Effect of Mg Concentration on the Aluminothermic Reduction of Mn2O3 Particles Obtained from Cathodes of Discharged Alkaline Batteries: Mathematical Modeling and Experimental Results
Abstract
:1. Introduction
ΔG°1073 K = −529.41 kJ
2. Materials and Methods
3. Results and Discussion
3.1. Thermodynamics in the Aluminothermic Reduction Process of Mn2O3
ΔG°1073 K = −1336.71 kJ
ΔG°1073 K = −529.41 kJ
2Al + Mg + Mn3O4 = 3Mn + MgAl2O4
ΔG°1073 K = −423.14 kJ
ΔG°1073 K = −212.40 kJ
ΔG°1073 K = −203.16 kJ
3.2. X-Ray Diffraction of Reaction Products in the Aluminothermic Reduction Process of Mn2O3
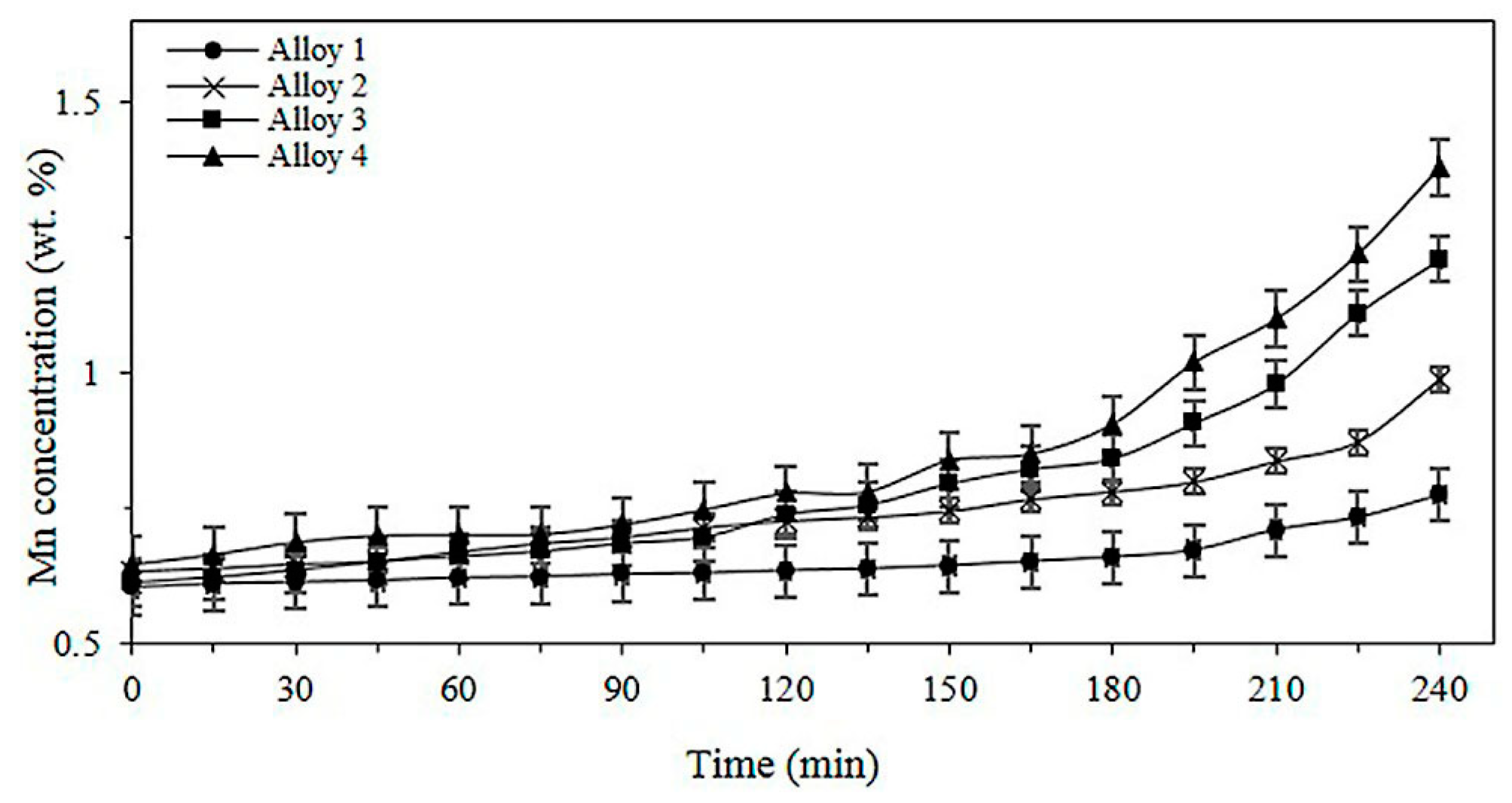
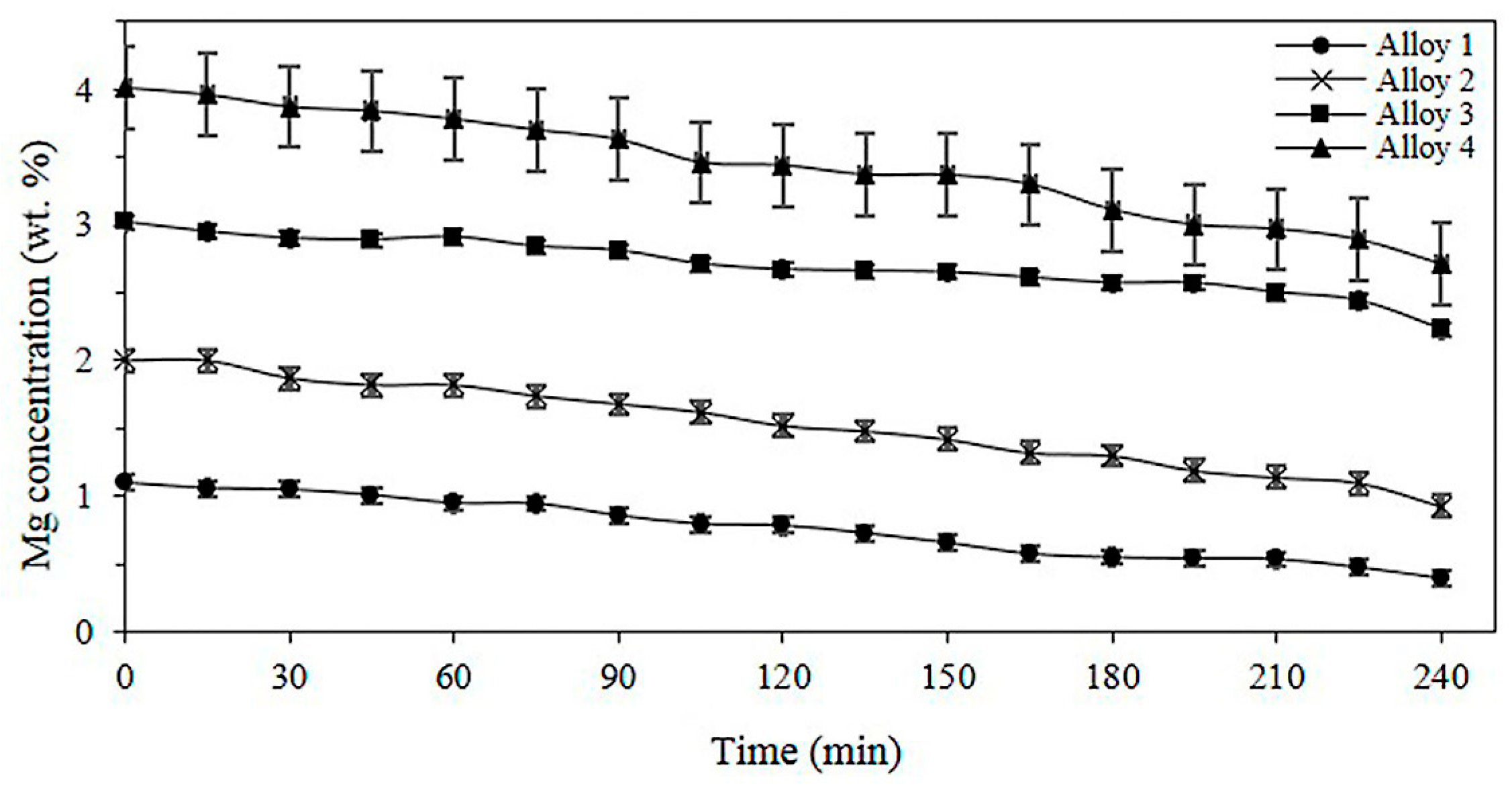
3.3. Effect of Mg on the Aluminothermic Reduction of Solid Particles of Mn2O3
3.4. Kinetics of the Aluminothermic Reduction of Mn2O3 for Different Mg Contents in the Alloy
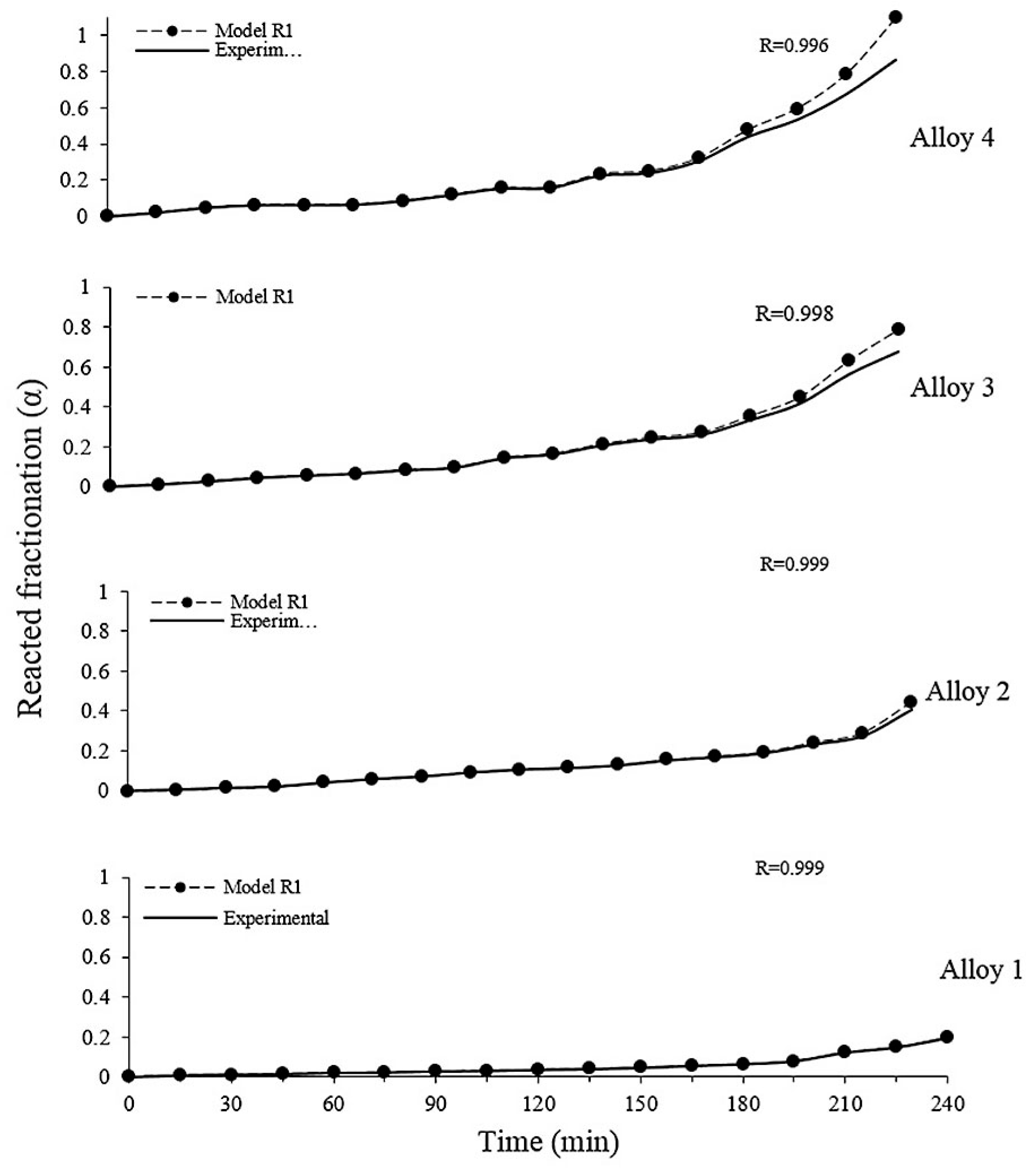
3.5. Mathematical Modelin
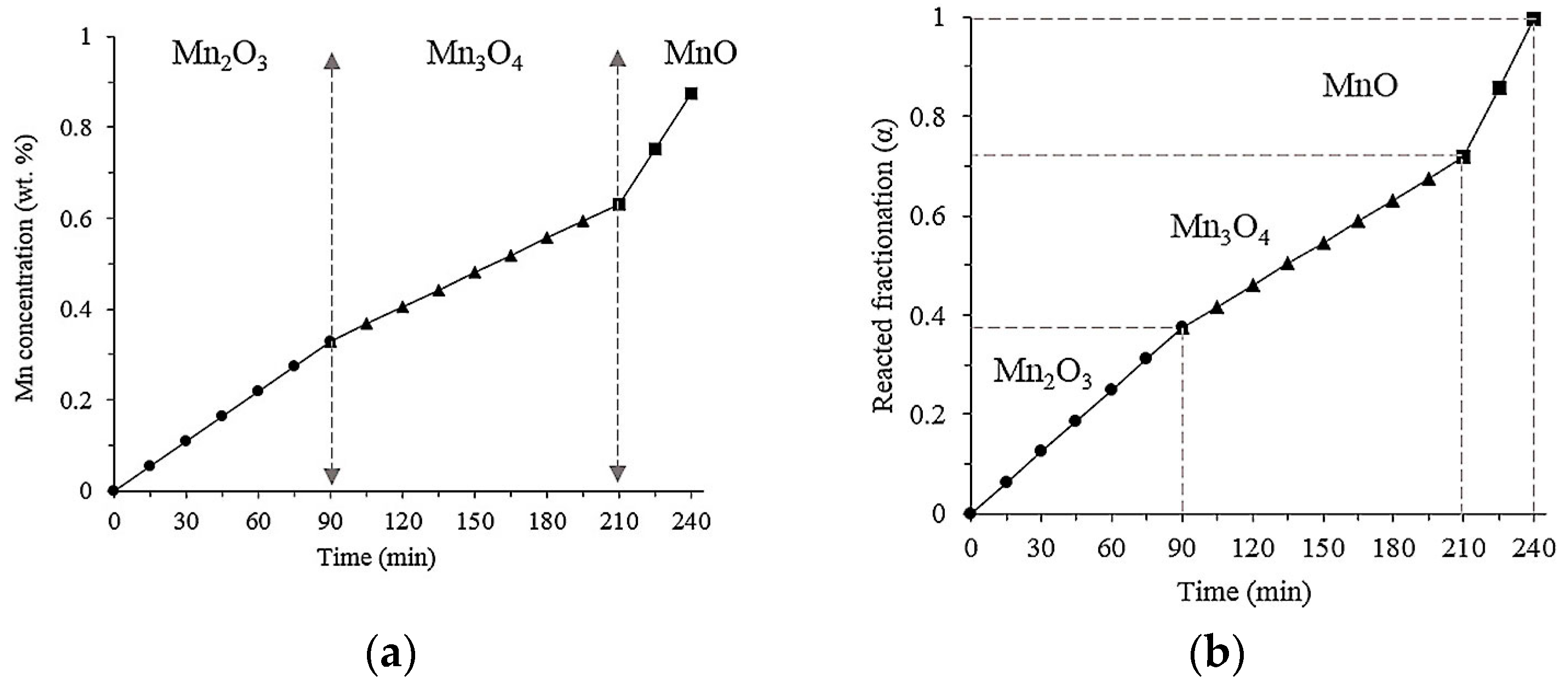
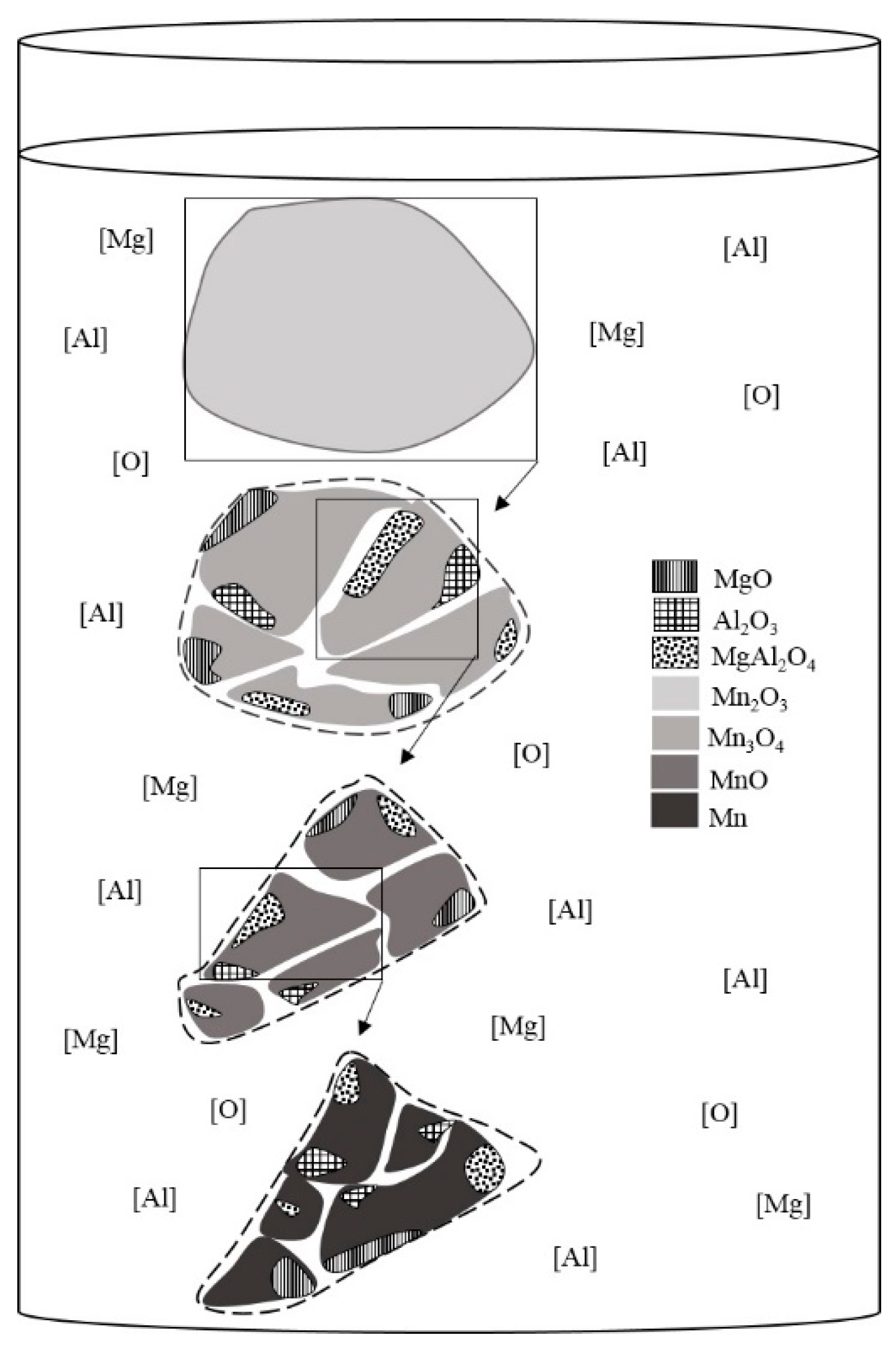
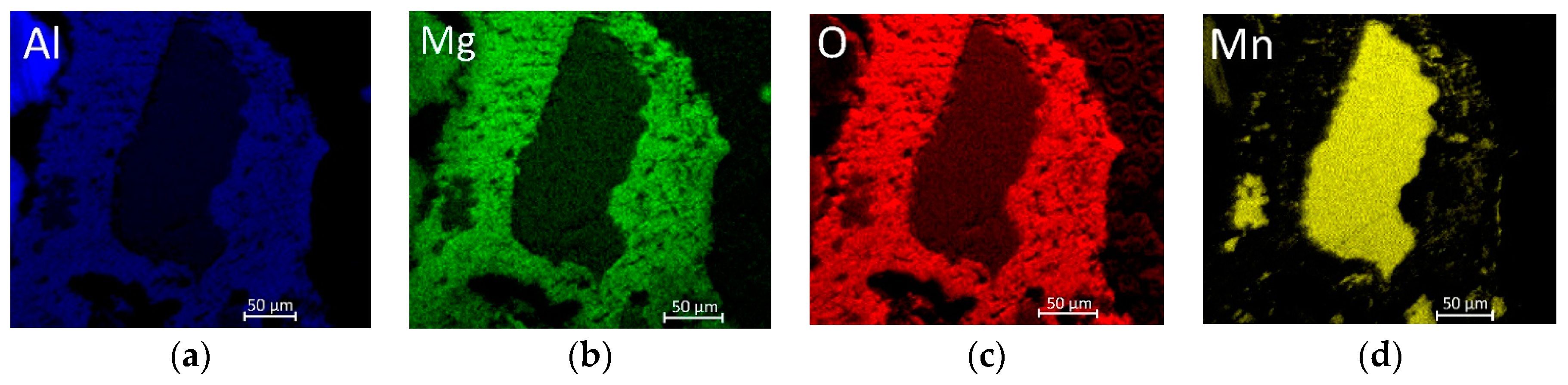
3.6. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castro, J.; Diaz, M.L. La Contaminacion por pilas y Baterias en México. Gaceta Ecologica 2004, 72, 53–70. [Google Scholar]
- Torres, L.G. Recuperacion de Zinc y Manganeso de Baterias Alcalinas Descargadas Utilizando Lixiviacion. Master’s Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2008. [Google Scholar]
- Ochoa, R.M. Utilización de Electrodos de Baterías Alcalinas Descargadas como Materia Prima para la Elaboración de Aleaciones Al-Zn-Mg y Al-Mn’. Master’s Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2009. [Google Scholar]
- Ochoa, R. Relación Microestructura—Propiedades Mecánicas de las Aleaciones Al-Zn-Mg-Cu y Al-Zn-Mg-Cu-1% Li Elaboradas por Reducción Aluminotérmica del Ánodo de Pilas Alcalinas Descargadas y Latas para Bebidas. Ph.D. Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2016. [Google Scholar]
- Luna, S.; Flores, A.; Muñiz, R.; Fernández, A.; Torres, J.; Rodriguez, N.; Ortíz, J.C.; Orozco, P. Cerium extraction by metallothermic reduction using cerium oxide powder injection. J. Rare Earths 2011, 29, 74–76. [Google Scholar] [CrossRef]
- Flores, A.; Ochoa, R.; Torres, J. Elaboration of alloys by aluminothermic reduction of Mn2O3, Aluminium Alloys. Mater. Today Proc. 2015, 2, 4964–4968. [Google Scholar] [CrossRef]
- Coudurier, L. Fundamentals of Metallurgical Processes; Pergamon Press: Oxford, UK, 1985; Volume 27, pp. 128–136. [Google Scholar]
- Langlais, J.; Harris, R. Strontium extraction by aluminothermic reduction. Can. Metall. Q. 1991, 31, 127–131. [Google Scholar] [CrossRef]
- Garcia, C.; Louis, E.; Pamies, A. Surface tension of binary and ternary aluminium alloys of the systems Al-Si-Mg and Al-Zn-Mg. J. Mater. Sci. 1992, 27, 5247–5251. [Google Scholar]
- Barrett, H.A.; Borkiewicz, O.; Krekeler, P.S. An investigation of zincite from spent anodic portions of alkaline batteries: An industrial mineral approach for evaluating stock material for recycling potential. J. Power Sources 2011, 508. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; Mc Graw Hill: New York, NY, USA, 2002. [Google Scholar]
- Candan, E.; Atkinson, H.V.; Turen, Y.; Salaoru, I.; Candan, S. Wettability of Aluminum–Magnesium Alloys on Silicon Carbide Substrates. J. Am. Cerm. Soc. 2011, 94, 867–871. [Google Scholar] [CrossRef]
- Afshar, S.; Allaire, C. Furnaces: Improving low cement castables by non-wetting additives. JOM 2001, 24–27. [Google Scholar] [CrossRef]
- Cotton, A.; Wikinson, G. Química Inórganica Avanzada, 8th ed.; Limusa: México City, México, 1998; pp. 20–29. [Google Scholar]
- Sharpe, A.G. Química inorgánica, 3rd ed.; Reverté: Barcelona, España, 1993; pp. 275–419. [Google Scholar]
- Levenspiel, O. Ingeniería de las Reacciones Químicas, 3rd ed.; Limusa: México City, México, 2015; pp. 369–415. [Google Scholar]
- Rosenquist, T. Cinética de la Reacción en Fundamentos de Metalurgia Extractiva. Limusa, México City, México, 1987, Volume 2, pp. 127–139.
- Romero, M. Expresiones analíticas de los modelos cinéticos para la reducción a temperatura programada en la ecuación general de Kissinger. Minera Geología 2004, 20, 78–86. [Google Scholar]
- Brown, M. Handbook of Thermal Analysis and Calorimetry; Elsevier: New York, NY, USA, 1998; Volume 1, pp. 136–208. [Google Scholar]
- Zhong, W.M.; L’Espérance, G.L.; Suery, M. Interfacial reactions in Al-Mg (5083)/SiCp composites during fabrication and remelting. Metall. Mater. Trans. 1995, 26, 2625–2635. [Google Scholar] [CrossRef]
- Mcleod, A.D.; Gabryel, C.M. Kinetics of the growth of spinel, MgAl2O4, on alumina particulate in aluminum alloys containing magnesium on. Metall. Mater. Trans. A 1992, 23, 1279–1283. [Google Scholar] [CrossRef]
- HSC Chemistry Software, Version 6.12. Outotec, 2007. Available online: https://www.outotec.com (accessed on 9 July 2018).




| Materials | Si | Fe | Cu | Mn | Mg | Ni | Zn | Al |
|---|---|---|---|---|---|---|---|---|
| Aluminum cans | 0.26 | 0.64 | 0.24 | 0.62 | 1.07 | 0.09 | 0.10 | Balance |
| Mg Scrap | 0.01 | 0.01 | - | - | Balance | - | 0.02 | 3.28 |
| Alloys of Al | Si | Fe | Cu | Mn | Mg | Ni | Zn | Al |
|---|---|---|---|---|---|---|---|---|
| Alloy 1 | 0.26 | 0.64 | 0.24 | 0.62 | 1.07 | 0.09 | 0.10 | Balance |
| Alloy 2 | 0.26 | 0.63 | 0.24 | 0.62 | 2.05 | 0.09 | 0.10 | Balance |
| Alloy 3 | 0.25 | 0.62 | 0.24 | 0.60 | 3.02 | 0.09 | 0.10 | Balance |
| Alloy 4 | 0.25 | 0.62 | 0.23 | 0.59 | 4.01 | 0.09 | 0.10 | Balance |
| Treatment Time (min) | Aggregate Quantity A1 Mn2O3 (g) | Grams Mn in Mn2O3 (β Mn2O3) | Mn Dissolved in Alloy θ (wt %) | Dissolved Mn Increase (wt %) | Reacted Fraction (Mn2O3) | Reacted Fraction by Applying the R1 Model |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 0.00 | 0.00 | 0.62 | 0.00 | 0.00 |
| 15 | 7.88 | 2.74 | 0.05 | 0.67 | 0.06 | 0.06 |
| 30 | 15.75 | 5.48 | 0.11 | 0.73 | 0.12 | 0.13 |
| 45 | 23.63 | 8.22 | 0.16 | 0.78 | 0.19 | 0.19 |
| 60 | 31.50 | 10.96 | 0.22 | 0.84 | 0.25 | 0.26 |
| 75 | 39.38 | 13.70 | 0.27 | 0.89 | 0.31 | 0.33 |
| 90 | 47.25 | 16.44 | 0.33 | 0.95 | 0.37 | 0.40 |
| 105 | 55.13 | 19.19 | 0.38 | 1.00 | 0.44 | 0.48 |
| 120 | 63.00 | 21.93 | 0.44 | 1.06 | 0.50 | 0.55 |
| 135 | 70.88 | 24.67 | 0.49 | 1.11 | 0.56 | 0.63 |
| 150 | 78.75 | 27.41 | 0.55 | 1.17 | 0.62 | 0.72 |
| 165 | 86.63 | 30.15 | 0.60 | 1.22 | 0.69 | 0.81 |
| 180 | 94.50 | 32.89 | 0.66 | 1.28 | 0.75 | 0.90 |
| 195 | 102.38 | 35.63 | 0.71 | 1.33 | 0.81 | 1.00 |
| 210 | 110.25 | 38.37 | 0.77 | 1.39 | 0.87 | 1.12 |
| 225 | 118.13 | 41.11 | 0.82 | 1.44 | 0.93 | 1.26 |
| 240 | 126.00 | 43.85 | 0.88 | 1.50 | 1.00 | 1.47 |
| Treatment Time (min) | Aggregate Quantity Mn3O4 (g) | Grams Mn in Mn3O4 (β Mn3O4) | Mn Dissolved in Alloy θ (wt %) | Dissolved Mn Increase (wt %) | Reacted Fraction (Mn3O4) | Reacted Fraction by Applying the R1 Model |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 0.00 | 0.00 | 0.62 | 0.00 | 0.00 |
| 15 | 7.88 | 1.89 | 0.04 | 0.66 | 0.04 | 0.04 |
| 30 | 15.75 | 3.78 | 0.08 | 0.70 | 0.09 | 0.09 |
| 45 | 23.63 | 5.67 | 0.11 | 0.73 | 0.13 | 0.13 |
| 60 | 31.50 | 7.56 | 0.15 | 0.77 | 0.17 | 0.18 |
| 75 | 39.38 | 9.46 | 0.19 | 0.81 | 0.21 | 0.22 |
| 90 | 47.25 | 11.35 | 0.23 | 0.85 | 0.26 | 0.27 |
| 105 | 55.13 | 13.24 | 0.26 | 0.88 | 0.30 | 0.32 |
| 120 | 63.00 | 15.13 | 0.30 | 0.92 | 0.34 | 0.37 |
| 135 | 70.88 | 17.02 | 0.34 | 0.96 | 0.39 | 0.42 |
| 150 | 78.75 | 18.91 | 0.38 | 1.00 | 0.43 | 0.47 |
| 165 | 86.63 | 20.80 | 0.42 | 1.04 | 0.47 | 0.52 |
| 180 | 94.50 | 22.69 | 0.45 | 1.07 | 0.52 | 0.57 |
| 195 | 102.38 | 24.58 | 0.49 | 1.11 | 0.56 | 0.63 |
| 210 | 110.25 | 26.47 | 0.53 | 1.15 | 0.60 | 0.69 |
| 225 | 118.13 | 28.37 | 0.57 | 1.19 | 0.64 | 0.75 |
| 240 | 126.00 | 30.26 | 0.61 | 1.23 | 0.69 | 0.81 |
| Treatment Time (min) | Aggregate Quantity MnO (g) | Grams Mn in MnO (β MnO) | Mn Dissolved in Alloy θ (wt %) | Dissolved Mn Increase (wt %) | Reacted Fraction (MnO) | Reacted Fraction by Applying the R1 Model |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 0.00 | 0.00 | 0.62 | 0.00 | 0.00 |
| 15 | 7.88 | 6.10 | 0.12 | 0.74 | 0.14 | 0.14 |
| 30 | 15.75 | 12.20 | 0.24 | 0.86 | 0.28 | 0.29 |
| 45 | 23.63 | 18.30 | 0.37 | 0.99 | 0.42 | 0.45 |
| 60 | 31.50 | 24.40 | 0.49 | 1.11 | 0.55 | 0.63 |
| 75 | 39.38 | 30.50 | 0.61 | 1.23 | 0.69 | 0.82 |
| 90 | 47.25 | 36.60 | 0.73 | 1.35 | 0.83 | 1.04 |
| 105 | 55.13 | 42.70 | 0.85 | 1.47 | 0.97 | 1.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila, O.F.; Torres, J.T.; Valdes, A.F. Effect of Mg Concentration on the Aluminothermic Reduction of Mn2O3 Particles Obtained from Cathodes of Discharged Alkaline Batteries: Mathematical Modeling and Experimental Results. Metals 2019, 9, 49. https://doi.org/10.3390/met9010049
Dávila OF, Torres JT, Valdes AF. Effect of Mg Concentration on the Aluminothermic Reduction of Mn2O3 Particles Obtained from Cathodes of Discharged Alkaline Batteries: Mathematical Modeling and Experimental Results. Metals. 2019; 9(1):49. https://doi.org/10.3390/met9010049
Chicago/Turabian StyleDávila, Orlando Flores, Jesús Torres Torres, and Alfredo Flores Valdes. 2019. "Effect of Mg Concentration on the Aluminothermic Reduction of Mn2O3 Particles Obtained from Cathodes of Discharged Alkaline Batteries: Mathematical Modeling and Experimental Results" Metals 9, no. 1: 49. https://doi.org/10.3390/met9010049





