Recovery of Soluble Potassium from Alunite by Thermal Decomposition: Effect of CaO and Phase Transformation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Thermal Decomposition Experiment
2.2.3. Water Leaching and Lixivium Crystallization
3. Results and Discussion
3.1. Recovery of the Soluble Potassium from Alunite
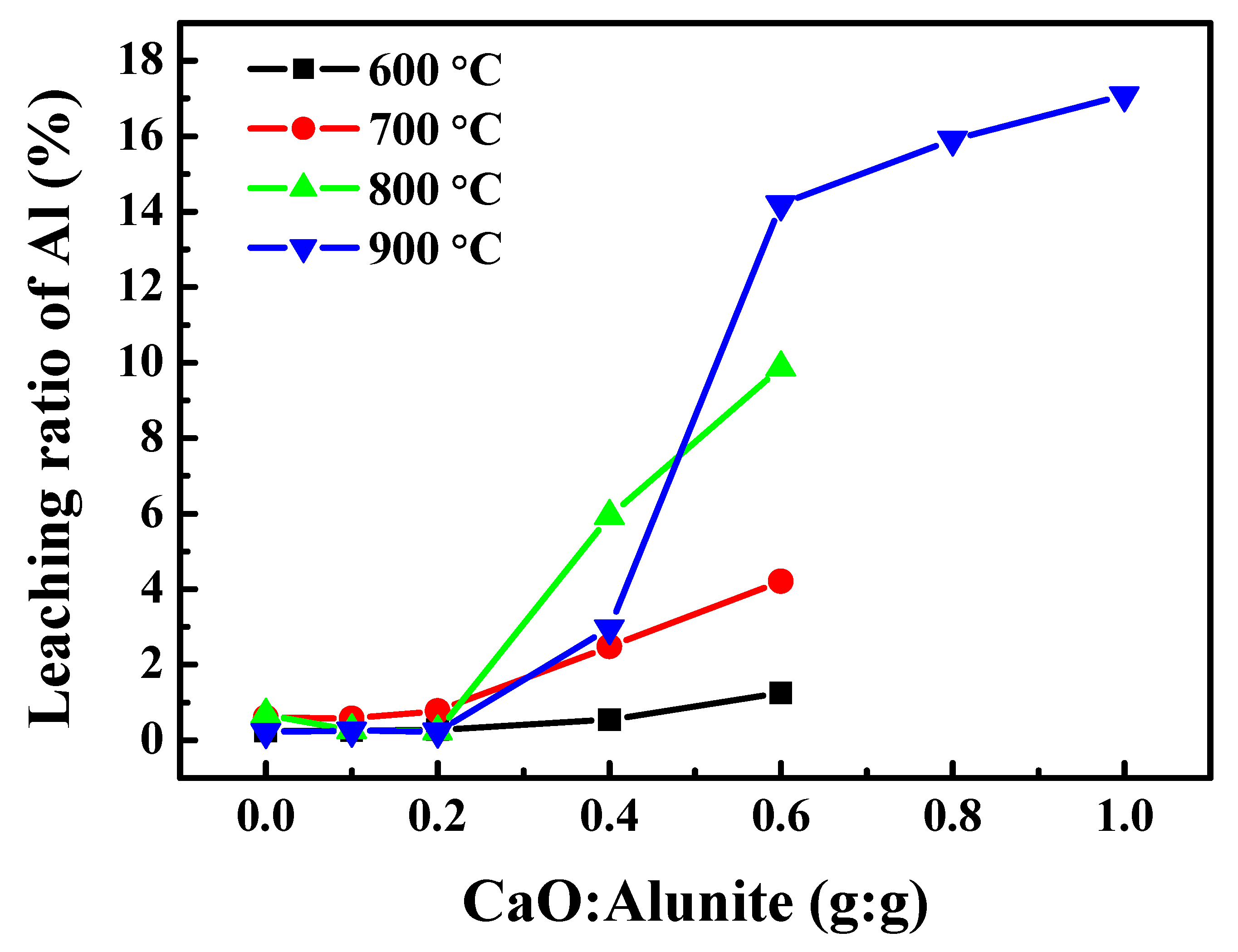
3.1.1. Effect of CaO Addition on the Recovery Ratio
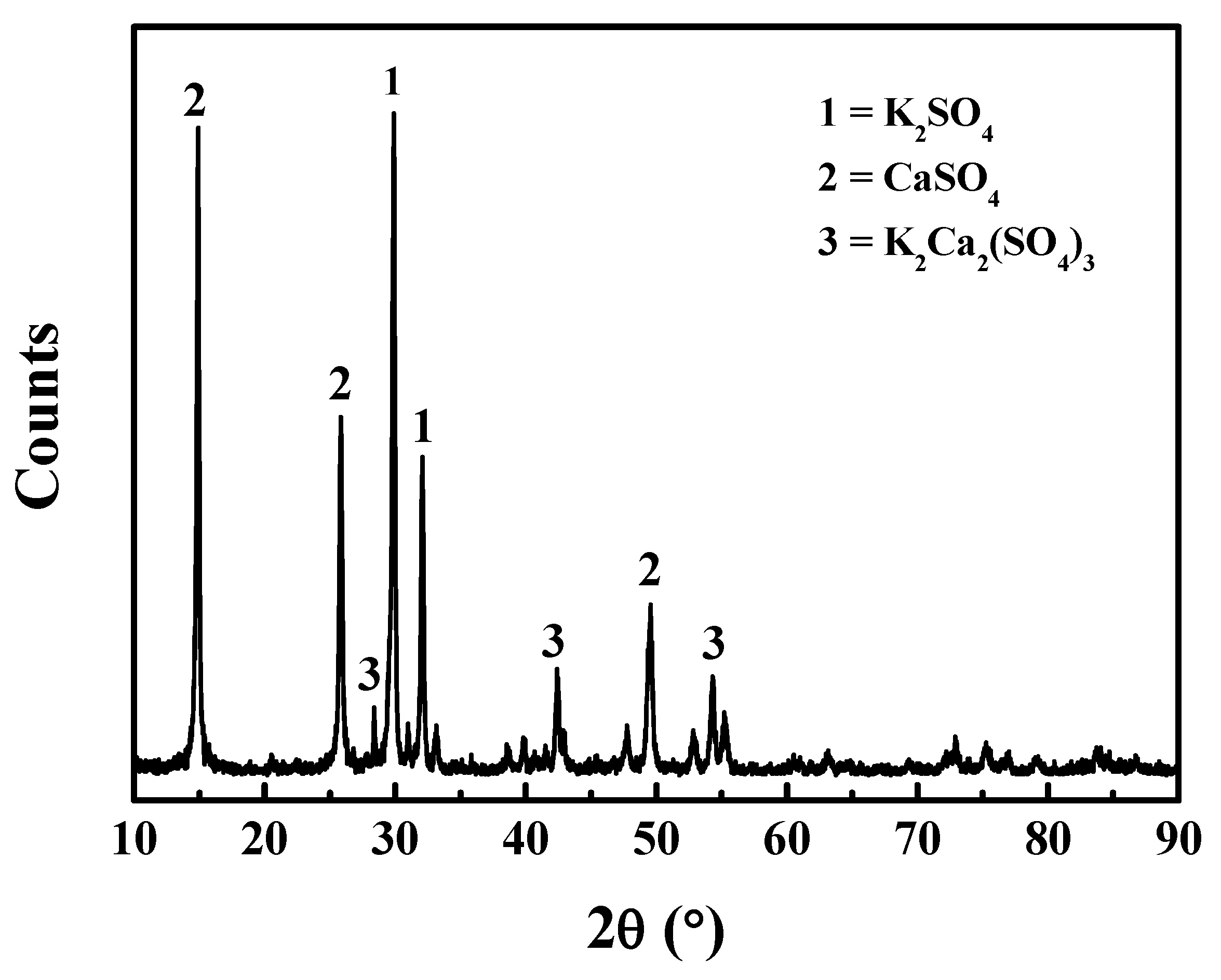
3.1.2. Effect of CaO Addition on Water Leaching Product
3.2. Effect of CaO on the Desulfurization of Alunite
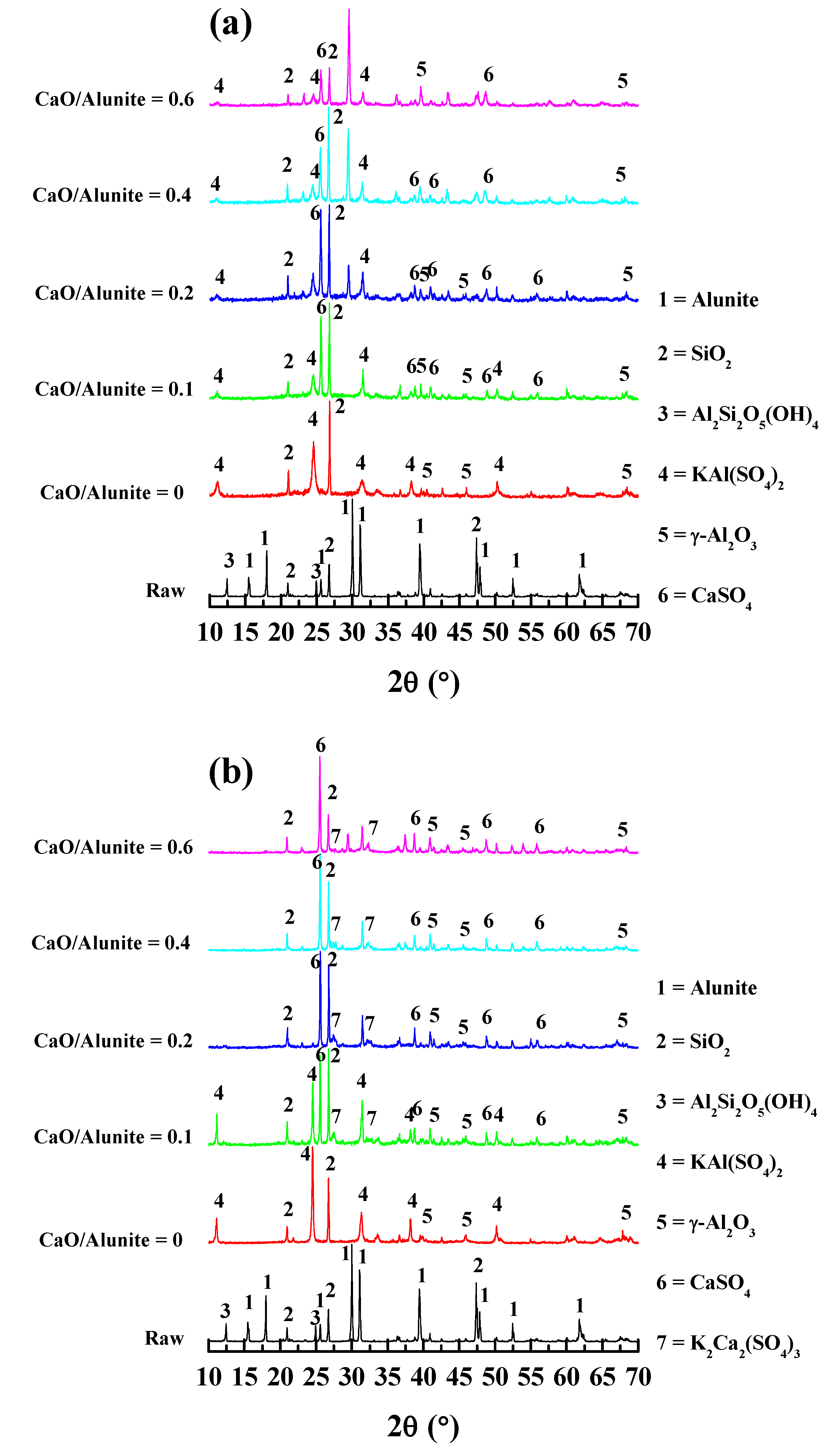
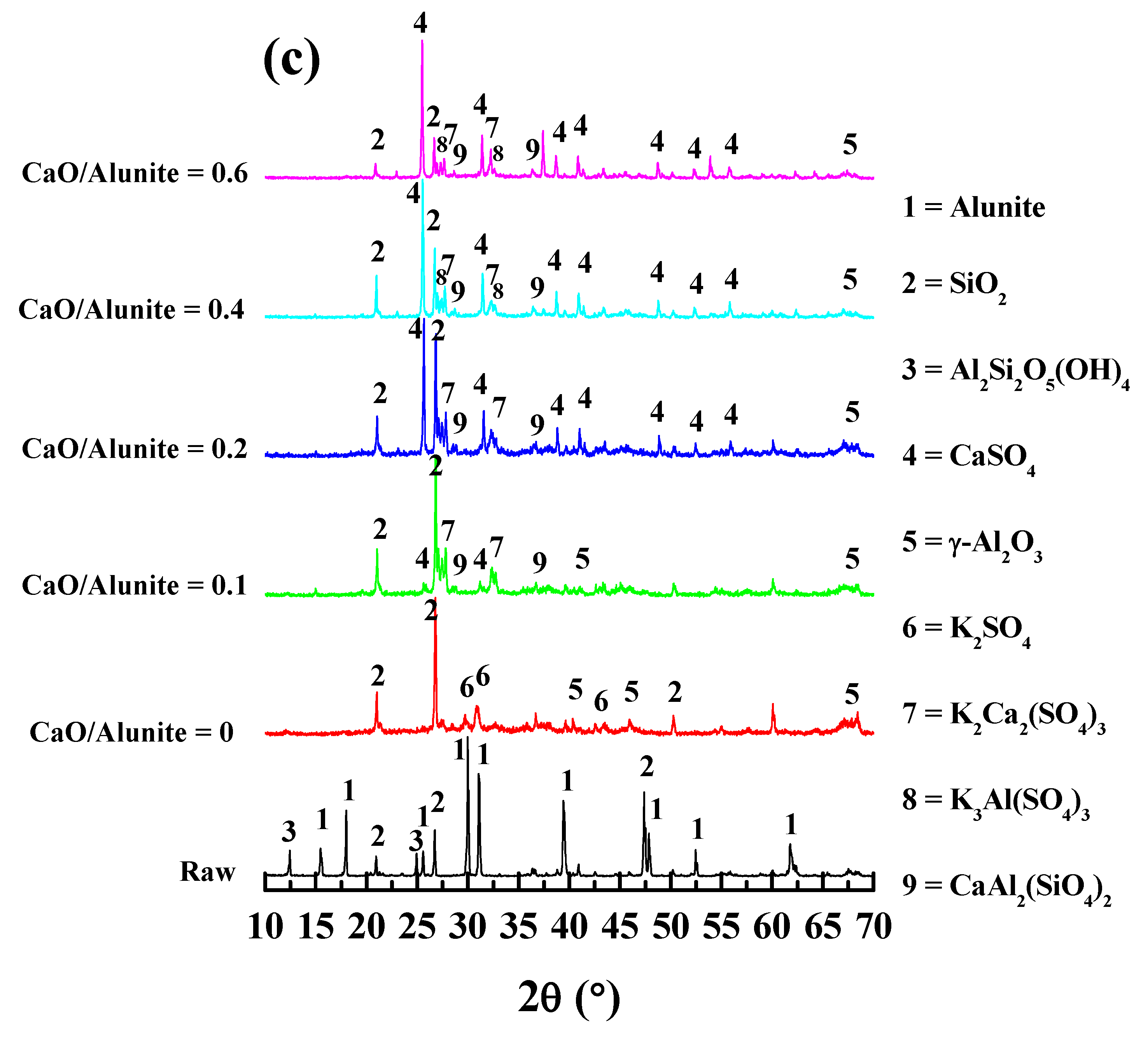
3.2.1. X-ray Diffraction (XRD) Analysis
3.2.2. Fourier-transform Infrared Spectrometry (FTIR) Results
3.2.3. Phase Structure Evolution
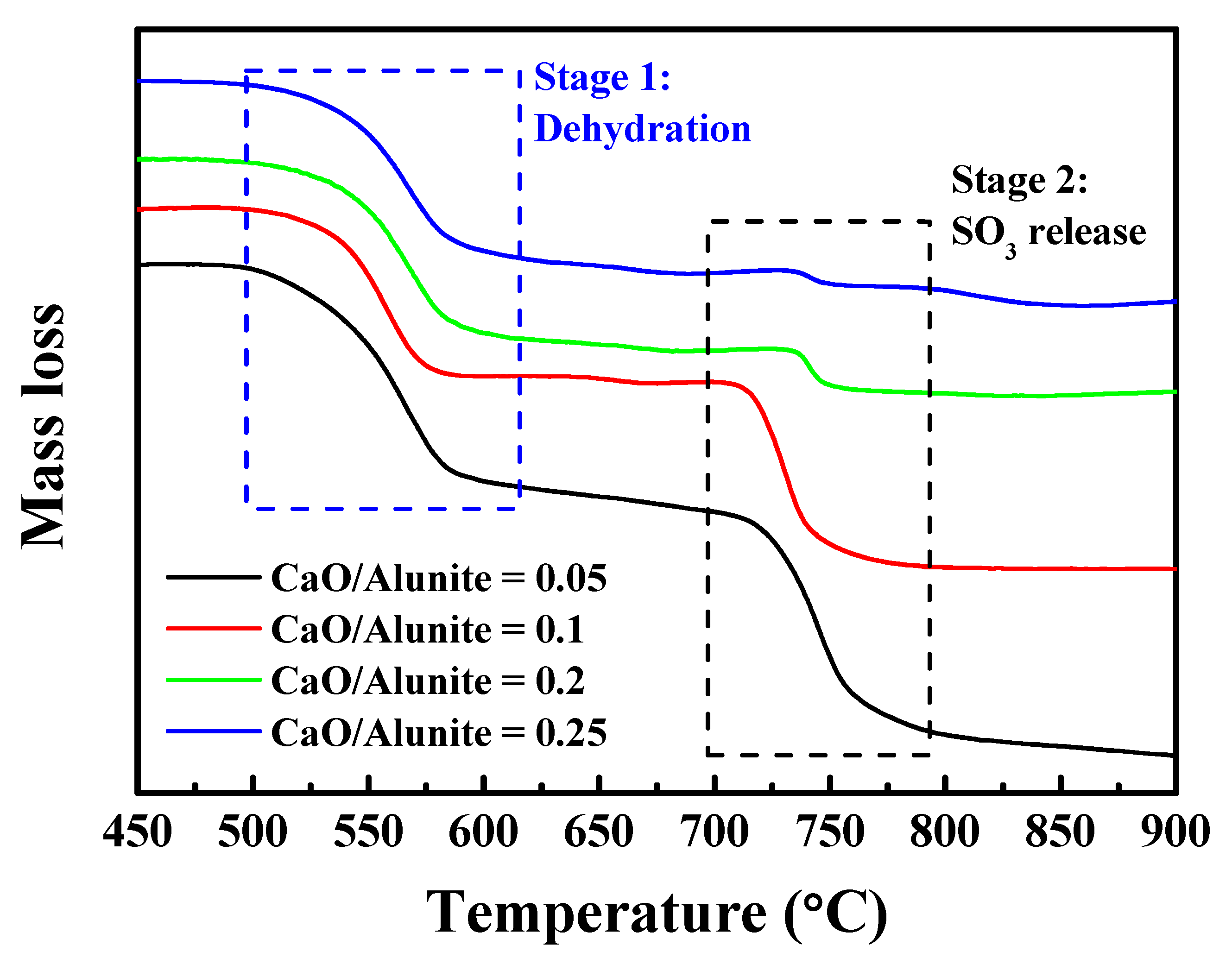
3.3. Effect of CaO on Thermal Behavior of Alunite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2013–2017; International Fertilizer Industry Association (IFA): Paris, France, 2013. [Google Scholar]
- Ma, H.; Yang, J.; Su, S.; Liu, M.; Zheng, H.; Wang, Y.; Qi, H.; Zhang, P.; Yao, W. 20 years advances in preparation of potassium salts from potassic rocks: A review. Acta Geol. Sin. 2015, 89, 2058–2071. [Google Scholar]
- Stoffregen, R.E.; Alpers, C.N.; Jambor, J.L. Alunite-jarosite crystallography, thermodynamics and geochronology. Rev. Mineral. Geochem. 2000, 40, 453–479. [Google Scholar] [CrossRef]
- Küçük, A.; Gülaboǧlu, M.Ş. Thermal decomposition of Şaphane alunite ore. Ind. Eng. Chem. Res. 2002, 41, 6028–6032. [Google Scholar] [CrossRef]
- Küçük, A.; Gülaboǧlu, M.Ş.; Bayrakcüeken, S. Dehydration kinetics of Şebinkarahisar (Gedehor) alunite ore in a fluidized-bed reactor. Ind. Eng. Chem. Res. 2004, 43, 962–968. [Google Scholar] [CrossRef]
- Küçük, F.; Yildiz, K. The decomposition kinetics of mechanically activated alunite. Thermochim. Acta 2006, 448, 107–110. [Google Scholar] [CrossRef]
- Frost, R.L.; Wain, D.L.; Wills, R.A.; Musemeci, A.; Martens, W. A thermogravimetric study of the alunites of sodium, potassium and ammonium. Thermochim. Acta 2006, 443, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Wain, D. A thermogravimetric and infrared emission spectroscopic study of alunite. J. Therm. Anal. Calorim. 2008, 91, 267–274. [Google Scholar] [CrossRef]
- Kristóf, J.; Frost, R.L.; Palmer, S.J.; Horváth, E.; Jakab, E. Thermoanalytical studies of natural potassium, sodium and ammonium alunites. J. Therm. Anal. Calorim. 2010, 100, 961–966. [Google Scholar] [CrossRef]
- Mohammadi, M.; Salarirad, M.M. Kinetics of direct leaching of natural alunite in KOH. Ind. Eng. Chem. Res. 2013, 52, 14359–14365. [Google Scholar] [CrossRef]
- Ozacar, M.; Sengil, I. Optimum conditions for leaching calcined alunite ore in strong NaOH. Can. Metall. Q. 1999, 38, 249–255. [Google Scholar] [CrossRef]
- Ozdemir, M.; Cetisli, H. Extraction kinetics of alunite in sulfuric acid and hydrochloric acid. Hydrometallurgy 2005, 76, 217–224. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, X.; Zhong, S.; Zhu, Y.; Yang, X.; Yi, L.; Li, G.; Song, J.; Yu, H.; Ruan, R.; et al. Extraction of Al and K salts from associated alunite tailings by an acid calcination-water leaching method. J. Clean. Prod. 2015, 107, 786–792. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, J.; Meng, L.; Guo, Z. Phase transformation and non-isothermal kinetics studies on thermal decomposition of alunite. J. Alloys Compd. 2017, 710, 182–190. [Google Scholar] [CrossRef]
- Clarke, L.; Partridge, E.P. Potassium sulfate from syngenite by high-temperature extraction with water. Ind. Eng. Chem. Res. 1934, 26, 897–903. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, J.; Chen, P.; Guo, Z. Recovery of potassium from K-feldspar by thermal decomposition with flue gas desulfurization gypsum and CaCO3: Analysis of mechanism and kinetics. Energy Fuels 2017, 31, 699–707. [Google Scholar] [CrossRef]
- Wen, L.; Liang, W.X.; Zhang, Z.G.; Huang, J.C. The Infrared Spectroscopy of Minerals; Chongqing University Press: Chongqing, China, 1988. [Google Scholar]
- Lane, M.D. Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. Am. Mineral. 2007, 92, 1–18. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Priya, G.K.; Padmaja, P.; Warrier, K.G.K.; Damodaran, A.D.; Aruldhas, G. Dehydroxylation and high temperature phase formation in sol-gel boehmite characterized by fourier transform infrared spectroscopy. J. Mater. Sci. Lett. 1997, 16, 1584–1587. [Google Scholar] [CrossRef]
- Aronne, A.; Esposito, S.; Pernice, P. FTIR and DTA study of lanthanum aluminosilicate glasses. Mater. Chem. Phys. 1997, 51, 163–168. [Google Scholar] [CrossRef]
- Navrotsky, A.; Geisinger, K.L.; McMillan, P.F.; Gibbs, G.V. The tetrahedral framework in glasses and melts: Influence from molecular orbital calculations and implications for structure, thermodynamics and physical properties. Phys. Chem. Miner. 1985, 11, 284–298. [Google Scholar] [CrossRef]
- Barashkov, M.V.; Komyak, A.I.; Shashkov, S.N. Vibrational spectra and structure of potassium alum. J. Appl. Spectrosc. 2004, 71, 328–333. [Google Scholar] [CrossRef]
- Criado, J.; Morales, J. On the thermal decomposition mechanism for dehydroxylation of alkaline-earth hydroxides. J. Thermal Anal. 1976, 10, 103–110. [Google Scholar] [CrossRef]
- Ma, L.; Ning, P.; Zheng, S.; Niu, X.; Zhang, W.; Du, Y. Reaction mechanism and kinetic analysis of the decomposition of phosphogypsum via a solid-state reaction. Ind. Eng. Chem. Res. 2010, 49, 3597–3602. [Google Scholar] [CrossRef]




| Component | K2O | SO3 | CaO | Al2O3 | Na2O | SiO2 | Fe2O3 |
|---|---|---|---|---|---|---|---|
| Content (wt %) | 40.42 | 47.23 | 7.33 | 3.61 | 1.07 | 0.27 | 0.07 |
| Element (atom %) | Point 1 | Point 2 | Point 3 |
|---|---|---|---|
| K | 7.98 | 14.06 | - |
| Ca | 0.38 | 17.05 | 23.21 |
| Al | 30.88 | 1.27 | - |
| S | 8.89 | 20.46 | 18.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Qiu, X.; Gao, J.; Meng, L.; Guo, Z. Recovery of Soluble Potassium from Alunite by Thermal Decomposition: Effect of CaO and Phase Transformation. Metals 2019, 9, 337. https://doi.org/10.3390/met9030337
Zhong Y, Qiu X, Gao J, Meng L, Guo Z. Recovery of Soluble Potassium from Alunite by Thermal Decomposition: Effect of CaO and Phase Transformation. Metals. 2019; 9(3):337. https://doi.org/10.3390/met9030337
Chicago/Turabian StyleZhong, Yiwei, Xinle Qiu, Jintao Gao, Long Meng, and Zhancheng Guo. 2019. "Recovery of Soluble Potassium from Alunite by Thermal Decomposition: Effect of CaO and Phase Transformation" Metals 9, no. 3: 337. https://doi.org/10.3390/met9030337




