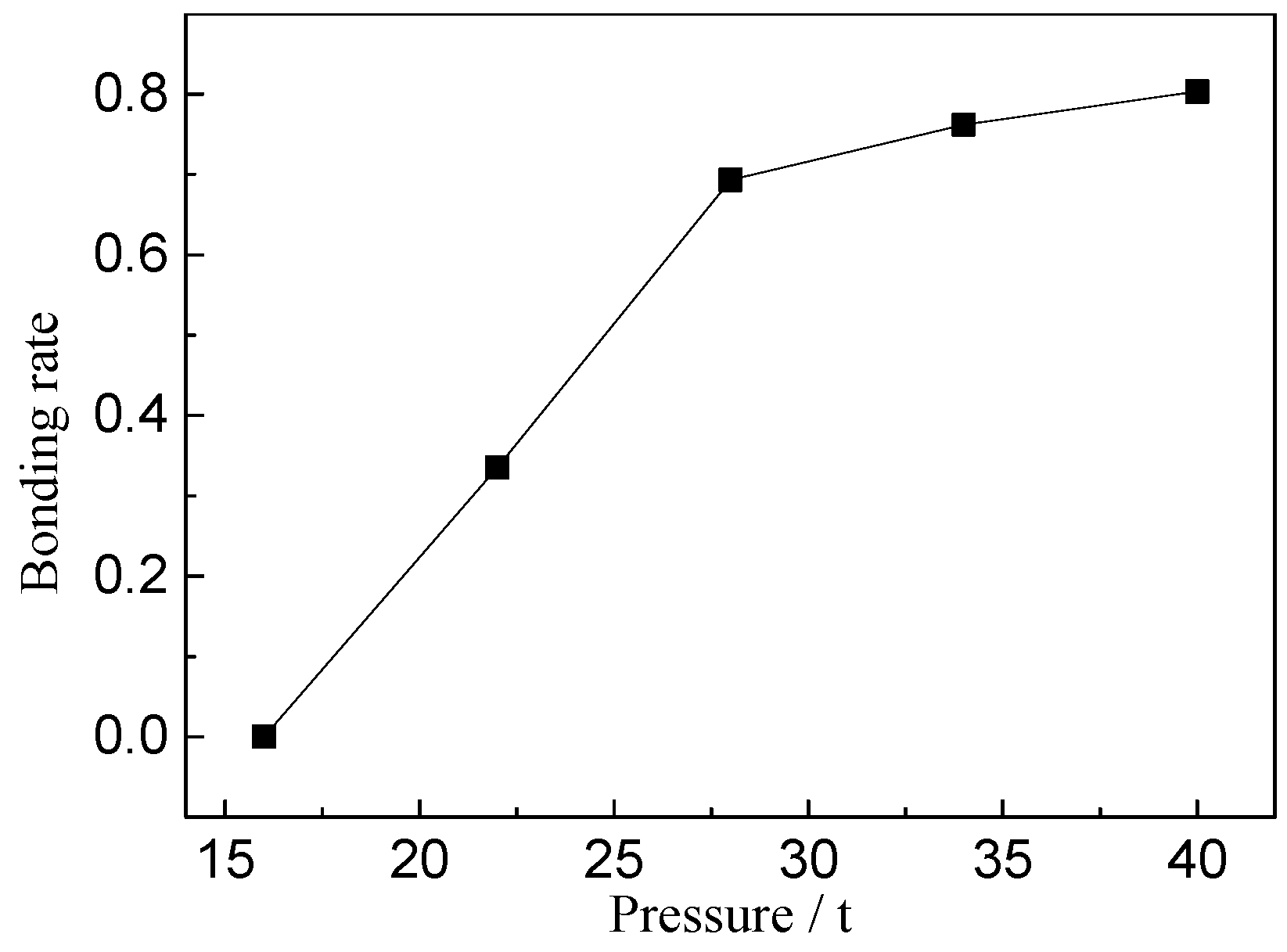
2.2. Closure Process
3. Multiscale Numerical Models
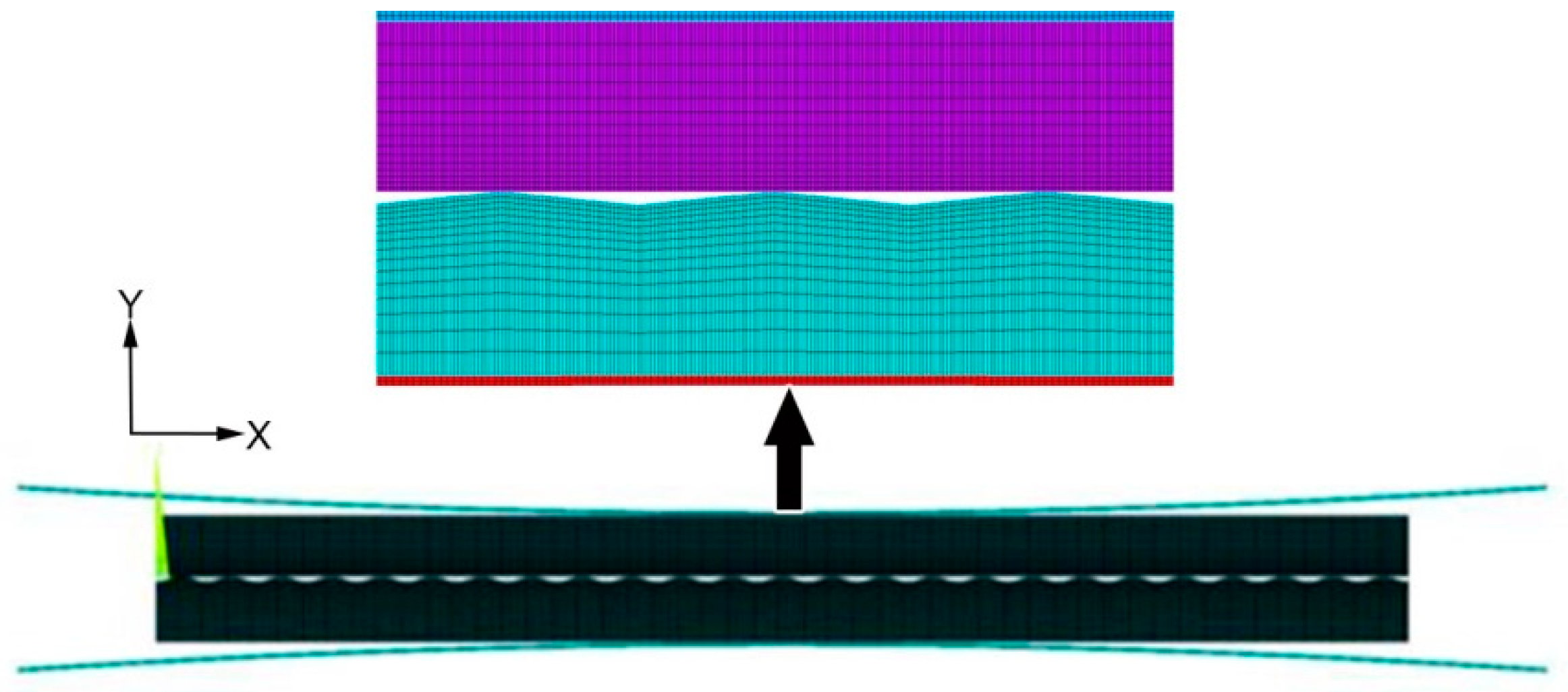
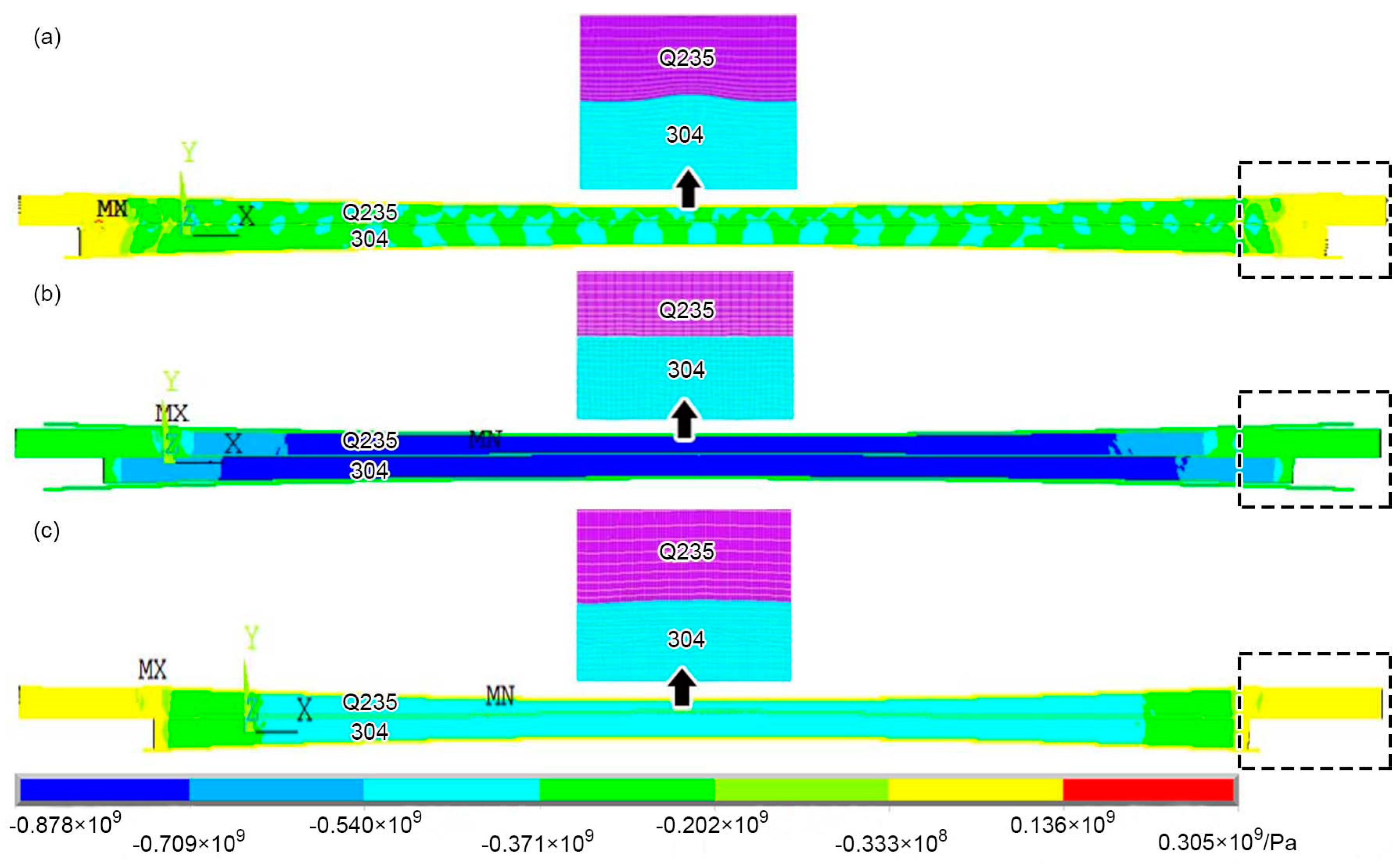
3.1. Finite Element Simulation at the Macro-scale
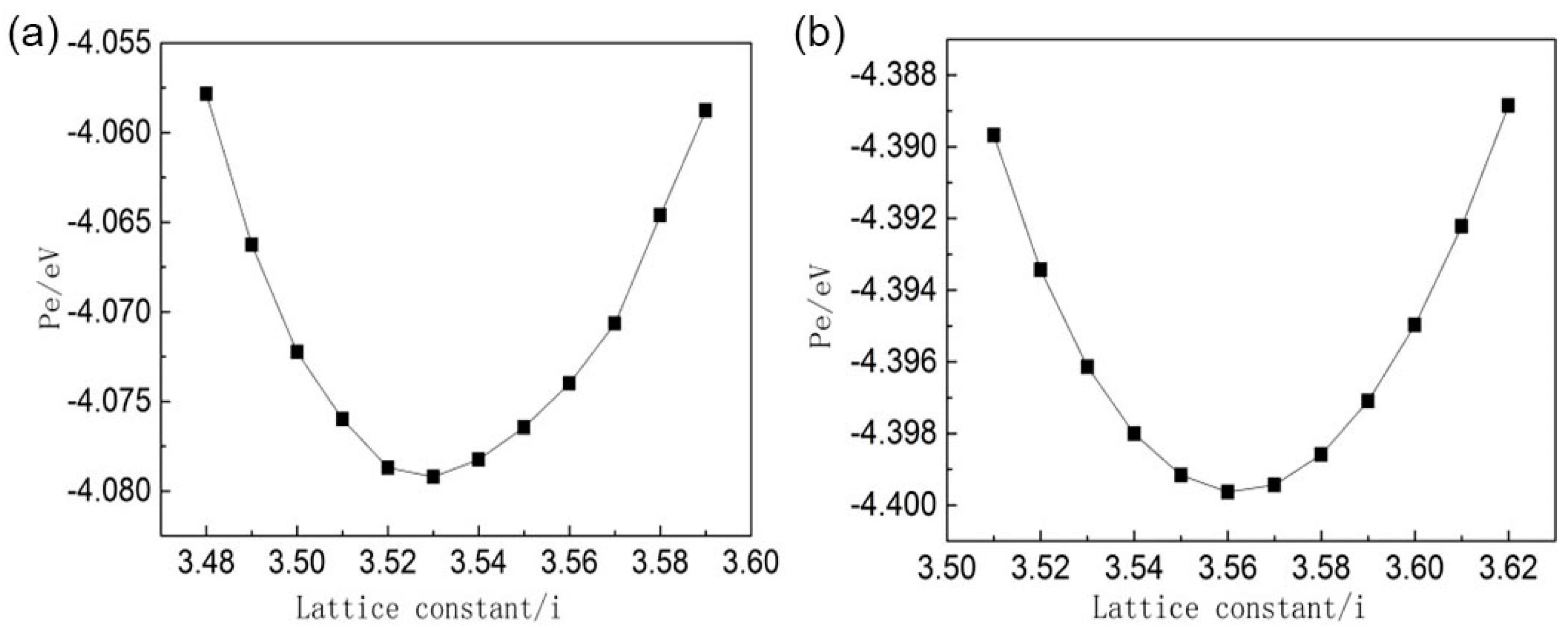
3.2. Molecular Dynamics Simulation at the Micro-scale
4. Closure Law and Mechanism of Interface Voids
4.1. Contact Deformation Law and Mechanism at the Macro-scale
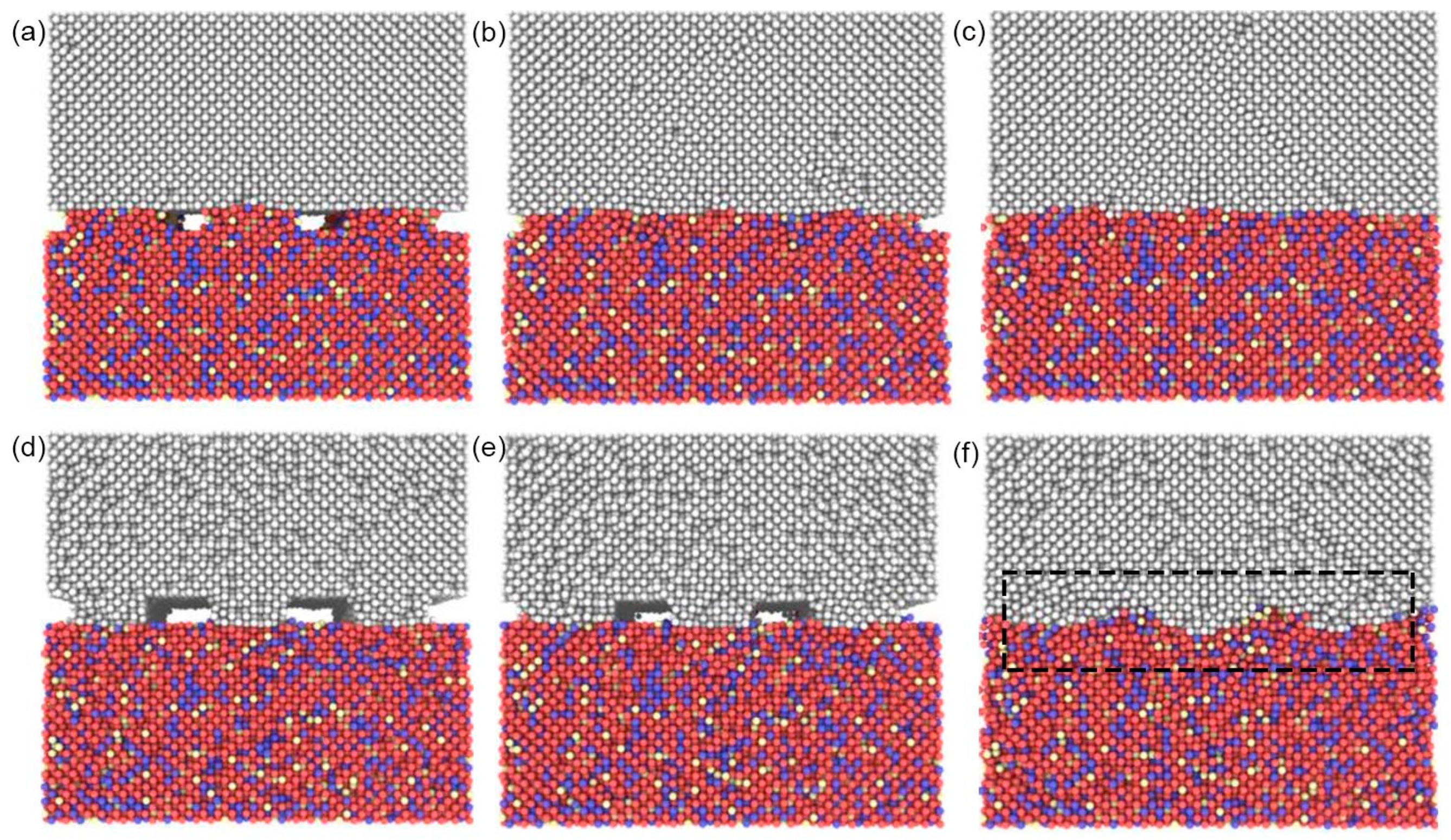
4.2. Atomic Bonding Process at the Micro-scale
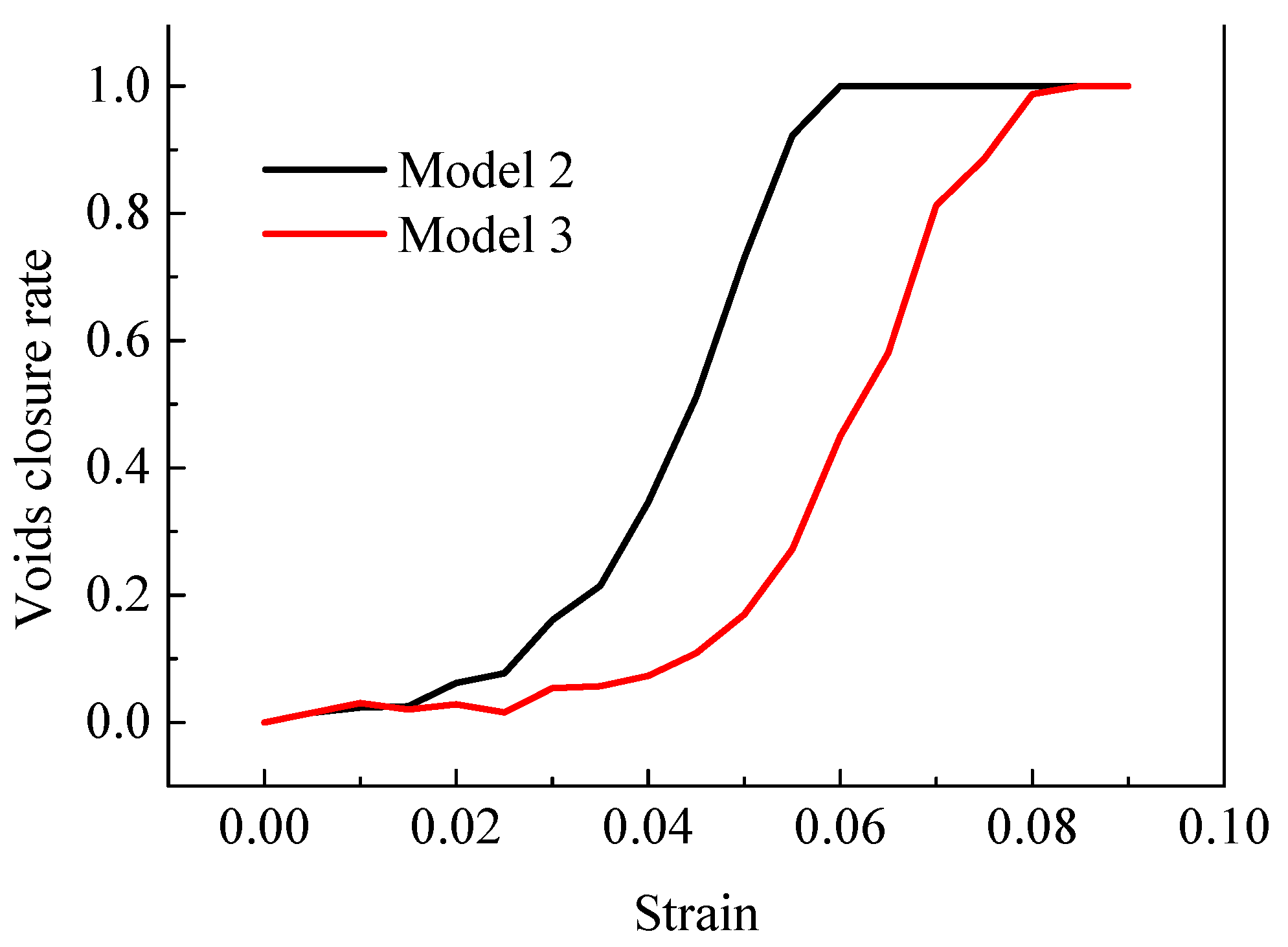
4.2.1. Closure Law of Interfacial Voids
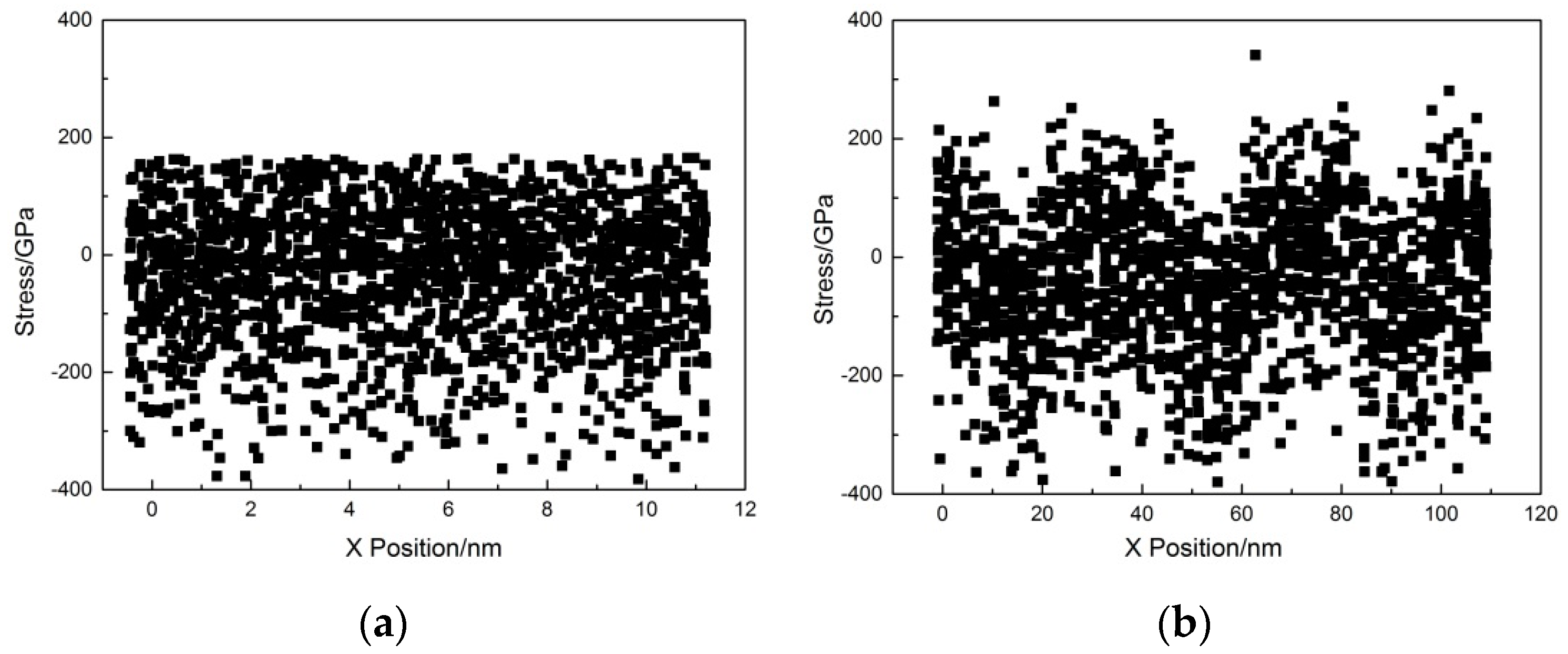
4.2.2. Closure Mechanism of Interface Voids
5. Conclusions
- (1)
- At the macro-scale, the closure rate of voids decreases with the increase of the real contact area between interfaces. The shape of voids changes from rectangular to circular or elliptical, and finally disappears completely. At the micro-scale, the arrangement of atoms near the interface become disordered under a certain pressure and move towards the voids, finally becoming ordered again with the voids completely healed.
- (2)
- The deformation law of surface roughness peaks at the macro- and micro-scales is different. The interface morphology after roll-bonding at the macro-scale was determined by the morphology of 304 stainless steel with a larger yield strength ratio, while the interface morphology at the micro-scale was mainly determined by the morphology of Q235 carbon steel with a higher yield strength.
- (3)
- At the micro-scale, the surface roughness affects the mechanical behavior of the material during deformation. Disordered atoms due to surface roughness hinder dislocation propagation through the interface, making the rough surface more plastically deformable than the ideal plane.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.D.; Li, S.; Liu, J.Y.; Wang, Y.N.; Zhang, B.Y.; Zhang, L.Y. Study of a Bimetallic Interfacial Bonding Process Based on Ultrasonic Quantitative Evaluation. Metals 2018, 8, 329. [Google Scholar] [CrossRef]
- Christian, W.S.; Patrick, K.; Heinz, W.H.; Mathias, G.P. Based Alloying by Accumulative Roll Bonding in the System Al-Cu. Metals 2011, 1, 65–78. [Google Scholar]
- Yilmaz, O.; Çelik, H. Electrical and thermal properties of the interface at diffusion-bonded and soldered 304 stainless steel and copper bimetal. J. Mater. Process. Technol. 2003, 141, 67–76. [Google Scholar] [CrossRef]
- Yao, G.C.; Mei, Q.S.; Li, J.Y.; Li, C.L.; Ma, Y.; Chen, F.; Zhang, G.D.; Yang, B. Hard copper with good electrical conductivity fabricated by accumulative roll-bonding to ultrahigh strains. Metals 2016, 6, 115. [Google Scholar] [CrossRef]
- Wang, A.; Ohashi, O.; Ueno, K. Effect of surface asperity on diffusion bonding. Mater. Trans. 2006, 47, 179. [Google Scholar] [CrossRef]
- Bahramian, A. The effect of heat treatment on the surface structure of polyaniline nanostructured film: An experimental and molecular dynamics approach. Appl. Surf. Sci. 2014, 311, 508–520. [Google Scholar] [CrossRef]
- Sayles, R.S.; Thomas, T.R. Surface topography as a nonstationary random process. Nature 1978, 271, 431–434. [Google Scholar] [CrossRef]
- Hill, A.; Wallach, E.R. Modelling solid-state diffusion bonding. Acta. Metall. 1989, 37, 2425–2437. [Google Scholar] [CrossRef]
- Ma, R.F.; Li, M.Q.; Li, H.; Yu, W.X. Modeling of void closure in diffusion bonding process based on dynamic conditions. Sci. China. Ser. E 2012, 55, 2420–2431. [Google Scholar] [CrossRef]
- Alegria, J.; Miranda, R.M.; Gomez, D.S.; Jose, M.; Fernandes, A.A. Modelling of voids closure in the diffusion bonding process. Mater. Sci. Forum 2008, 587–588, 731–735. [Google Scholar] [CrossRef]
- Rapaport, D.C. Multibillion-atom molecular dynamics simulation: Design considerations for vector-parallel processing. Comput. Phys. Commun. 2006, 174, 521–529. [Google Scholar] [CrossRef]
- Song, J.; Srolovitz, D.J. Molecular dynamics investigation of patterning via cold welding. J. Mech. Phys. Solids 2009, 57, 776–787. [Google Scholar] [CrossRef]
- Chen, S.D.; Ke, F.J.; Zhou, M.; Bai, Y.L. Atomistic investigation of the effects of temperature and surface roughness on diffusion bonding between Cu and Al. Acta. Mater. 2007, 55, 3169–3175. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.; Song, C.; Lin, T.; He, P. Molecular dynamics simulation of the effect of surface roughness and pore on linear friction welding between Ni and Al. Comput. Mater. Sci. 2011, 50, 3385–3389. [Google Scholar] [CrossRef]
- Medyanik, S.N.; Shao, S. Strengthening effects of coherent interfaces in nanoscale metallic bilayers. Comput. Mater. Sci. 2009, 45, 1129–1133. [Google Scholar] [CrossRef]
- Misra, A.; Hirth, J.P.; Hoagland, R.G. Length-scale-dependent deformation mechanisms in incoherent metallic multilayered composites. Acta. Mater. 2005, 53, 4817–4824. [Google Scholar] [CrossRef]
- Wang, J.; Misra, A. An overview of interface-dominated deformation mechanisms in metallic multilayers. Curr. Opin. Solid St. M. 2011, 15, 20–28. [Google Scholar] [CrossRef]
- Rao, S.I.; Hazzledine, P.M. Atomistic simulations of dislocation–interface interactions in the Cu-Ni multilayer system. MRS Proceedings 1999, 578, 2011–2040. [Google Scholar] [CrossRef]
- Shao, S.; Wang, J.; Misra, A.; Hoagland, R.G. Spiral patterns of dislocations at nodes in (111) semi-coherent FCC interfaces. Sci. Rep. 2013, 3, 2448. [Google Scholar] [CrossRef]
- Hoagland, R.G.; Kurtz, R.J.; Henager, C.H. Slip resistance of interfaces and the strength of metallic multilayer composites. Scripta Mater. 2004, 50, 775–779. [Google Scholar] [CrossRef]
- Weng, S.; Ning, H.; Hu, N.; Cheng, Y.; Fu, T.; Peng, X.H.; Fu, S.Y.; Zhang, J.Y.; Xu, C.H.; Sun, D.Y.; et al. Strengthening effects of twin interface in Cu/Ni multilayer thin films – A molecular dynamics study. Mater. Des. 2016, 111, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Misra, A.; Wang, H.; Shen, T.D.; Nastasi, M.; Mitchell, T.E.; Hirth, J.P.; Hoagland, R.G.; Embury, J.D. Enhanced hardening in Cu/330 stainless steel multilayers by nanoscale twinning. Acta. Mater. 2004, 52, 995–1002. [Google Scholar] [CrossRef]
- López de Lacalle, L.N.; Rodríguez, A.; Lamikiz, A.; Celaya, A.; Alberdi, R. Five-axis machining and burnishing of complex parts for the improvement of surface roughness. Adv. Manuf. Processes 2011, 26, 997–1003. [Google Scholar] [CrossRef]
- Pozo, D.D.; López de Lacalle, L.N.; López, J.M.; Hernández, A. Prediction of press/die deformation for an accurate manufacturing of drawing dies. Int. J. of Adv. Manuf. Tech. 2008, 37, 649–656. [Google Scholar] [CrossRef]
- Bonny, G.; Castin, N.; Terentyev, D. Interatomic potential for studying ageing under irradiation in stainless steels: the FeNiCr model alloy. Model. Simul. Mater. Sci. Eng. 2013, 21, 5897. [Google Scholar] [CrossRef]
- Mishin, Y.; Mehl, M.J.; Papaconstantopoulos, D.A.; Voter, A.F.; Kress, J.D. Structural stability and lattice defects in copper: Ab initio, tight-binding, and embedded-atom calculations. Phys. Rev. B 2001, 63, 224106. [Google Scholar] [CrossRef]
- Cohen, A.J.; Gordon, R.G. Theory of the lattice energy, equilibrium structure, elastic constants, and pressure-induced phase transitions in alkali-halide crystals. Phys. Rev. B 1975, 12, 3228–3241. [Google Scholar] [CrossRef]
- Yu, J.; Xin, L.; Wang, J.; Chen, J.; Huang, W. First-principles study of the relaxation and energy of bcc-Fe, fcc-Fe and AISI-304 stainless steel surfaces. Appl. Surf. Sci. 2009, 255, 9032–9039. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. IEEE T. Fuzzy Syst. 2010, 18, 2154–2162. [Google Scholar] [CrossRef]
- Moxnes, E.D.; Kristiansen, N.I.; Stohl, A.; Clarisse, L.; Durant, A.; Weber, K. Extracting dislocations and non-dislocation crystal defects from atomistic simulation data. Model. Simul. Mater. Sci. Eng. 2010, 18, 2131–2145. [Google Scholar]









| Material | Cr | Ni | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| AISI304 | 18.97 | 8.86 | 0.04 | 1.00 | 2.00 | 0.035 | 0.03 | Bal |
| Q235A | - | - | 0.22 | 0.30 | 0.43 | 0.04 | 0.05 | Bal |
| Material | Young’s Modulus (E/MPa) | Poisson’s Ratio (γ) | ||
|---|---|---|---|---|
| AISI304 | 67 | 2.02 × 105 | 0.3 | 7.9 × 103 |
| Q235A | 78 | 2.1 × 105 | 0.3 | 7.8 × 103 |
| Group | Surface of AISI304 | Surface of Q235A | Strain |
|---|---|---|---|
| 1 | Smooth | Rough | 0.5 |
| 2 | Smooth | Smooth | 0.5 |
| 3 | Rough | Smooth | 0.5 |
| 4 | Rough | Smooth | 0.1 |
| 5 | Rough | Smooth | 0.2 |
| 6 | Rough | Smooth | 0.3 |
| 7 | Rough | Smooth | 0.4 |
| 8 | Rough | Smooth | 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, S.; Li, R.; Zhang, B. Multiscale Comparison Study of Void Closure Law and Mechanism in the Bimetal Roll-Bonding Process. Metals 2019, 9, 343. https://doi.org/10.3390/met9030343
Zhang Q, Li S, Li R, Zhang B. Multiscale Comparison Study of Void Closure Law and Mechanism in the Bimetal Roll-Bonding Process. Metals. 2019; 9(3):343. https://doi.org/10.3390/met9030343
Chicago/Turabian StyleZhang, Qingdong, Shuo Li, Rui Li, and Boyang Zhang. 2019. "Multiscale Comparison Study of Void Closure Law and Mechanism in the Bimetal Roll-Bonding Process" Metals 9, no. 3: 343. https://doi.org/10.3390/met9030343





