Effect of N/C Ratio on Precipitation Behavior of (Cr,Fe)23C6 Carbide in Novel Cast Austenitic Heat-Resistant Steels during Directional Solidification
Abstract
1. Introduction
2. Materials and Methods
3. Results
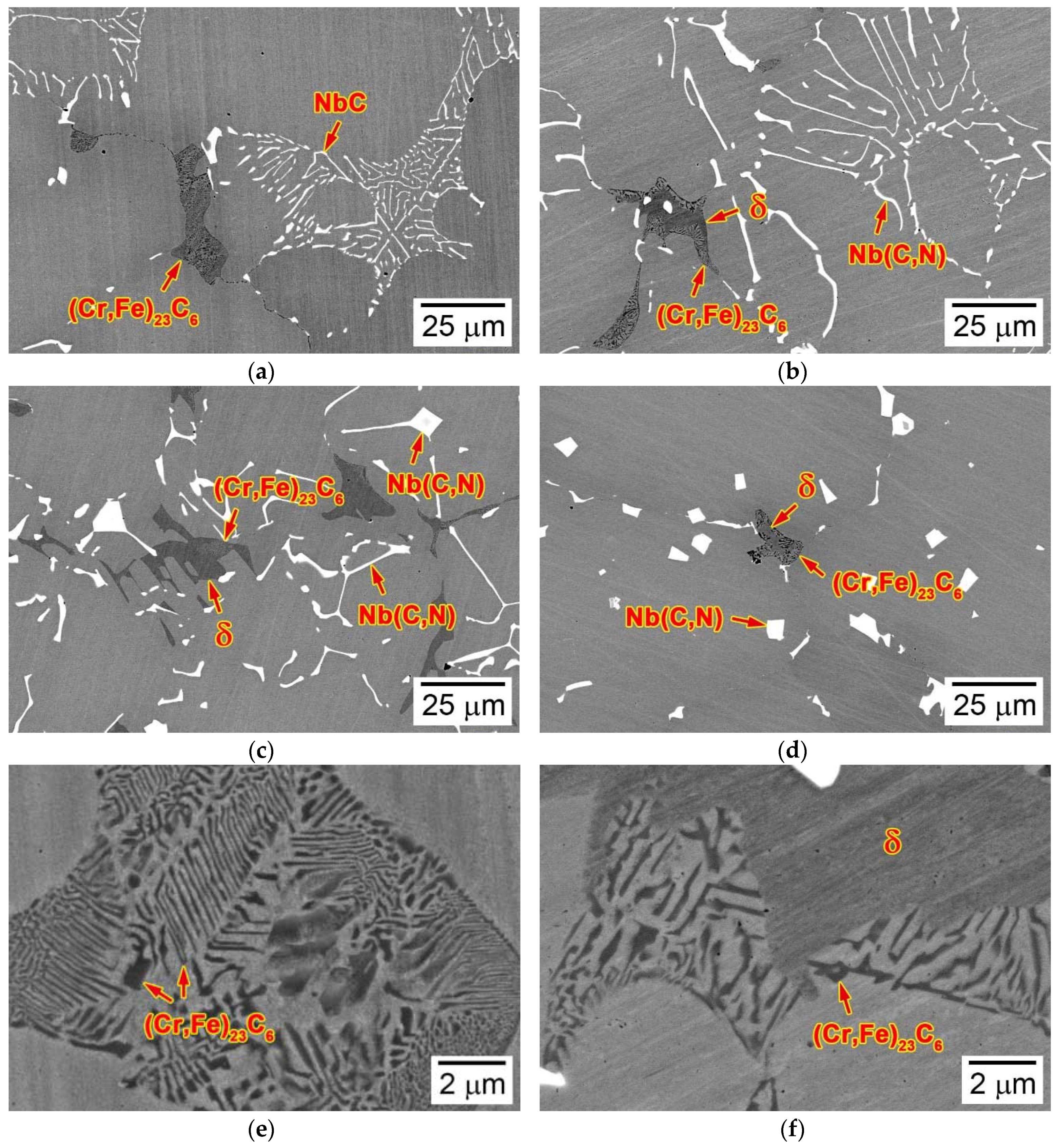
3.1. Microstructures of as-Cast Ingots
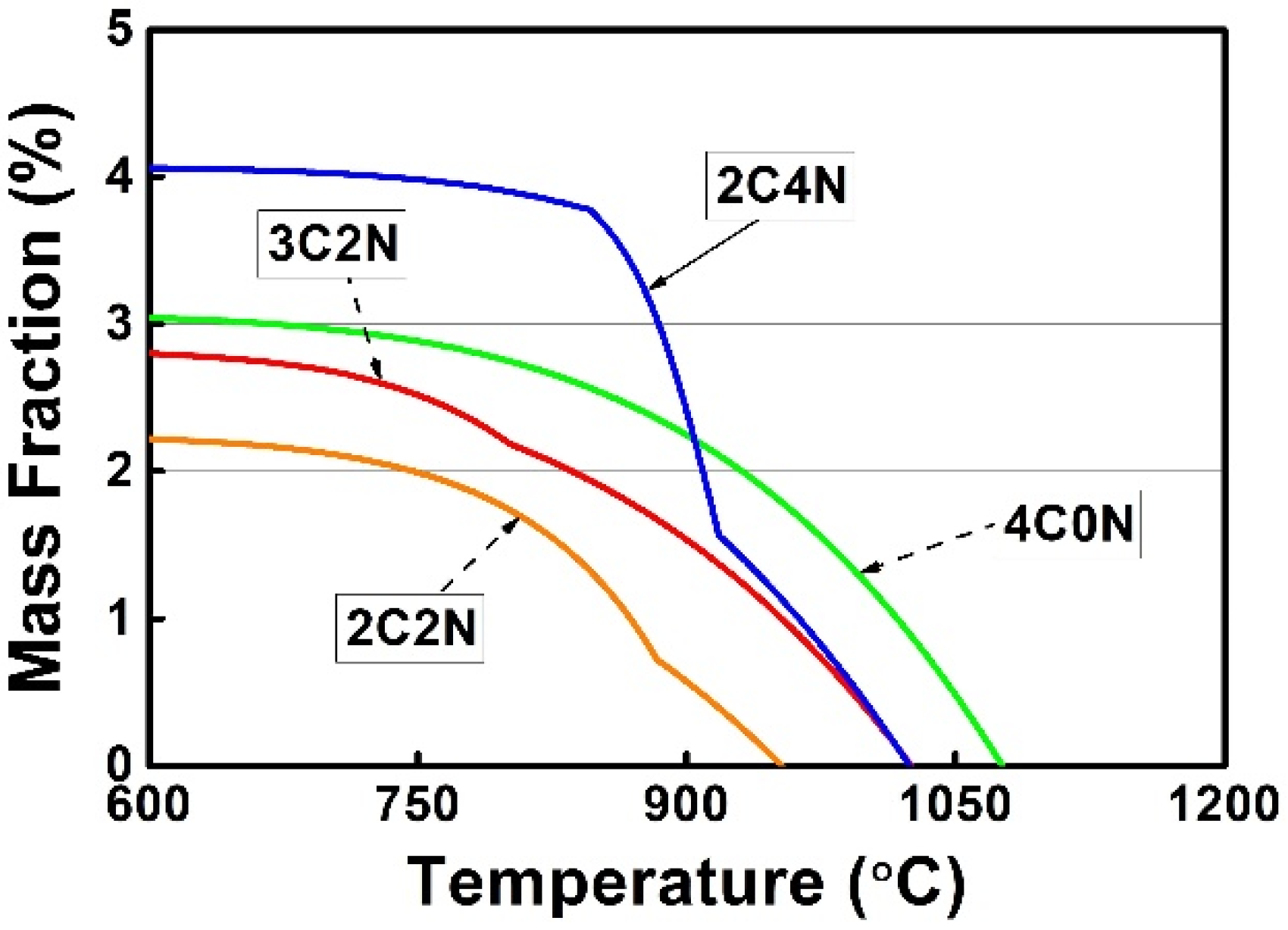
3.2. Solidification Calculations
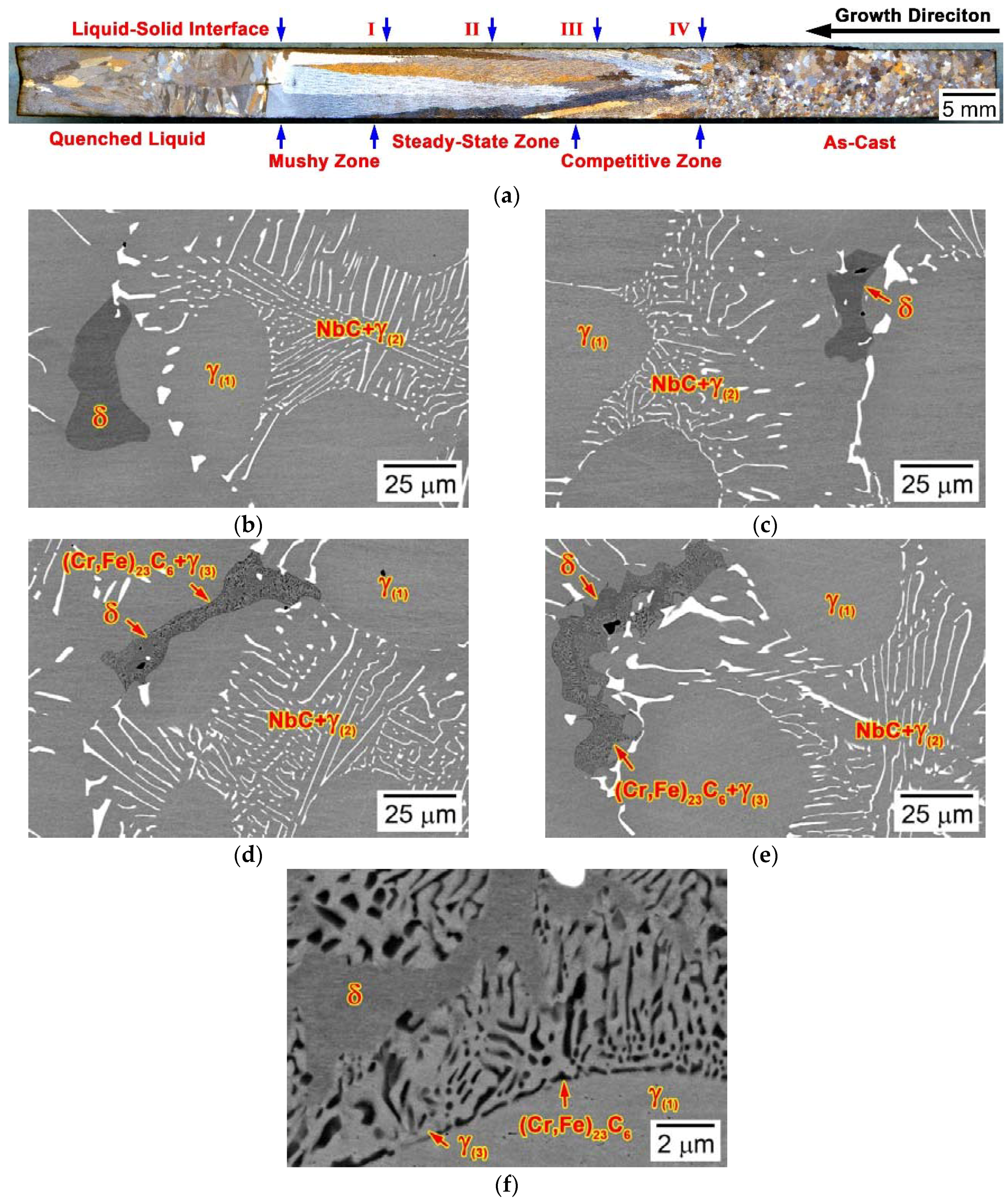
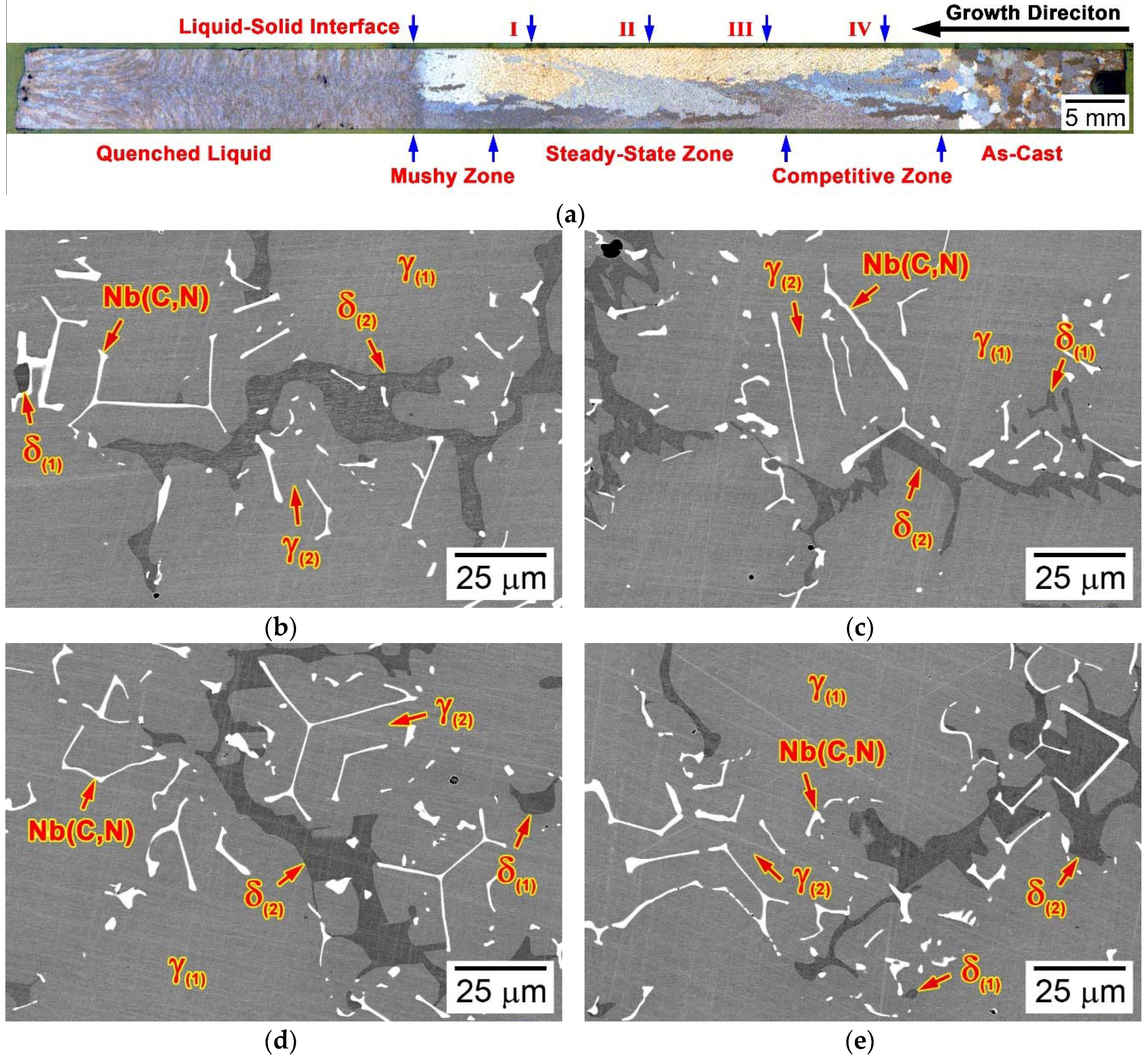
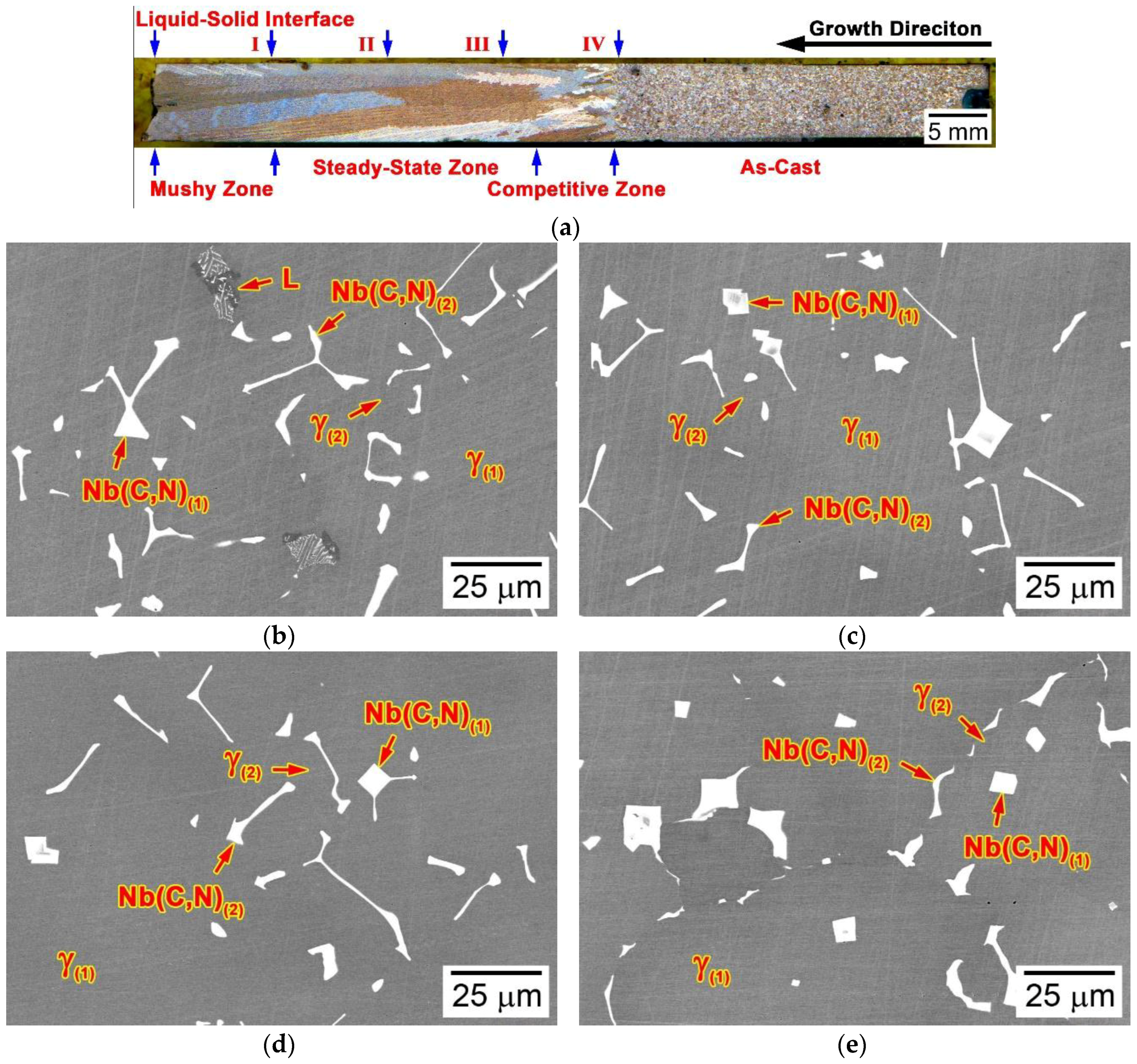
3.3. Microstructural Developments during Directional Solidification
3.3.1. Alloy 4C0N (Script Model)
3.3.2. Alloy 3C2N (Script Model)
3.3.3. Alloy 2C2N (Flake-Blocky Model)
3.3.4. Alloy 2C4N (Blocky Model)
3.4. Composition of (Cr,Fe)23C6 Carbide and δ-Ferrite
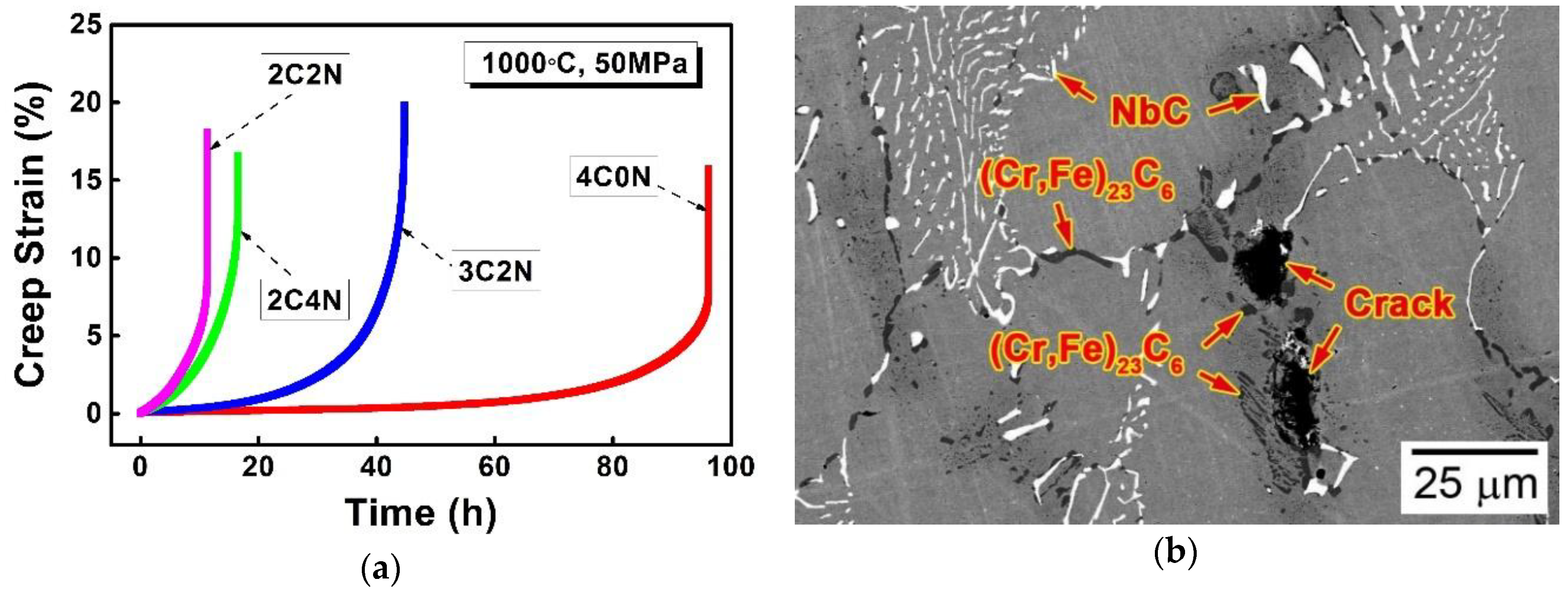
4. Discussion
5. Conclusions
- Different from the NbC/Nb(C,N) carbonitride and δ-ferrite that formed in the mushy zones, the (Cr,Fe)23C6 carbide formed in the steady-state zone and the competitive zone upon cooling to room temperature, after the solidification finished.
- Both the (Cr,Fe)23C6 carbide and δ-ferrite were enriched in Cr and depleted in Nb, Ni, Mn, and N, whereas the two phases showed different concentrations of C and Si, depending on which they were recognized as carbide and δ-ferrite.
- The (Cr,Fe)23C6 carbide grew in the colony of the δ-ferrite through the eutectoid reaction, forming fine rods and sheets that comprised the cellular structure, and moving the growth front towards the δ-ferrite.
- The precipitation temperature of (Cr,Fe)23C6 carbide decreased significantly with increasing the N/C ratio, giving rise to the prevention of this carbide precipitation during the liquid metal cooling directional solidification process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kunanoppadon, J. Thermal efficiency of a combined turbocharger set with gasoline engine. Am. J. Eng. Appl. Sci. 2010, 3, 342–349. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tojo, M.; Jinnai, Y.; Hayashi, N.; Ibaraki, S. Development of compact and high-performance turbocharger for 1050 °C exhaust gas. Mitsubishi Heavy Ind. Tech. Rev. 2008, 45, 1–5. [Google Scholar]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Creep behavior at 1273 K (1000 °C) in Nb-bearing austenitic heat-resistant cast steels developed for exhaust component applications. Metall. Mater. Trans. A 2016, 47, 3289–3294. [Google Scholar] [CrossRef]
- Zhao, H.L.; Engler-Pinto, C.C.; Tong, J.Y.; Godlewski, L.A.; Zindel, J.W.; Li, L.F.; Li, M.; Feng, Q. Mechanical response and dislocation substructure of a cast austenitic steel under low cycle fatigue at elevated temperatures. Mater. Sci. Eng. A 2017, 703, 422–429. [Google Scholar] [CrossRef]
- Shingledecker, J.P.; Maziasz, P.J.; Evans, N.D.; Pollard, M.J. Creep behavior of a new cast austenitic alloy. Int. J. Press. Vessels Pip. 2007, 84, 21–28. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Buchanan, K.G.; Kral, M.V.; Bishop, C. Crystallography and morphology of MC carbides in niobium-titanium modified as-cast HP alloys. Metall. Mater. Trans. A 2014, 45, 3373–3385. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effects of N on creep properties of austenitic heat-resistant cast steels developed for exhaust component applications at 1000 °C. Acta Metall. Sin. 2016, 52, 661–671. [Google Scholar]
- Zhang, Y.H.; Feng, Q. Effects of W on creep behaviors of novel Nb-bearing austenitic heat-resistant cast steels at 1000 °C. Acta Metall. Sin. 2017, 53, 1025–1037. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effective design of new austenitic cast steels for ultra-high temperature automotive exhaust components through combined CALPHAD and experimental approaches. Mater. Sci. Eng. A 2017, 683, 195–206. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effects of W on creep behaviors of novel Nb-bearing high nitrogen austenitic heat-resistant cast steels at 1000 °C. Mater. Charact. 2018, 139, 19–29. [Google Scholar] [CrossRef]
- Sourmail, T. Precipitation in creep resistant austenitic stainless steels. Mater. Sci. Technol. 2001, 17, 1–14. [Google Scholar] [CrossRef]
- McGuire, M.F. Stainless Steels for Design Engineers; ASM International: Materials Park, OH, USA, 2008; pp. 69–78. [Google Scholar]
- Erneman, J.; Schwind, M.; Liu, P.; Nilsson, J.O.; Andrén, H.O.; Ågren, J. Precipitation reactions caused by nitrogen uptake during service at high temperatures of a niobium stabilised austenitic stainless steel. Acta Mater. 2004, 52, 4337–4350. [Google Scholar] [CrossRef]
- Wasnik, D.N.; Dey, G.K.; Kain, V.; Samajdar, I. Precipitation stages in a 316L austenitic stainless steel. Scr. Mater. 2003, 49, 135–141. [Google Scholar] [CrossRef]
- Padilha, A.F.; Rios, P.R. Decomposition of austenite in austenitic stainless steels. ISIJ Int. 2002, 42, 325–327. [Google Scholar] [CrossRef]
- Hong, H.U.; Nam, S.W. The occurrence of grain boundary serration and its effect on the M23C6 carbide characteristics in an AISI 316 stainless steel. Mater. Sci. Eng. A 2002, 332, 255–261. [Google Scholar] [CrossRef]
- Kim, K.J.; Hong, H.U.; Min, K.S.; Nam, S.W. Correlation between the carbide morphology and cavity nucleation in an austenitic stainless steels under creep-fatigue. Mater. Sci. Eng. A 2004, 387–389, 531–535. [Google Scholar] [CrossRef]
- Kaneko, K.; Fukunaga, T.; Yamada, K.; Nakada, N.; Kikuchi, M.; Saghi, Z.; Barnard, J.S.; Midgley, P.A. Formation of M23C6-type precipitates and chromium-depleted zones in austenite stainless steel. Scr. Mater. 2011, 65, 509–512. [Google Scholar] [CrossRef]
- Ding, X.F.; Liu, D.F.; Zheng, Y.R.; Feng, Q. Effect of B micro-alloying on micro-porosities in as-cast HK40 alloys. Acta Metall. Sin. 2015, 51, 1121–1128. [Google Scholar]
- Ding, X.F.; Liu, D.F.; Guo, P.L.; Zheng, Y.R.; Feng, Q. Solidification microstructure formation in HK40 and HH40 alloys. Int. J. Min. Met. Mater. 2016, 23, 442–448. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effects of N/C ratio on solidification behaviors of novel Nb-bearing austenitic heat-resistant cast steels for exhaust components of gasoline engines. Metall. Mater. Trans. A 2017, 48, 1151–1162. [Google Scholar] [CrossRef]
- Miller, J.D.; Pollock, T.M. Stability of dendrite growth during directional solidification in the presence of a non-axial thermal field. Acta Mater. 2014, 78, 23–36. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Zhang, L.Q.; Wang, H.L.; Hao, G.J.; Chen, G.L. Microstructure development during directional solidification of Ti–45Al–8Nb alloy. J. Alloy. Compd. 2010, 506, 115–119. [Google Scholar] [CrossRef]





| Alloy | Fe | Cr | Ni | Si | Mn | Nb | S | P | C | N | N+C | N/C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4C0N | Bal. | 18.42 | 9.16 | 0.49 | 0.86 | 2.00 | 0.007 | 0.013 | 0.40 | 0.01 | 0.41 | 0 |
| 3C2N | Bal. | 19.68 | 10.12 | 0.80 | 0.93 | 2.09 | 0.008 | 0.013 | 0.29 | 0.15 | 0.44 | 0.52 |
| 2C2N | Bal. | 20.91 | 9.60 | 0.86 | 0.95 | 2.16 | 0.008 | 0.014 | 0.21 | 0.22 | 0.43 | 1.05 |
| 2C4N | Bal. | 21.32 | 9.88 | 0.92 | 0.95 | 2.18 | 0.007 | 0.014 | 0.23 | 0.36 | 0.59 | 1.57 |
| Alloy | NbC/Nb(C,N) | (Cr,Fe)23C6 | δ |
|---|---|---|---|
| 4C0N | 2.9 | 1.1 | 0.0 |
| 3C2N | 2.8 | 1.4 | 0.1 |
| 2C2N | 2.8 | 0.5 | 3.1 |
| 2C4N | 2.9 | 0.4 | Trace |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, J. Effect of N/C Ratio on Precipitation Behavior of (Cr,Fe)23C6 Carbide in Novel Cast Austenitic Heat-Resistant Steels during Directional Solidification. Metals 2018, 8, 678. https://doi.org/10.3390/met8090678
Zhang Y, Yang J. Effect of N/C Ratio on Precipitation Behavior of (Cr,Fe)23C6 Carbide in Novel Cast Austenitic Heat-Resistant Steels during Directional Solidification. Metals. 2018; 8(9):678. https://doi.org/10.3390/met8090678
Chicago/Turabian StyleZhang, Yinhui, and Jian Yang. 2018. "Effect of N/C Ratio on Precipitation Behavior of (Cr,Fe)23C6 Carbide in Novel Cast Austenitic Heat-Resistant Steels during Directional Solidification" Metals 8, no. 9: 678. https://doi.org/10.3390/met8090678
APA StyleZhang, Y., & Yang, J. (2018). Effect of N/C Ratio on Precipitation Behavior of (Cr,Fe)23C6 Carbide in Novel Cast Austenitic Heat-Resistant Steels during Directional Solidification. Metals, 8(9), 678. https://doi.org/10.3390/met8090678




