Influences of Alloying Elements on Continuous Cooling Phase Transformation and Microstructures of Extremely Fine Pearlite
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Transformation Microstructure
3.2. Thermal Expansion and CCT Curves
3.3. Lamellar Spacing and Hardness of the Pearlite.
4. Discussion
4.1. Effects of Co and Al on the Structure and Lamellar Spacing of Pearlite
4.2. Effects of Co and Al on Kinetics and Thermodynamics of the Pearlite Transformation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhadeshia, H.K. Bainite in Steels, 2nd ed.; IOM Communications: London, UK, 2001. [Google Scholar]
- Bhadeshia, H.K. Nanostructured bainite. Proc. R. Soc. A 2010, 466, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Caballero, F.G.; Bhadeshia, H.K. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mateo, C.; Caballero, F.G. Ultra-high-strength bainitic steels. ISIJ Int. 2005, 45, 1736–1740. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K. Mechanical Properties of Low-Temperature Bainite. Mater. Sci. Forum. 2005, 500, 495–501. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Beladi, H.; Xiong, X.Y.; Adachi, Y.; Hodgson, P.D. Nanoscale microstructural characterization of a nanobainitic steel. Acta Mater. 2011, 59, 5511–5522. [Google Scholar] [CrossRef]
- Sherif, M.Y. Characterisation and Development of Nanostructured, Ultrahigh Strength and Ductile Bainitic Steels. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2006. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K. Acceleration of low-temperature bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Jaramillo, R.A.; Babu, S.S.; Ludtka, G.M.; Kisner, R.A.; Wilgen, J.B.; Mackiewicz-Ludtka, G.; Nicholson, D.M.; Kelly, S.M.; Murugananth, M.; Bhadeshia, H.K. Effect of 30T magnetic field on transformations in a novel bainitic steel. Scrip. Mater. 2005, 52, 461–466. [Google Scholar] [CrossRef]
- Shi, S.Q. Phase Transformation and Mechanical Properties of Fe-1.5C-1.5Cr-xAl Ultra High Carbon Steel. Ph.D. Thesis, Nanjing University of Science, Nanjing, China, 2006. [Google Scholar]
- Houin, J.P.; Simon, A.; Beck, G. Relationship between Structure and Mechanical Properties of Pearlite between 0.2% and 0.8%C. Trans. ISIJ 1981, 21, 726–731. [Google Scholar] [CrossRef]
- Wang, B.Q.; Song, X.Y.; Li, H.J.; Gu, N.J. Microstructure Ultra-fining Treatment of Ultrahigh Carbon Steel. J. Iron Steel Res. 2004, 16, 5–9. [Google Scholar]
- Sun, S.H.; Xiong, Y.; Fu, W.T.; Xing, G.Z.; Furuhara, T.; Maki, T. Microstructure changes of eutectoid pearlitic steel during cold rolling. Acta Metall. Sin. 2005, 41, 267–270. [Google Scholar]
- Yan, Z.G.; Xue, H.J. Test research on 2000 MPa 7 mm diameter steel wire for hutong Changjiang River bridge. J. China Railw. Soc. 2018, 7, 115–120. [Google Scholar]
- ISO 9042. Steels—Manual Point Counting Method for Statistically Estimating the Volume Fraction of a Constituent with A Point Grid; IHS: Zurich, Switzerland, 1988. [Google Scholar]
- Caballero, F.G.; Capdevila, C.; Garcia de Andres, C. Modeling of the interlamellar spacing of isothermally formed pearlite in a eutectoid steel. Scrip. Mater. 2000, 42, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Peet, M.; Bhadeshia, H.K. Materials Algorithms Project. Available online: www.msm.cam.ac.uk/map/steel/tar/mucg83.exe. (accessed on 11 January 2019).
- Takahashi, M. Reaustenitization from Bainite in Steels. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1992. [Google Scholar]
- Capdevila, C.; Caballero, F.G.; Garcia de Andres, C. Neural network model for isothermal pearlite transformation. Part I: Interlamellar spacing. ISIJ Int. 2005, 45, 229–237. [Google Scholar] [CrossRef]
- Hu, F.; Wu, K.M.; Shirzadi, A.A. Influence of Co and Al on pearlitic transformation in superbainitic steels. Ironmak. Steelmak. 2012, 39, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.M.; Bhadeshia, H.K. Extremely fine pearlite by continuous cooling transformation. Scrip. Mater. 2012, 67, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Straumal, B.B.; Kucheev, Y.O.; Efron, L.I.; Petelin, A.L.; Majumdar, J.D.; Manna, I. Complete and incomplete wetting of ferrite grain boundaries by austenite in the low-alloyed ferritic steel. J. Mater. Eng. Perform. 2012, 21, 667–670. [Google Scholar] [CrossRef]
- Straumal, A.B.; Bokstein, B.S.; Petelin, A.L.; Straumal, B.B.; Baretzky, B.; Rodin, A.O.; Nekrasov, A.N. Apparently complete grain boundary wetting in Cu–In alloys. J. Mater. Sci. 2012, 24, 8336–8343. [Google Scholar] [CrossRef]
- Yi, H.L. Full Pearlite Obtained by Slow Cooling in Medium Carbon Steel. Mater. Sci. Eng. A 2010, 527, 7600–7604. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.; Honeycombe, R.W.K. Steels: Microstructure and Properties, 3rd ed.; Butterworth-Heinemann: London, UK, 2006. [Google Scholar]




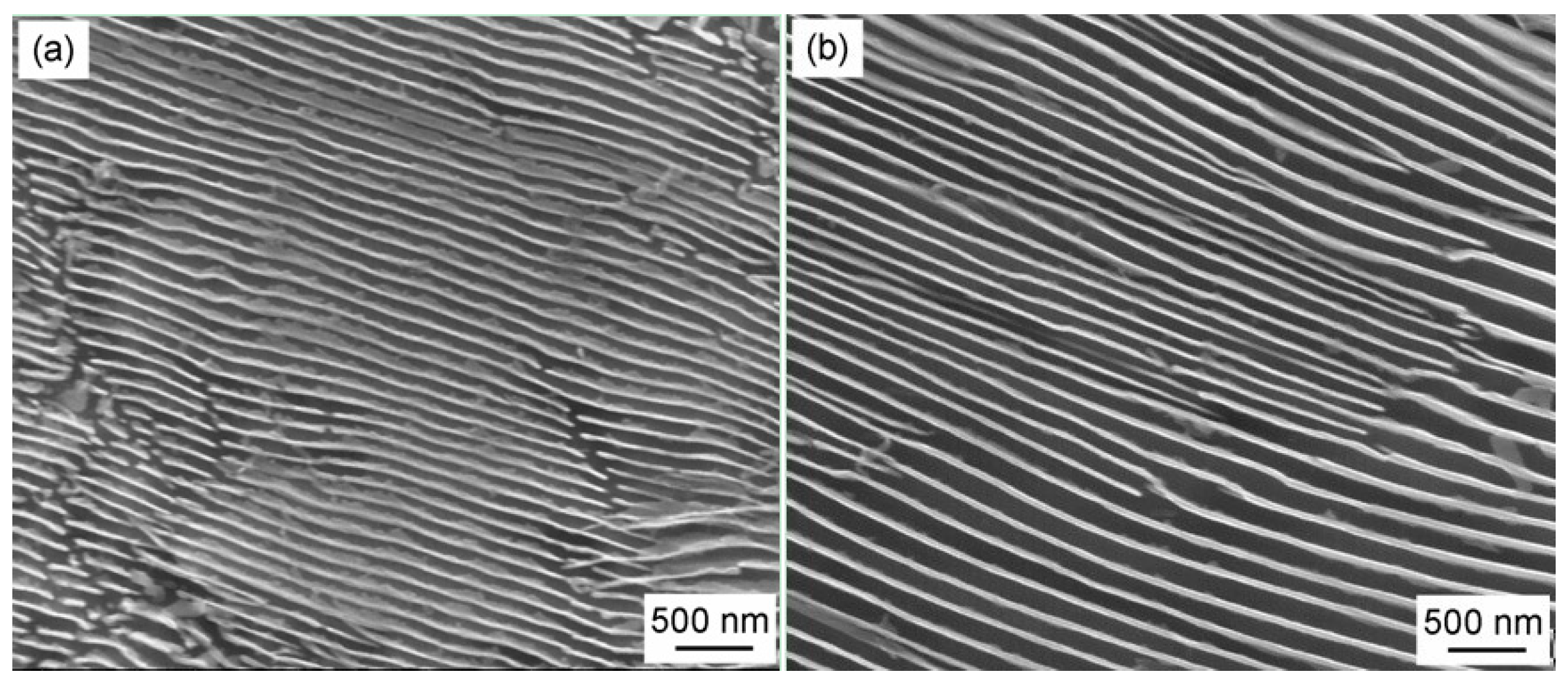
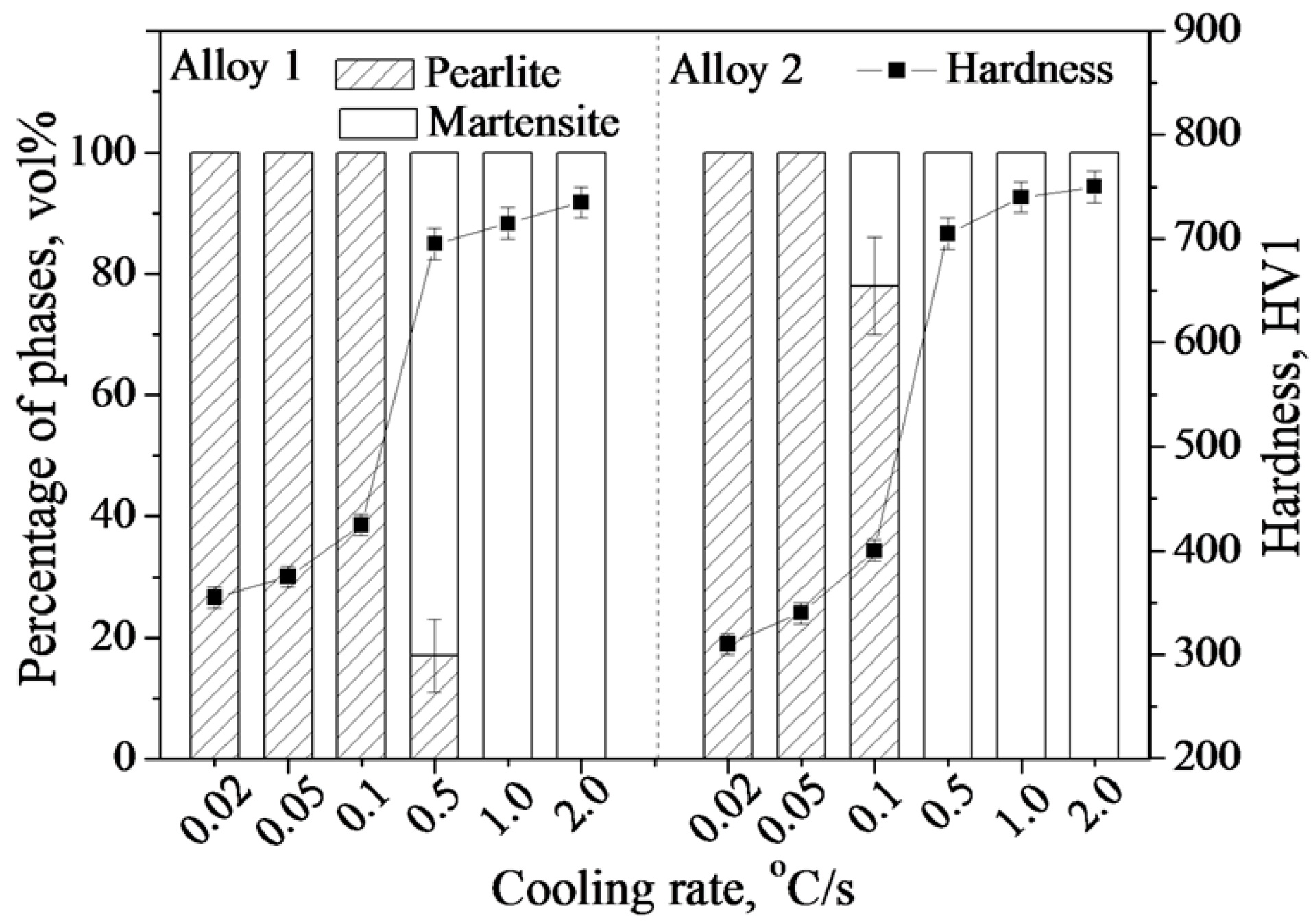
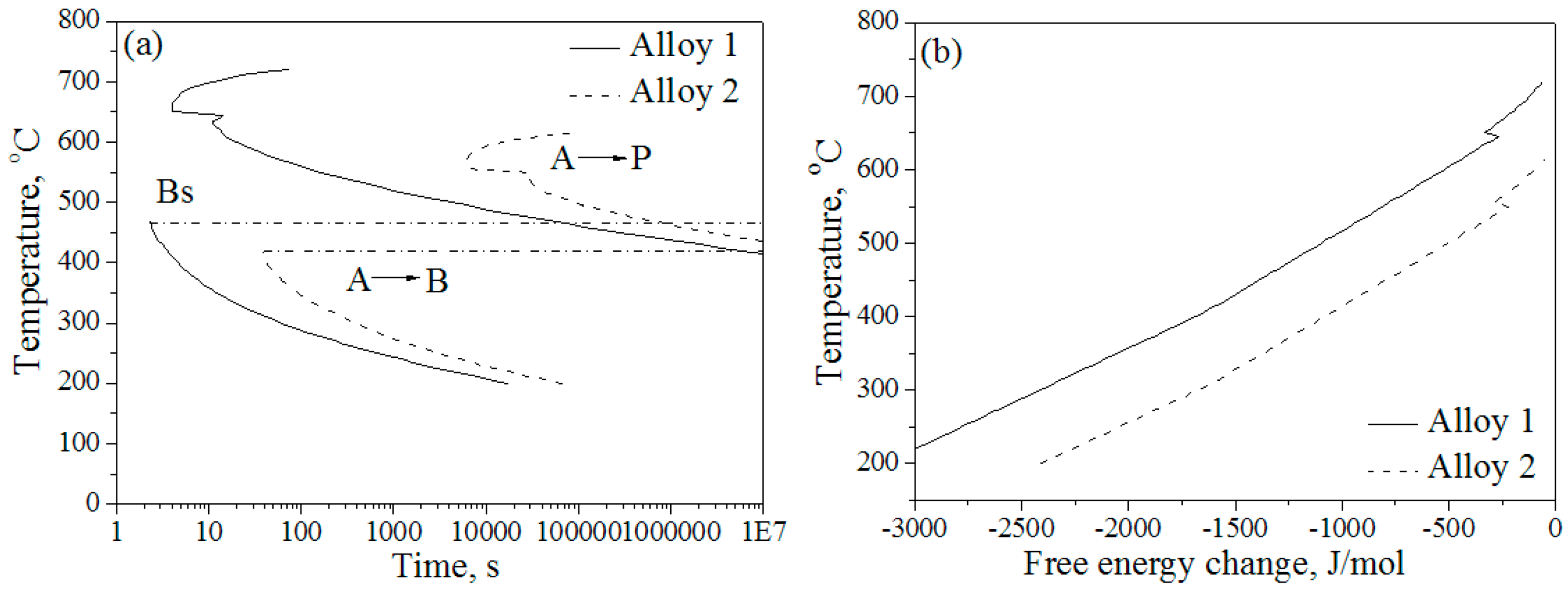


| Alloys | C | Si | Mn | Mo | Cr | Co | Al |
|---|---|---|---|---|---|---|---|
| Alloy 1 | 0.78 | 1.60 | 2.02 | 0.24 | 1.01 | 3.87 | 1.37 |
| Alloy 2 | 0.79 | 1.59 | 1.94 | 0.30 | 1.33 | - | 0.01 |
| Sample | Alloy 1 | Alloy 2 | ||||
|---|---|---|---|---|---|---|
| Cooling Rate, °C/s | 0.02 | 0.05 | 0.1 | 0.02 | 0.05 | 0.1 |
| Interlamellar Spacing, nm | 95 ± 15 | 65 ± 10 | 45 ± 10 | 130 ± 15 | 100 ± 15 | 80 ± 10 |
| Hardness, HV1 | 355 ± 10 | 375 ± 10 | 425 ± 10 | 310 ± 10 | 340 ± 10 | 400 ± 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Hu, F.; Zhou, W.; Ke, R.; Zhang, G.; Wu, K.; Qiao, W. Influences of Alloying Elements on Continuous Cooling Phase Transformation and Microstructures of Extremely Fine Pearlite. Metals 2019, 9, 70. https://doi.org/10.3390/met9010070
Feng L, Hu F, Zhou W, Ke R, Zhang G, Wu K, Qiao W. Influences of Alloying Elements on Continuous Cooling Phase Transformation and Microstructures of Extremely Fine Pearlite. Metals. 2019; 9(1):70. https://doi.org/10.3390/met9010070
Chicago/Turabian StyleFeng, Lulu, Feng Hu, Wen Zhou, Rui Ke, Guohong Zhang, Kaiming Wu, and Wenwei Qiao. 2019. "Influences of Alloying Elements on Continuous Cooling Phase Transformation and Microstructures of Extremely Fine Pearlite" Metals 9, no. 1: 70. https://doi.org/10.3390/met9010070




