Interfacial Characterization and Bonding Properties of Copper/Aluminum Clad Sheets Processed by Horizontal Twin-Roll Casting, Multi-Pass Rolling, and Annealing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
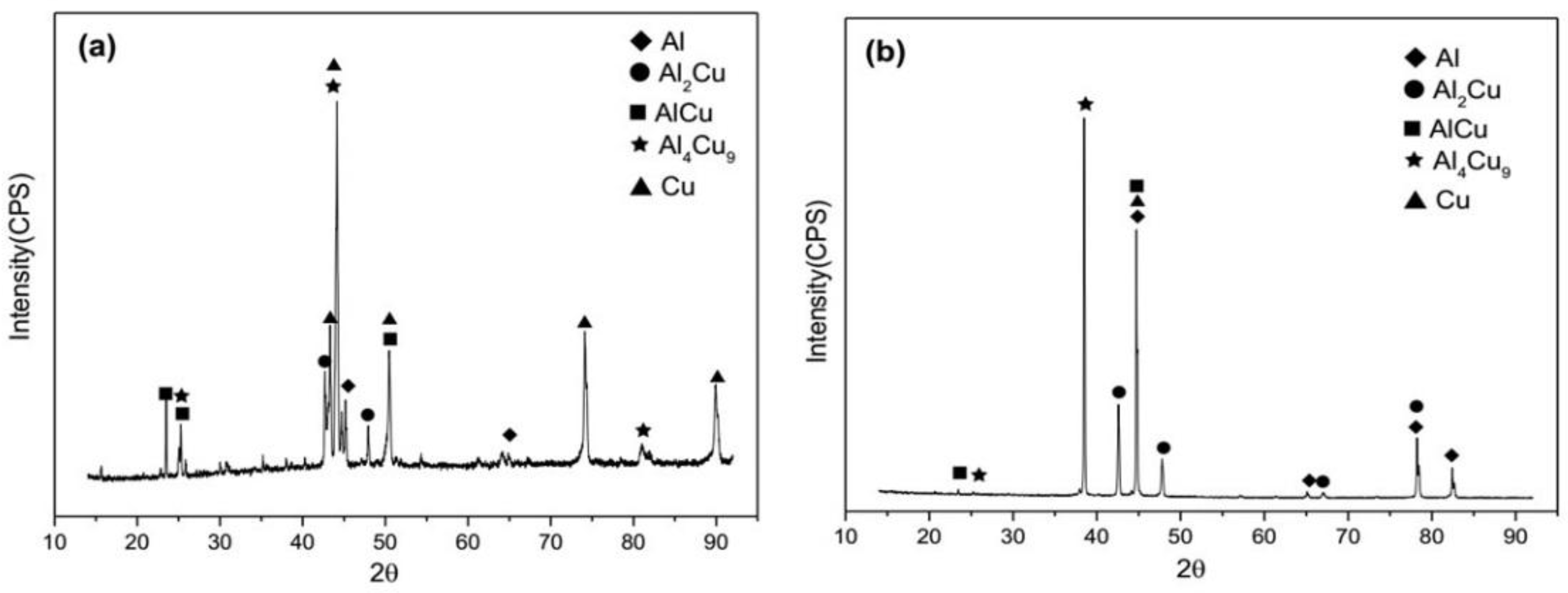
3.1. Analysis of the As-Cast Cu/Al Clad Sheet Interface
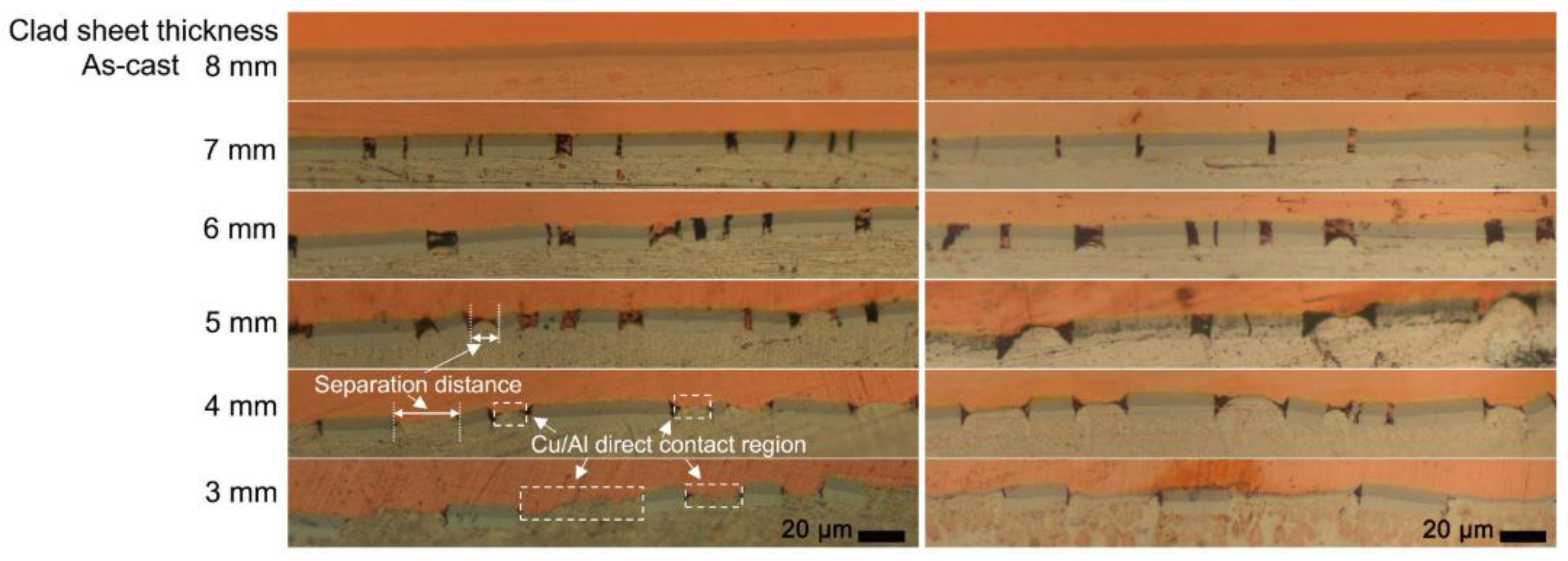
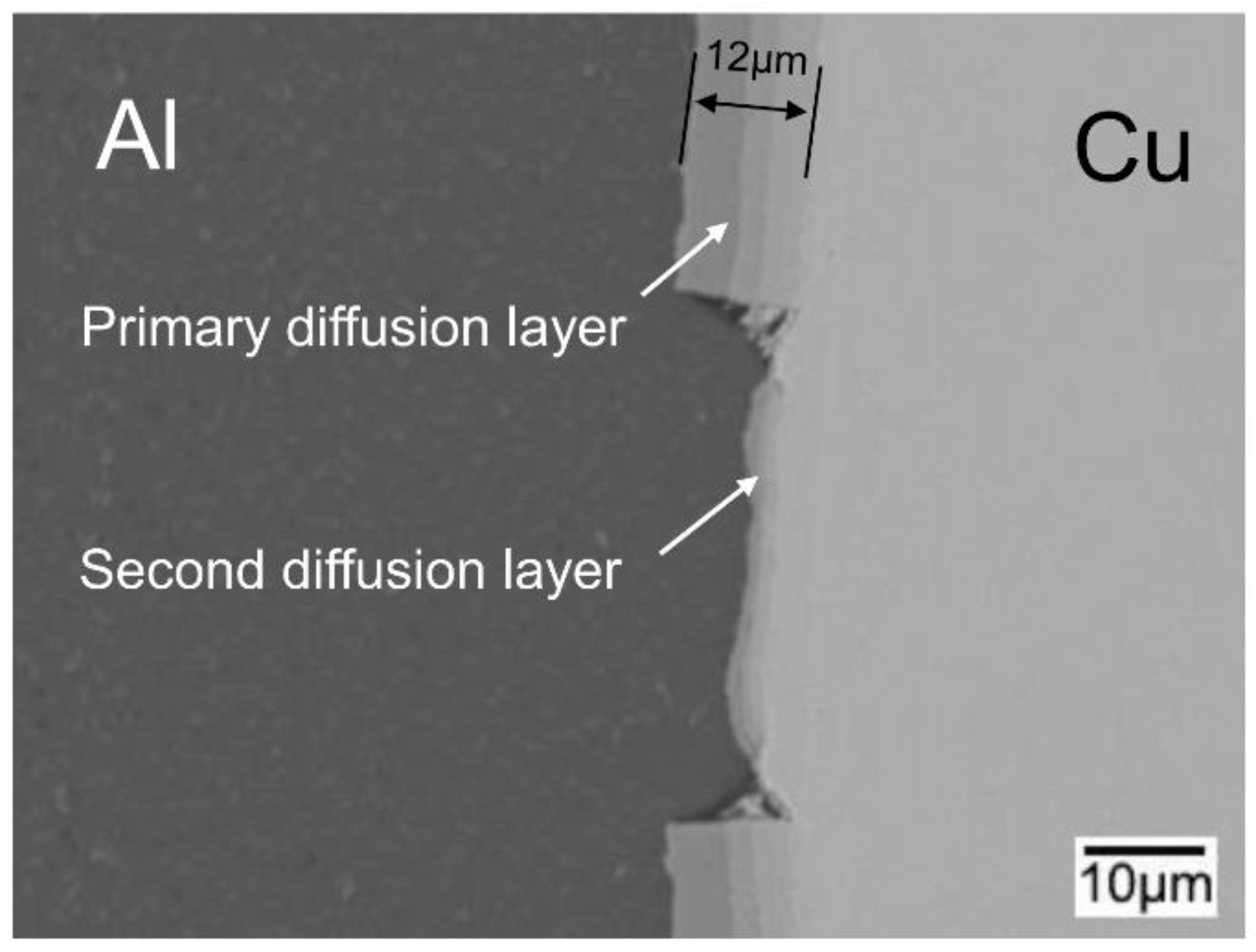
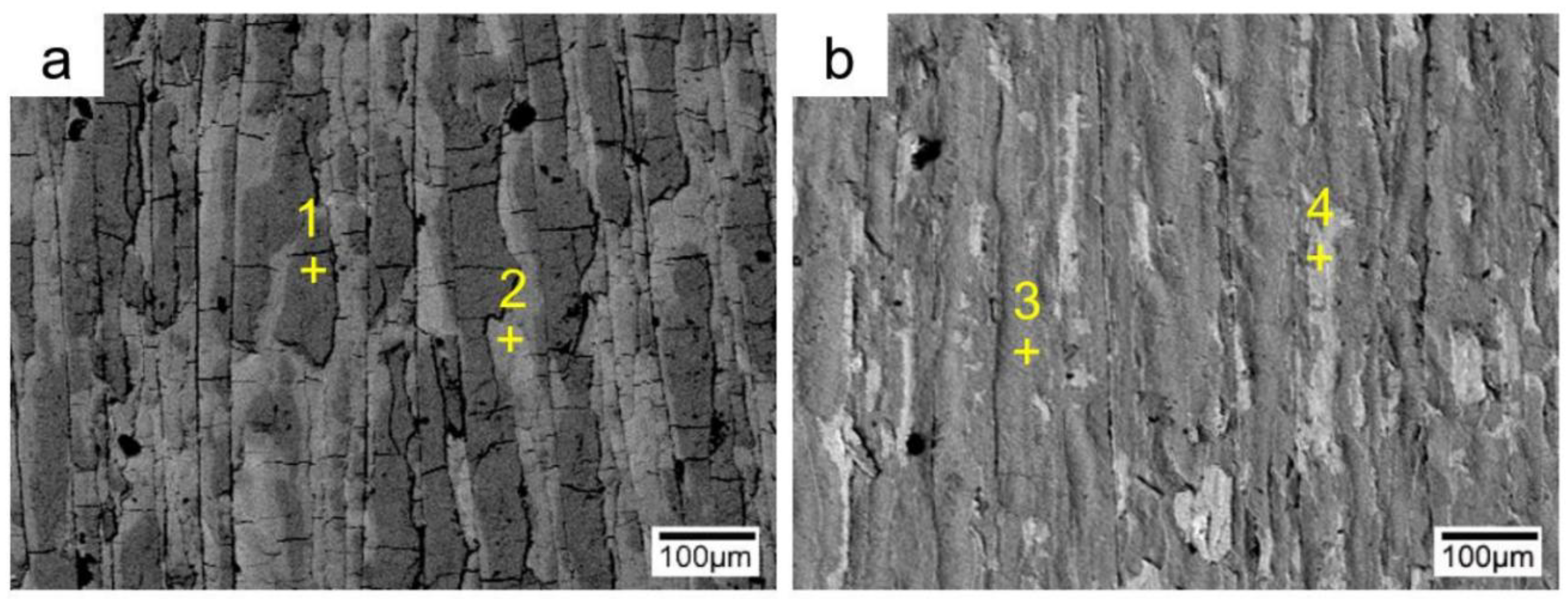
3.2. Interfacial Microstructure of the Clad Sheets after Multi-Pass Rolling and Annealing
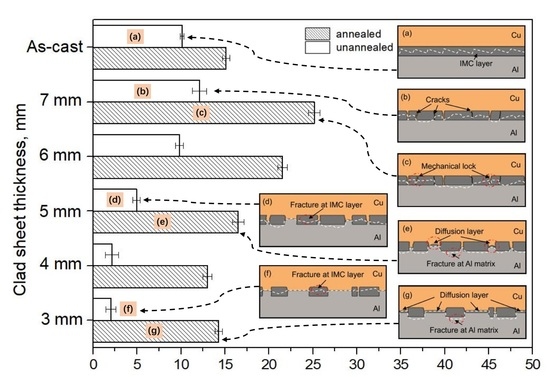
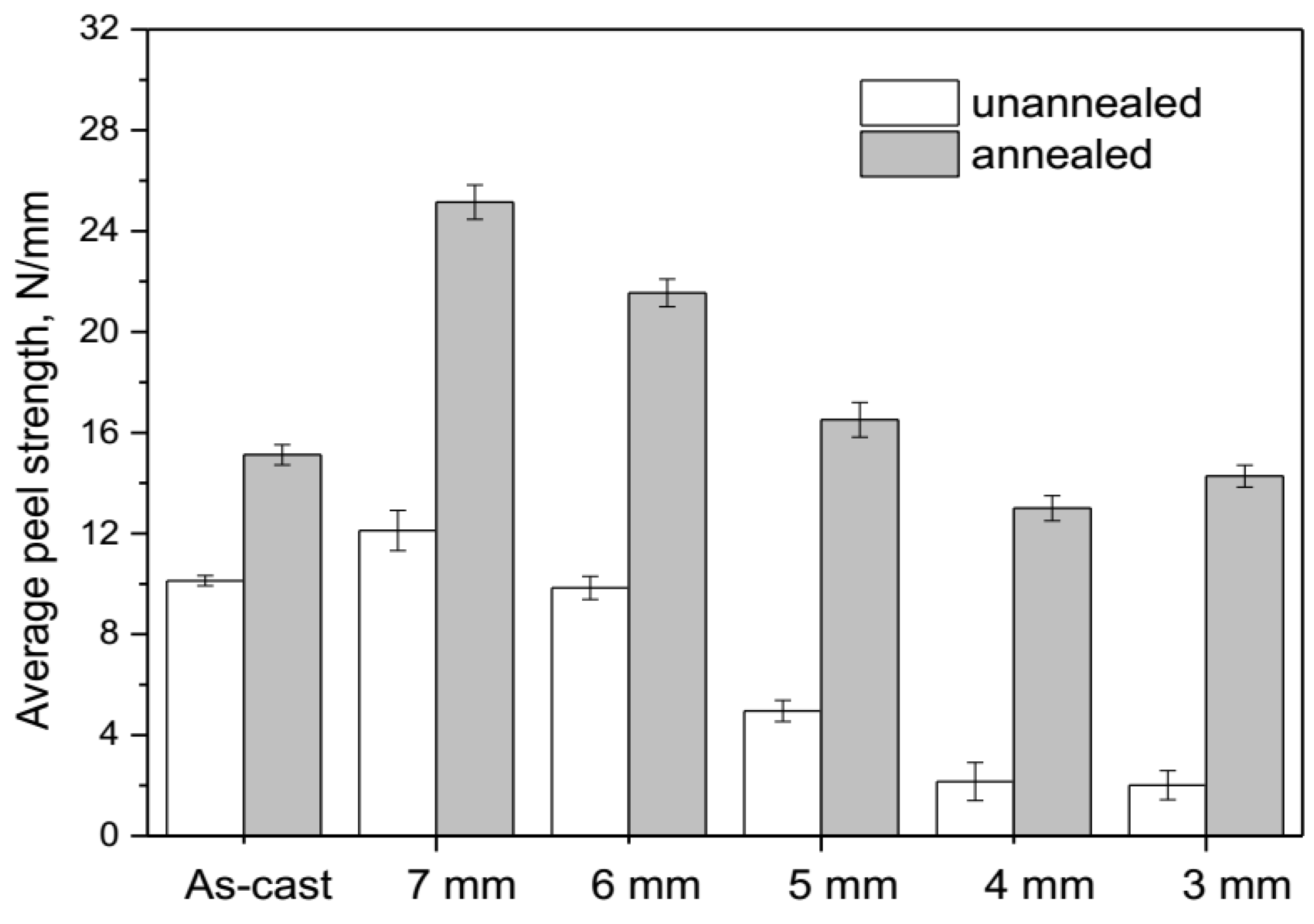
3.3. Peel Strength
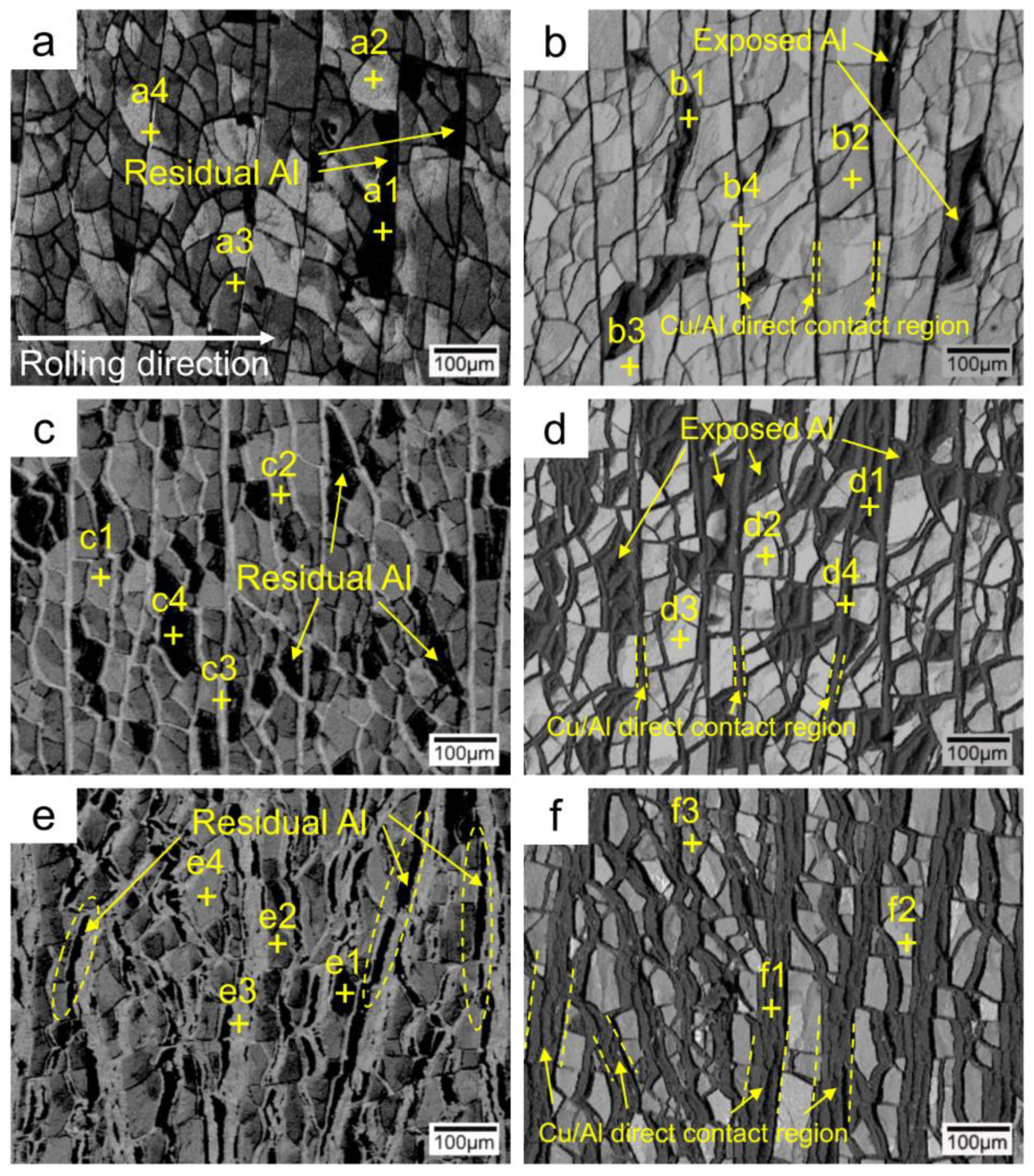
3.4. Peeled Surface Morphology
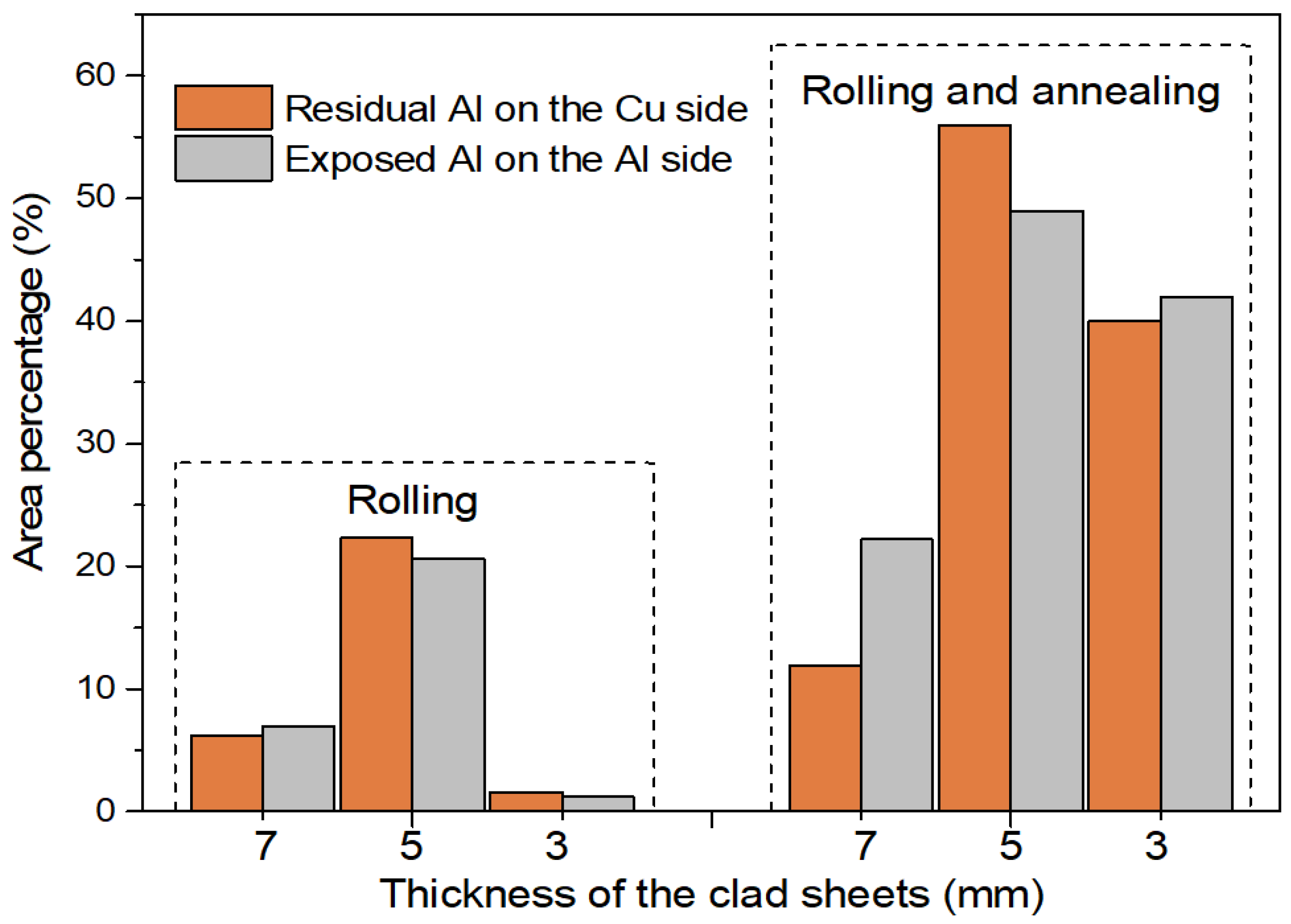
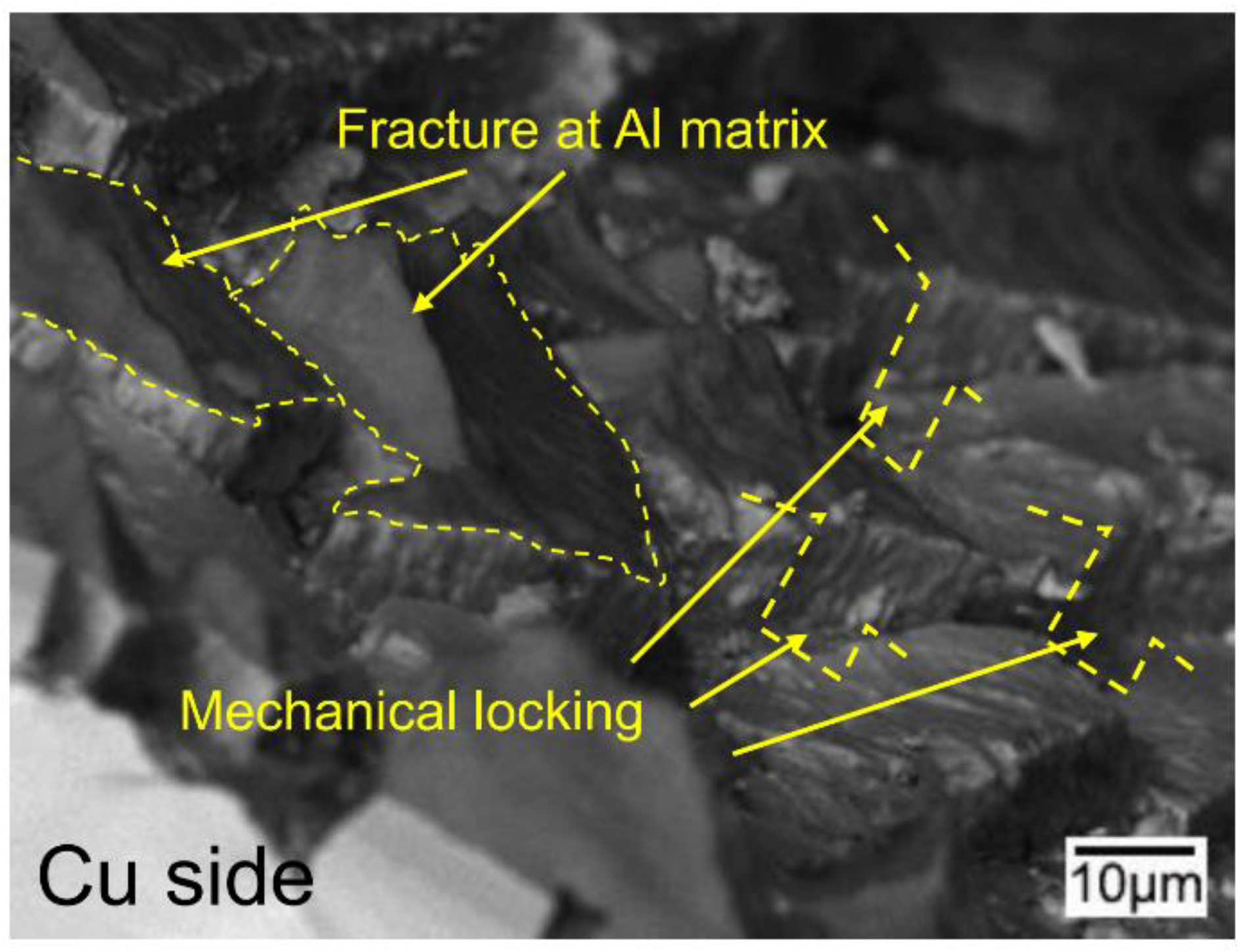
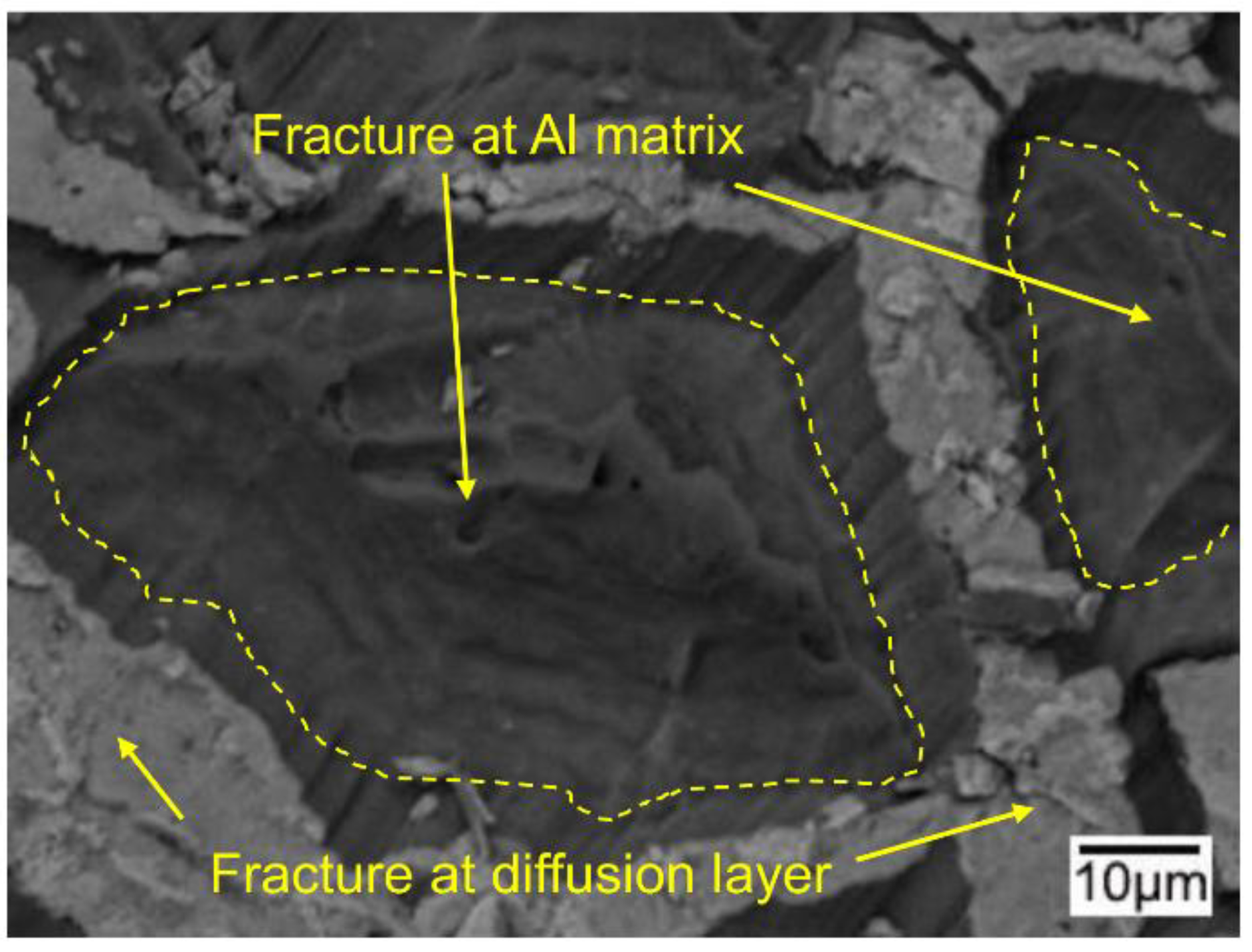
3.5. The Bonding Mechanism of the Cu/Al Clad Sheets
4. Conclusions
- (1)
- The Cu/Al clad sheets were produced with a horizontal twin-roll caster. The interface thickness of the as-cast Cu/Al clad sheet reached 9 μm to 10 μm and the interface was composed of three IMC layers including Al2Cu, AlCu, and Al4Cu9.
- (2)
- During multi-pass rolling, the peel strength first slightly increased and then gradually decreased with the increase of the rolling pass number. The IMC layer was broken and became increasingly separated with the increase of the rolling pass number. Mechanical locking formed in the broken region of the IMC layer. The mechanical locking resisted crack propagation and enhanced the peel strength, but the effect of this locking diminished with the increase of the rolling pass number.
- (3)
- During annealing, the bonding force between the extruded metals and cracking surface of the primary diffusion layer can enhance by thermal diffusion. Therefore, this leads to further increasing of the mechanical locking force. Meanwhile, the integrity of the IMC layer was improved, which propagates more peeling cracks in the Al matrix. The second diffusion layer can form at the Cu/Al direct contact region. All these effects of annealing improved the interfacial microstructure and enhanced the bonding strength of clad sheets. After rolling to 7 mm and annealing at 350 °C for 2 h, the clad sheet had the highest APS of 25 N/mm. This process can be used as the method to improve the peel strength of the Cu/Al clad sheets.
- (4)
- The improvement in the peel strength is mainly based on the following three factors: mechanical locking formed in the Cu/Al direct contact region after rolling, fracture occurred in the Al matrix, and a mechanical biting occurred from the Cu/Al direct contact region. These strengthening mechanisms of the clad sheets with a hard interface layer should be considered during the rolling and annealing processes in future applications.
Author Contributions
Funding
Conflicts of Interest
References
- Mai, T.A.; Spowage, A.C. Characterization of dissimilar joints in laser welding of steel-kovar, copper–steel and copper–aluminum. Mater. Sci. Eng. A. 2004, 374, 224–233. [Google Scholar] [CrossRef]
- Tseng, H.C.; Hung, C.H.; Huang, C.C. An analysis of the formability of aluminum/copper clad metals with different thicknesses by the finite element method and experiment. Int. J. Adv. Manuf. Technol. 2010, 49, 1029–1036. [Google Scholar] [CrossRef]
- Eslami, P.; Karimi Taheri, A. An investigation on diffusion bonding of aluminum to copper using equal channel angular extrusion process. Mater. Lett. 2011, 65, 1862–1864. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.Y.; Yang, F.; Xi, T.F.; Lai, C.; Ye, H.Q. Influence of heat treatment on interface of Cu/Al bimetal composite fabricated by cold rolling. Compos. Part B Eng. 2011, 42, 1468–1473. [Google Scholar] [CrossRef]
- Xia, H.B.; Wang, S.G.; Ben, H.F. Microstructure and mechanical properties of Ti/Al explosive cladding. Mater. Des. 2014, 56, 1014–1019. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.D.; Sui, Y.D.; Wang, Q.G. Microstructure and Mechanical Properties of Overcast 6101–6101 Wrought Al Alloy Joint by Squeeze Casting. J. Mater. Sci. Technol. 2015, 32, 298–304. [Google Scholar] [CrossRef]
- Loureiro, A.; Verdera, D.; Gesto, D.; Rodrigues, D.M. Influence of Tool Offsetting on the Structure and Morphology of Dissimilar Aluminum to Copper Friction-Stir Welds. Metall. Mater. Trans. A. 2012, 43, 5096–5105. [Google Scholar] [CrossRef]
- Barekar, N.S.; Dhindaw, B.K. Twin-roll casting of Aluminum alloys—An overview. Mater. Manuf. Process. 2014, 29, 651–661. [Google Scholar] [CrossRef]
- Bae, J.H.; Prasada Rao, A.K.; Kim, K.H.; Kim, N.J. Cladding of Mg alloy with Al by twin-roll casting. Scr. Mater. 2011, 64, 836–839. [Google Scholar] [CrossRef]
- Grydin, O.; Gerstein, G.; Nürnberger, F.; Schaper, M.; Danchenko, V. Twin-roll casting of aluminum–steel clad strips. J. Manuf. Process. 2013, 15, 501–507. [Google Scholar] [CrossRef]
- Huang, H.G.; Dong, Y.K.; Yang, M.; Du, F.S. Evolution of bonding interface in solid-liquid cast-rolling bonding of Cu/Al clad strip. Trans. Nonferrous Met. Soc. China 2017, 27, 1019–1025. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, H.L.; Hwang, W.S. Influence of interfacial structure development on the fracture mechanism and bond strength of aluminum/copper bimetal plate. Mater. Trans. 2006, 47, 1232–1239. [Google Scholar] [CrossRef]
- Wu, B.; Li, L.; Xia, C.D.; Guo, X.F.; Zhou, D.J. Effect of surface nitridingtreatment in a steel plate on the interfacial bonding strength of the aluminum/steel clad sheets by the cold roll bonding process. Mater. Sci. Eng. A. 2017, 682, 270–278. [Google Scholar] [CrossRef]
- Jing, Y.A.; Qin, Y.; Zang, X.M.; Li, Y.H. The bonding properties and interfacial morphologies of clad plate prepared by multiple passes hot rolling in a protective atmosphere. J. Mater. Process. Technol. 2014, 214, 1686–1695. [Google Scholar] [CrossRef]
- Li, X.B.; Zu, G.Y.; Ding, M.M.; Mu, Y.L.; Wang, P. Interfacial microstructure and mechanical properties of Cu/Al clad sheet fabricated by asymmetrical roll bonding and annealing. Mater. Sci. Eng. A. 2011, 529, 485–491. [Google Scholar] [CrossRef]
- Macwan, A.; Jiang, X.Q.; Li, C.; Chen, D.L. Effect of annealing on interface microstructures and tensile properties of rolled Al/Mg/Al tri-layer clad sheets. Mater. Sci. Eng. A. 2013, 587, 344–351. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.T.; Yu, H.L.; Su, L.H.; Xu, G.M.; Pan, J.S.; You, T.; Zhang, G.; Sun, K.M.; He, L.Z. Investigation on bonding strength of steel/aluminum clad sheet processed by horizontal twin-roll casting annealing and cold rolling. Mater. Des. 2016, 112, 263–274. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hwang, W.S. Effect of annealing on the interfacial structure of Aluminum-Copper joints. Mater. Trans. 2007, 48, 1938–1947. [Google Scholar] [CrossRef]
- Pelzer, R.; Nelhiebel, M.; Zink, R.; Wöhlert, S.; Lassnig, A.; Khatibi, G. High temperature storage reliability investigation of the Al-Cu wire bond interface. Microelectron. Reliab. 2012, 52, 1966–1970. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M.; Watanabe, Y. Growth behavior of compound layers during reactive diffusion between solid Cu and liquid Al. Mater. Sci. Eng. A 2007, 445–446, 355–363. [Google Scholar] [CrossRef]
- Peng, X.K.; Wuhrer, R.; Heness, G.; Yeung, W.Y. Rolling strain effects on the interlaminar properties of roll bonded copper/aluminium metal laminates. J. Mater. Sci. 2000, 35, 4357–4363. [Google Scholar] [CrossRef]
- Hannech, E.B.; Lamoudi, N.; Benslim, N.; Makhloufi, B. Intermetallic formation in the aluminum–copper system. Surf. Rev. Lett. 2003, 10, 677–683. [Google Scholar] [CrossRef]
- Danesh Manesh, H.; Karimi Taheri, A. The effect of annealing treatment on mechanical properties of aluminum clad steel sheet. Mater. Des. 2003, 24, 617–622. [Google Scholar] [CrossRef]








| Rolling Pass Number | Reduction Per Pass (%) | Total Reduction (%) | Clad Sheet Thickness (mm) |
|---|---|---|---|
| 1 | 12.5 | 12.5 | 7 |
| 2 | 14.3 | 25 | 6 |
| 3 | 16.7 | 37.5 | 5 |
| 4 | 20 | 50 | 4 |
| 5 | 25 | 62.5 | 3 |
| Element | 1 (at.%) | 2 (at.%) | 3 (at.%) |
|---|---|---|---|
| Cu | 62.50 | 43.29 | 31.67 |
| Al | 37.50 | 56.71 | 68.33 |
| Probable Phase | Al4Cu9 | AlCu | Al2Cu |
| Thickness of the Clad Sheets (mm) | Ratio of Cu/Al Direct Contact Region to the Interface Layer (%) | Average Width of Cu/Al Direct Contact Region (μm) | The Number of Cu/Al Direct Contact Region Per Millimeter |
|---|---|---|---|
| 7 | 19.12 | 7.2338 | 26 |
| 6 | 27.12 | 10.865 | 25 |
| 5 | 37.68 | 15.10 | 25 |
| 4 | 51.25 | 26.86 | 19 |
| 3 | 56.18 | 27.50 | 20 |
| Element | 1 (at.%) | 2 (at.%) | 3 (at.%) | 4 (at.%) |
|---|---|---|---|---|
| Cu | 29.49 | 45.92 | 29.70 | 52.36 |
| Al | 70.51 | 54.08 | 70.30 | 47.64 |
| Phase | Al2Cu | AlCu | Al2Cu | AlCu |
| Phase | Crystal Structure | Hardness/HV0.025 |
|---|---|---|
| Cu | Cubic (Fm3m, FCC) | 85–92 |
| Al | Cubic (Fm3m, FCC) | 45–48 |
| Al4Cu9 | Cubic (P43m) | 156 |
| AlCu | Monoclinic (C2/m) | 411 |
| Al2Cu | Tetragonal (I4/mcm) | 318 |
| Element | a1 | a2 | a3 | a4 | b1 | b2 | b3 | b4 |
|---|---|---|---|---|---|---|---|---|
| Cu | 01.58 | 41.05 | 27.98 | 92.47 | 01.28 | 29.33 | 52.41 | 01.58 |
| Al | 98.42 | 58.95 | 72.02 | 07.53 | 98.72 | 70.67 | 47.59 | 98.42 |
| Phase | Al | AlCu | Al2Cu | Cu | Al | Al2Cu | AlCu | Al |
| c1 | c2 | c3 | c4 | d1 | d2 | d3 | d4 | |
| Cu | 54.13 | 30.33 | 89.39 | 01.25 | 02.24 | 29.45 | 46.01 | 01.18 |
| Al | 45.87 | 69.67 | 10.61 | 98.75 | 97.76 | 70.55 | 53.99 | 98.82 |
| Phase | AlCu | Al2Cu | Cu | Al | Al | Al2Cu | AlCu | Al |
| e1 | e2 | e3 | e4 | f1 | f2 | f3 | ||
| Cu | 02.20 | 28.51 | 93.76 | 01.77 | 00.68 | 44.59 | 28.43 | |
| Al | 97.80 | 71.49 | 06.24 | 98.23 | 99.32 | 55.41 | 71.57 | |
| Phase | Al | Al2Cu | Cu | Al | Al | AlCu | Al2Cu |
| Element | a1 | a2 | a3 | a4 | b1 | b2 | b3 | b4 |
|---|---|---|---|---|---|---|---|---|
| Cu | 00.20 | 51.41 | 30.58 | 90.72 | 00.27 | 07.92 | 26.79 | 52.37 |
| Al | 99.80 | 48.59 | 69.42 | 09.28 | 99.73 | 92.08 | 73.21 | 47.63 |
| Phase | Al | AlCu | Al2Cu | Cu | Al | Al | Al2Cu | AlCu |
| c1 | c2 | c3 | d1 | d2 | d3 | |||
| Cu | 01.48 | 91.75 | 28.34 | 03.12 | 52.69 | 28.72 | ||
| Al | 98.52 | 08.25 | 71.66 | 96.88 | 47.31 | 71.28 | ||
| Phase | Al | Cu | Al2Cu | Al | AlCu | Al2Cu | ||
| e1 | e2 | e3 | f1 | f2 | f3 | |||
| Cu | 00.74 | 29.49 | 23.59 | 30.37 | 29.57 | 00.91 | ||
| Al | 99.26 | 70.51 | 76.41 | 69.63 | 70.43 | 99.09 | ||
| Phase | Al | Al2Cu | Al2Cu | Al2Cu | Al2Cu | Al |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Xie, J.; Wang, A.; Wang, W.; Ma, D. Interfacial Characterization and Bonding Properties of Copper/Aluminum Clad Sheets Processed by Horizontal Twin-Roll Casting, Multi-Pass Rolling, and Annealing. Metals 2018, 8, 645. https://doi.org/10.3390/met8080645
Mao Z, Xie J, Wang A, Wang W, Ma D. Interfacial Characterization and Bonding Properties of Copper/Aluminum Clad Sheets Processed by Horizontal Twin-Roll Casting, Multi-Pass Rolling, and Annealing. Metals. 2018; 8(8):645. https://doi.org/10.3390/met8080645
Chicago/Turabian StyleMao, Zhiping, Jingpei Xie, Aiqin Wang, Wenyan Wang, and Douqin Ma. 2018. "Interfacial Characterization and Bonding Properties of Copper/Aluminum Clad Sheets Processed by Horizontal Twin-Roll Casting, Multi-Pass Rolling, and Annealing" Metals 8, no. 8: 645. https://doi.org/10.3390/met8080645