Microstructure and Corrosion Resistance to H2S in the Welded Joints of X80 Pipeline Steel
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Microstructure and Micro-Morphologies of Corrosion Product Observations
2.3. Electrochemical Experiments
3. Results and Discussion
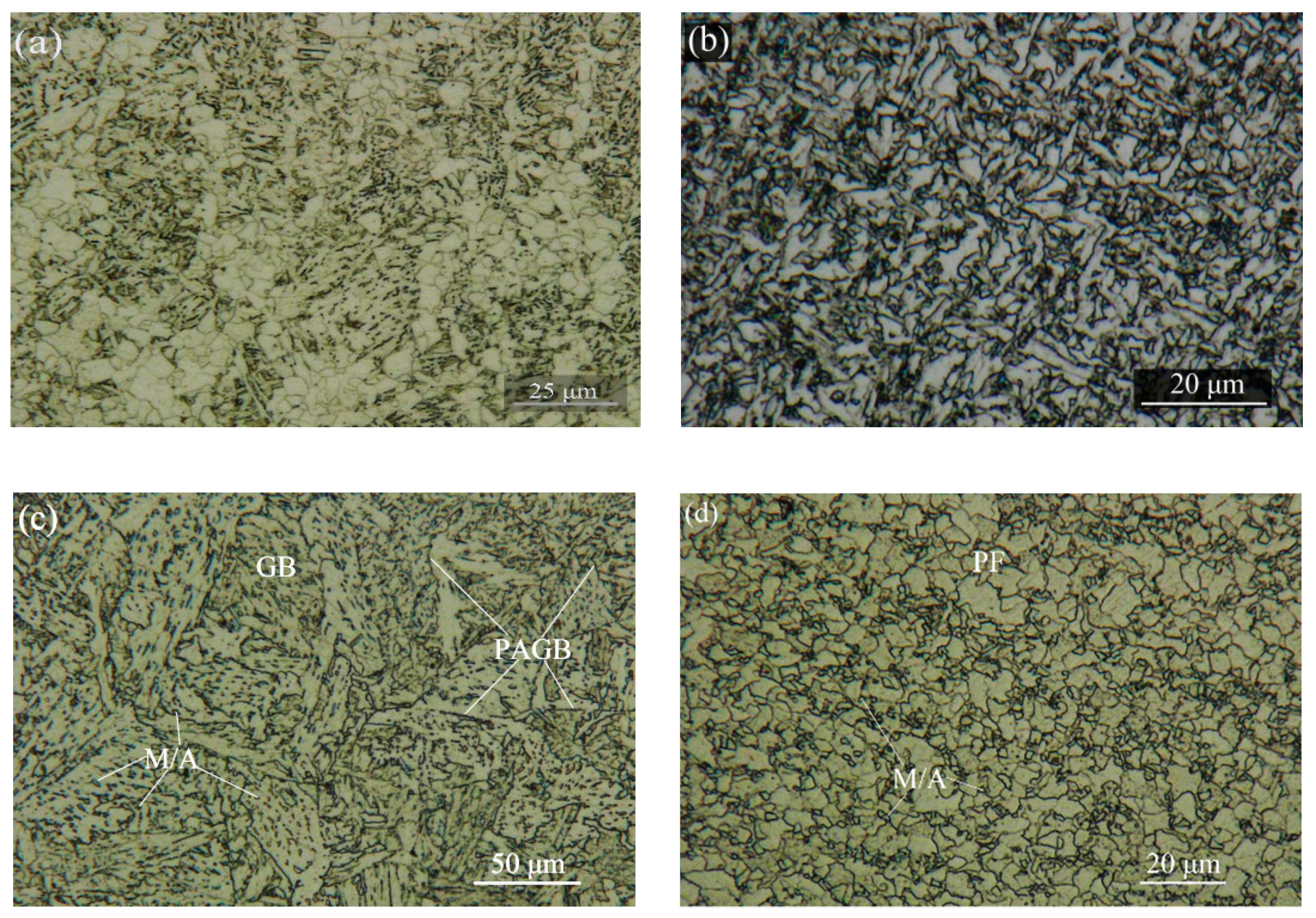
3.1. Microstructure Evolution
3.2. Corrosion Resistance Analysis in H2S Environments
3.2.1. Open Circuit Potential (OCP)
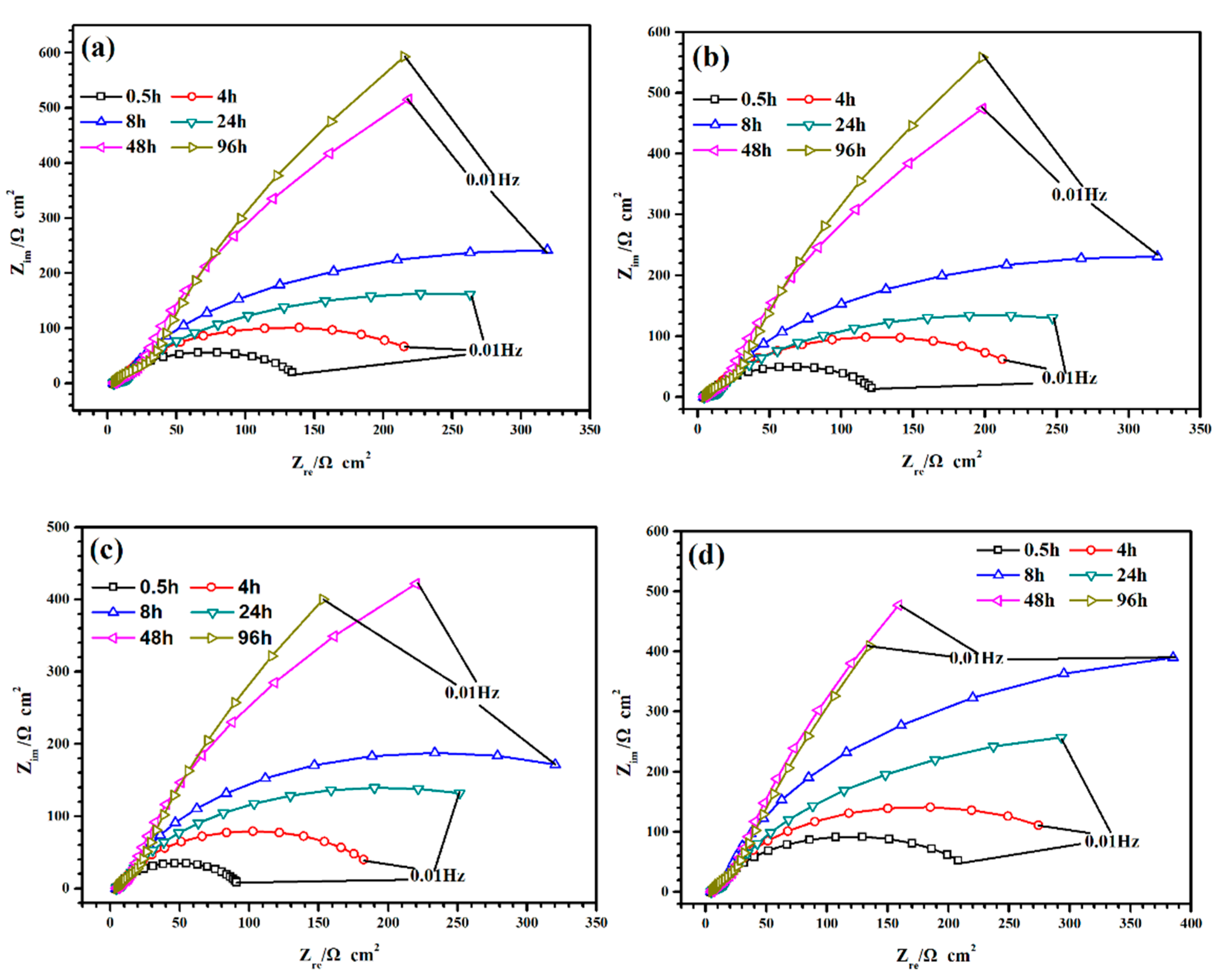
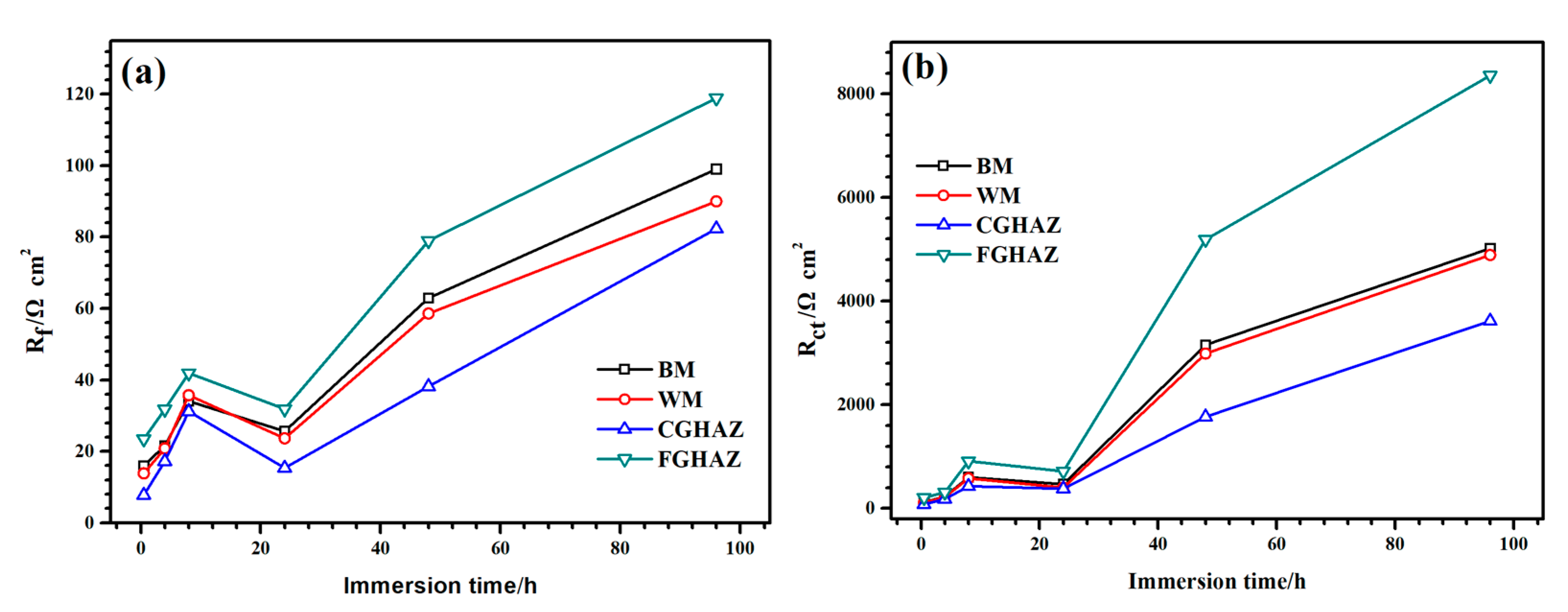
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
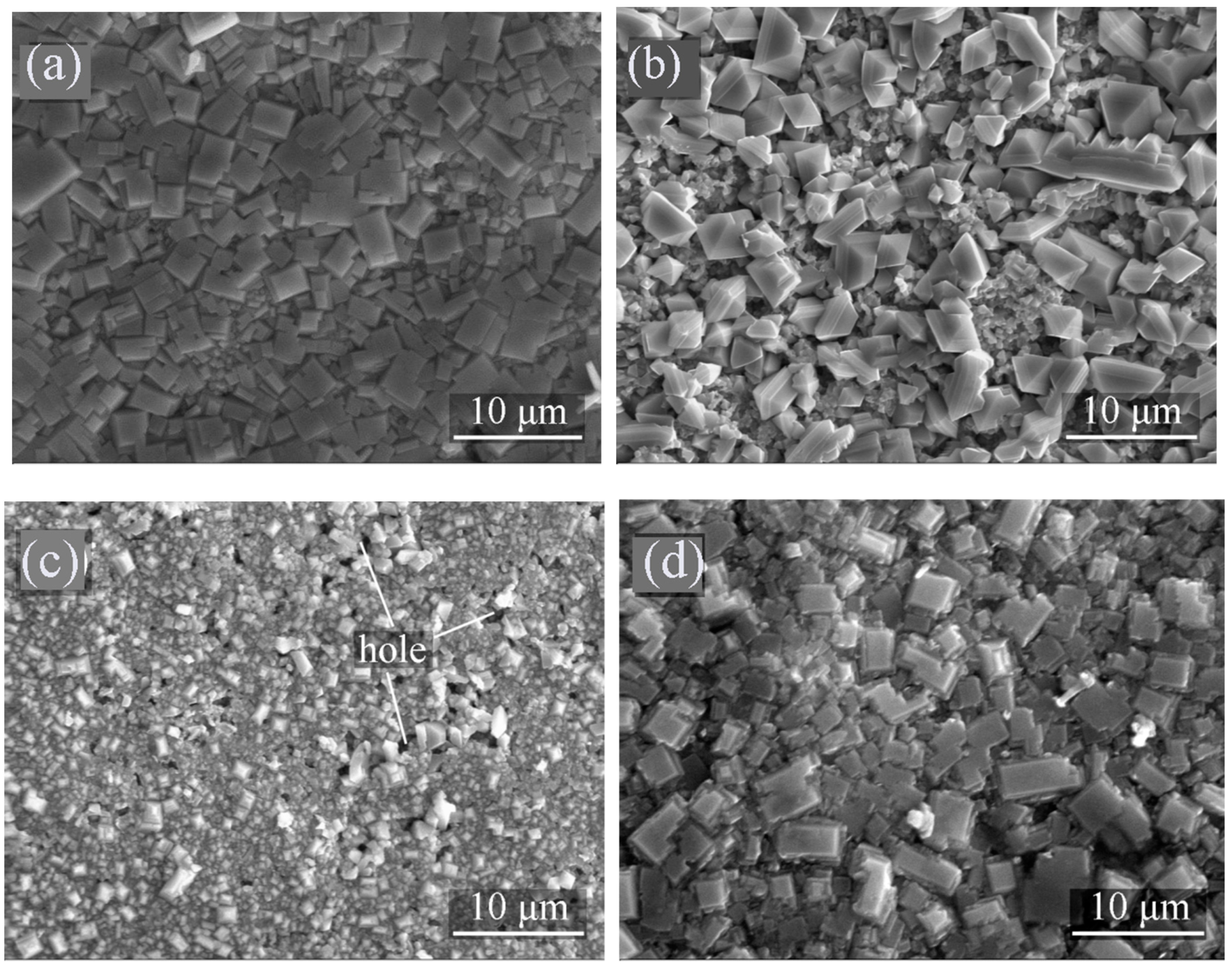
3.2.3. Characterization of the Corrosion Product Films
3.2.4. Analysis of the Differences in Corrosion Resistance for Different Zones
4. Conclusions
- (1)
- In the X80 pipeline steel submerged arc welded joint, the microstructure of BM, WM, CGHAZ, and FGHAZ was mainly PF + GB, AF, GB, PF + M/A constituents with fine grains, respectively.
- (2)
- The WM showed the highest hardness, and the HAZ near the BM showed the lowest hardness in the welded joint.
- (3)
- The CGHAZ showed the lowest open circuit potential, indicating that it would be attacked preferentially when the welded joint is immersed in the electrolyte as a whole.
- (4)
- In the H2S environments, the order of corrosion resistance of X80 pipeline steel submerged arc welded joints was FGHAZ > BM> WM > CGHAZ. The CGHAZ was the weak zone of corrosion resistance of the welded joint.
- (5)
- The composition and structure of the corrosion products were not affected by the difference of composition and crystal structure of the welded joint. But the micromorphology was quite different, which was the reason for the difference of corrosion resistance.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sha, Q.; Li, D. Microstructure, mechanical properties and hydrogen induced cracking susceptibility of X80 pipeline steel with reduced Mn content. Mater. Sci. Eng. A 2013, 585, 214–221. [Google Scholar] [CrossRef]
- Jia, Y.Z.; Wang, J.Q.; Han, E.H.; Ke, W. Stress corrosion cracking of X80 pipeline steel in near-neutral pH environment under constant load tests with and without preload. J. Mater. Sci. Technol. 2011, 27, 1039–1046. [Google Scholar] [CrossRef]
- Zhou, C.; Zheng, S.; Chen, C.; Lu, G. The effect of the partial pressure of H2S on the permeation of hydrogen in low carbon pipeline steel. Corros. Sci. 2013, 67, 184–192. [Google Scholar] [CrossRef]
- Al-Mansour, M.; Alfantazi, A.M.; El-Boujdaini, M. Sulfide stress cracking resistance of API-X100 high strength low alloy steel. Mater. Des. 2009, 30, 4088–4094. [Google Scholar] [CrossRef]
- Huang, F.; Liu, J.; Deng, Z.J.; Cheng, J.H.; Lu, Z.H.; Li, X.G. Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel. Mater. Sci. Eng. A 2010, 527, 6997–7001. [Google Scholar] [CrossRef]
- Qiu, Z.; Xiong, C.; Chang, Z.; Zhao, Z.; Zhao, C.; Ye, Z. Major corrosion factors in the CO2 and H2S coexistent environment and the relative anti-corrosion method: Taking Tazhong I gas field, Tarim Basin, as an example. Petrol. Explor. Dev. 2012, 39, 256–260. [Google Scholar] [CrossRef]
- Bi, F.Q.; Zhang, X.Y.; Yong, W.; Sun, L.L.; Yang, W. Research on Stress Corrosion Behavior of X70 Pipeline Steel in H2S Environment. J. Mater. Eng. 2009, 8, 18–23. [Google Scholar]
- Li, W.F.; Zhou, Y.J.; Xue, Y. Corrosion Behavior of 110S Tube Steel in Environments of High H2S and CO2 Content. J. Iron. Steel Res. Int. 2012, 19, 59–65. [Google Scholar] [CrossRef]
- Fei, X.D.; Ming, W. Effect of dissolved oxygen on corrosion behavior of X80 pipeline steel and its mechanism. J. Iron. Steel Res. Int. 2015, 27, 60–65. [Google Scholar]
- Ren, C.; Liu, D.; Bai, Z.; Li, T. Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater. Chem. Phys. 2005, 93, 305–309. [Google Scholar] [CrossRef]
- Han, Y.D.; Jing, H.Y.; Xu, L.Y. Welding heat input effect on the hydrogen permeation in the X80 steel welded joints. Mater. Chem. Phys. 2012, 132, 216–222. [Google Scholar] [CrossRef]
- Wang, H.K.; Luu, W.C.; Ho, K.F.; Wu, J.K. Hydrogen permeation in a submerged arc weldment of TMCP steel. Mater. Chem. Phys. 2003, 77, 447–454. [Google Scholar] [CrossRef]
- Bai, P.; Zheng, S.; Zhao, H.; Ding, Y.; Wu, J.; Chen, C. Investigations of the diverse corrosion products on steel in a hydrogen sulfide environment. Corros. Sci. 2014, 87, 397–406. [Google Scholar] [CrossRef]
- Ozekmekci, M.; Salkic, G.; Fellah, M.F. Use of zeolites for the removal of H2S: A mini-review. Fuel Process. Technol. 2015, 139, 49–60. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Z.Y.; Cui, Z.Y.; Du, C.W.; Wang, X.H.; Li, X.G. In situ corrosion characterization of simulated weld heat affected zone on API X80 pipeline steel. Corros. Sci. 2014, 85, 401–410. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Corrosion of the Heat-Affected Zones (HAZs) of API-X100 pipeline steel in dilute bicarbonate solutions at 90 °C-An electrochemical evaluation. Corros. Sci. 2013, 74, 297–307. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, L.; Feng, Z.; Frankel, G.S.; Lu, M.; Chang, W. Galvanic corrosion of a welded joint in 3Cr low alloy pipeline steel. Corros. Sci. 2016, 111, 391–403. [Google Scholar] [CrossRef]
- Wang, P.; Lv, Z.; Zheng, S.; Qi, Y.; Wang, J.; Zheng, Y. Tensile and impact properties of X70 pipeline steel exposed to wet H2S environments. Int. J. Hydrogen Energy 2015, 40, 11514–11521. [Google Scholar] [CrossRef]
- Papadakis, G. Major hazard pipelines: A comparative study of onshore transmission accidents. J. Loss Prev. Process Ind. 1999, 12, 91–107. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Z.Y.; Zhao, J.; Ju, D.; Yu, Z.G. Research of Corrosion Resistance of Welded API X80 Steel. Mater. Port. 2019, 52, 44–47. (In Chinese) [Google Scholar]
- Xing, Y.Y.; Liu, Z.Y.; Du, C.W.; Li, X.G.; Liu, R.K.; Zhu, M. Influence of H2S concentration and pH value on corrosion behavior of weld joint of X65 subsea pipeline steel. J. Chin. Soc. Corros. Prot. 2014, 34, 231–236. [Google Scholar]
- Hemmingsen, T.; Hovdan, H.; Sanni, P.; Aagotnes, N.O. The influence of electrolyte reduction potential on weld corrosion. Electrochim. Acta 2002, 47, 3949–3955. [Google Scholar] [CrossRef]
- Huang, H.H.; Wen, T.T.; Ju, T.L. The influences of microstructure and composition on the electrochemical behaviour of a516 steel weldment. Corros. Sci. 1994, 36, 1027–1038. [Google Scholar] [CrossRef]
- Bordbar, S.; Alizadeh, M.; Hashemi, S.H. Effects of microstructure alteration on corrosion behavior of welded joint in API X70 pipeline steel. Mater. Des. 2013, 45, 597–604. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bordbar, S. The influence of microstructure on the protective properties of the corrosion product layer generated on the welded API X70 steel in chloride solution. Corros. Sci. 2013, 70, 170–179. [Google Scholar] [CrossRef]
- Kong, D.J.; Wu, Y.Z.; Long, D. Stress Corrosion of X80 Pipeline Steel Welded Joints by Slow Strain Test in NACE H2S Solutions. J. Iron. Steel Res. Int. 2013, 20, 40–46. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization of corrosion of welded X70 pipeline steel in near-neutral pH solution. Corros. Sci. 2009, 51, 1714–1724. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, Y.; Matsuda, K.; Zou, Z. Corrosion behavior of reheated CGHAZ of X80 pipeline steel in H2S-containing environments. Mater. Des. 2016, 99, 44–56. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization and Mott–Schottky analysis of corrosion of welded X70 pipeline steel in carbonate/bicarbonate solution. Electrochim. Acta 2009, 55, 316–324. [Google Scholar] [CrossRef]
- Bai, P.; Zhou, H.; Zheng, S.; Chen, C. Initiation and development stages of steel corrosion in wet H2S environments. Corros. Sci. 2015, 93, 109–119. [Google Scholar] [CrossRef]
- Qi, Y.; Luo, H.; Zheng, S.; Chen, C.; Lv, Z.; Xiong, M. Effect of H2S partial pressure on the tensile properties of A350LF2 steel in the absence and presence of pre-immersion. Mater. Sci. Eng. A 2014, 609, 161–167. [Google Scholar] [CrossRef]
- Lucio-Garcia, M.A.; Gonzalez-Rodriguez, J.G.; Casales, M.; Martinez, L.; Chacon-Nava, J.G.; Neri-Flores, M.A. Effect of heat treatment on H2S corrosion of a micro-alloyed C-Mn steel. Corros. Sci. 2009, 51, 2380–2386. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175. [Google Scholar] [CrossRef]
- Bai, P.; Zheng, S.; Chen, C. Electrochemical characteristics of the early corrosion stages of API X52 steel exposed to H2S environments. Mater. Chem. Phys. 2015, 149–150, 295–301. [Google Scholar] [CrossRef]
- Rickard, D.; Morse, J.W. Acid volatile sulfide (AVS). Mar. Chem. 2005, 97, 141–197. [Google Scholar] [CrossRef]
- Andrzej, A.; Patrick, J.S. A computational approach to predicting the formation of iron sulfide species using stability diagrams. Comput. Geosci. 1997, 23, 647–658. [Google Scholar]
- Zhou, C.; Chen, X.; Wang, Z.; Zheng, S.; Li, X.; Zhang, L. Effects of environmental conditions on hydrogen permeation of X52 pipeline steel exposed to high H2S-containing solutions. Corros. Sci. 2014, 89, 30–37. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Matsuda, K.; Zou, Z. Characterization of the effects of hydrogen sulfide on the corrosion of X80 pipeline steel in saline solution. Corros. Sci. 2016, 102, 455–468. [Google Scholar] [CrossRef]
- Chen, X.W.; Liao, B.; Qiao, G.Y.; Gu, Y.; Wang, X.; Xiao, F.R. Effect of Nb on mechanical properties of HAZ for high-Nb X80 pipeline steels. J. Iron. Steel Res. Int. 2013, 20, 53–60. [Google Scholar] [CrossRef]



| Material | C | Si | Mn | Al | V | Ni | Cr | Mo | Ti | Cu | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM | 0.046 | 0.305 | 1.760 | 0.058 | 0.008 | 0.225 | 0.023 | 0.226 | 0.015 | 0.215 | 0.079 |
| WM | 0.062 | 0.380 | 1.830 | 0.030 | 0.004 | 0.190 | 0.204 | 0.090 | 0.013 | 0.200 | 0.040 |
| Material | BM | WM | CGHAZ | FGHAZ |
|---|---|---|---|---|
| Fe (at %) | 44.82 (1.21) | 46.83 (1.26) | 45.12 (1.15) | 45.83 (1.28) |
| S (at %) | 55.18 (1.21) | 53.17 (1.26) | 54.88 (1.15) | 54.17 (1.28) |
| Fe/S | 1.23 (0.11) | 1.14 (0.13) | 1.22 (0.09) | 1.18 (0.15) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-B.; Xiao, G.-C.; Zhao, W.; Zhang, B.-R.; Rao, W.-F. Microstructure and Corrosion Resistance to H2S in the Welded Joints of X80 Pipeline Steel. Metals 2019, 9, 1325. https://doi.org/10.3390/met9121325
Wang J-B, Xiao G-C, Zhao W, Zhang B-R, Rao W-F. Microstructure and Corrosion Resistance to H2S in the Welded Joints of X80 Pipeline Steel. Metals. 2019; 9(12):1325. https://doi.org/10.3390/met9121325
Chicago/Turabian StyleWang, Jian-Bao, Guang-Chun Xiao, Wei Zhao, Bing-Rong Zhang, and Wei-Feng Rao. 2019. "Microstructure and Corrosion Resistance to H2S in the Welded Joints of X80 Pipeline Steel" Metals 9, no. 12: 1325. https://doi.org/10.3390/met9121325




