Fabrication of Al2O3/SiC/Al Hybrid Nanocomposites Through Solidification Process for Improved Mechanical Properties
Abstract
:1. Introduction
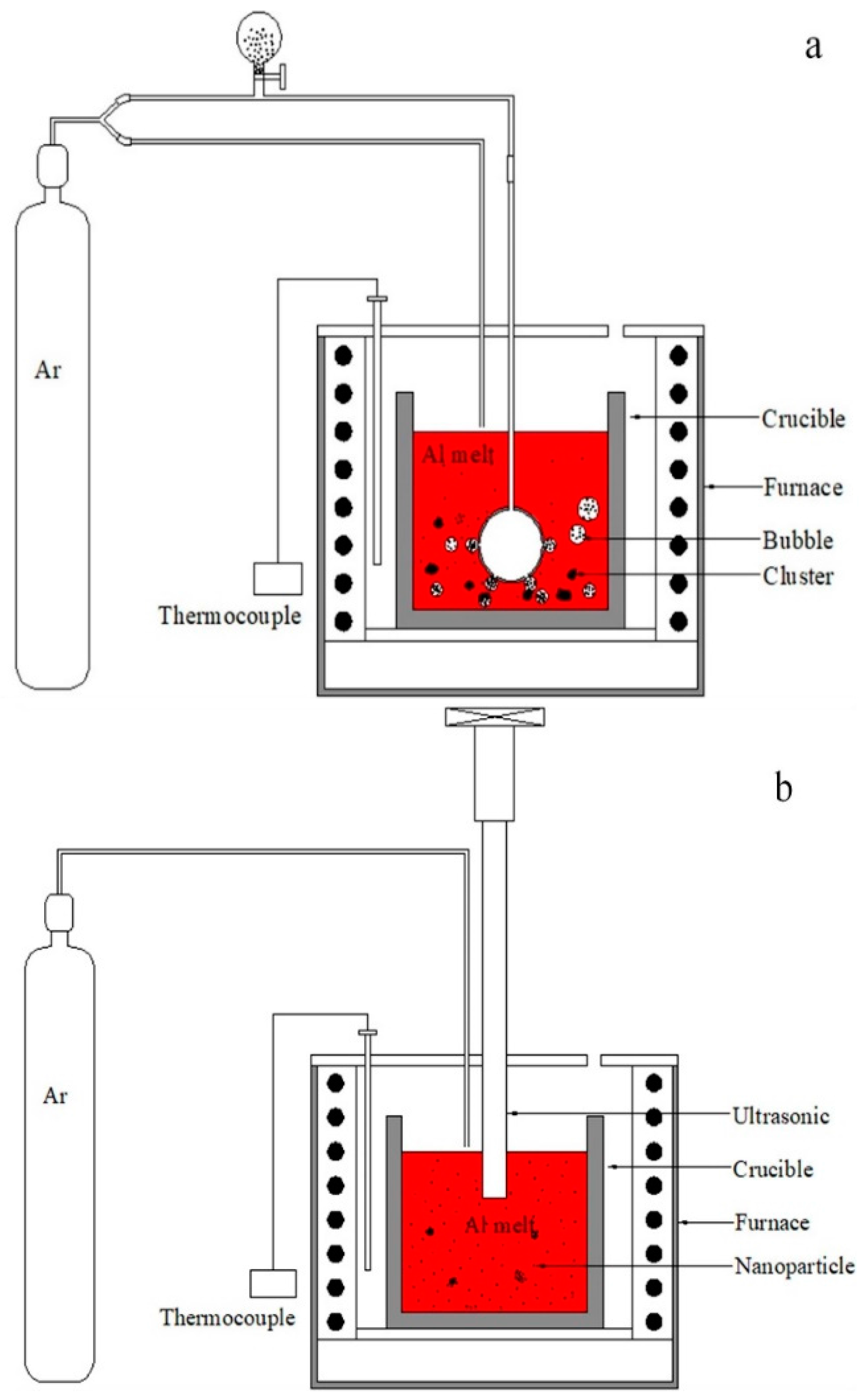
2. Materials and Methods
3. Results and Discussion
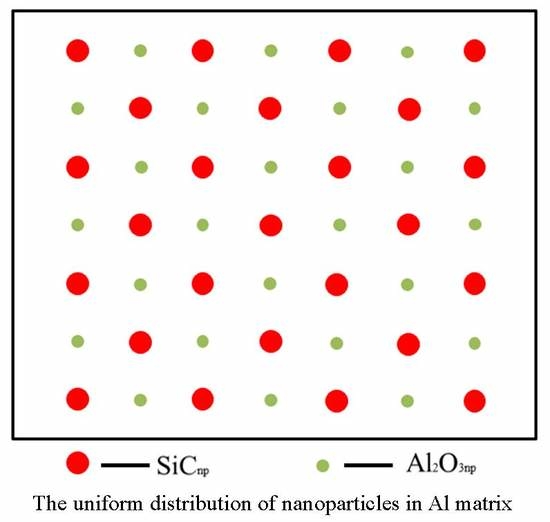
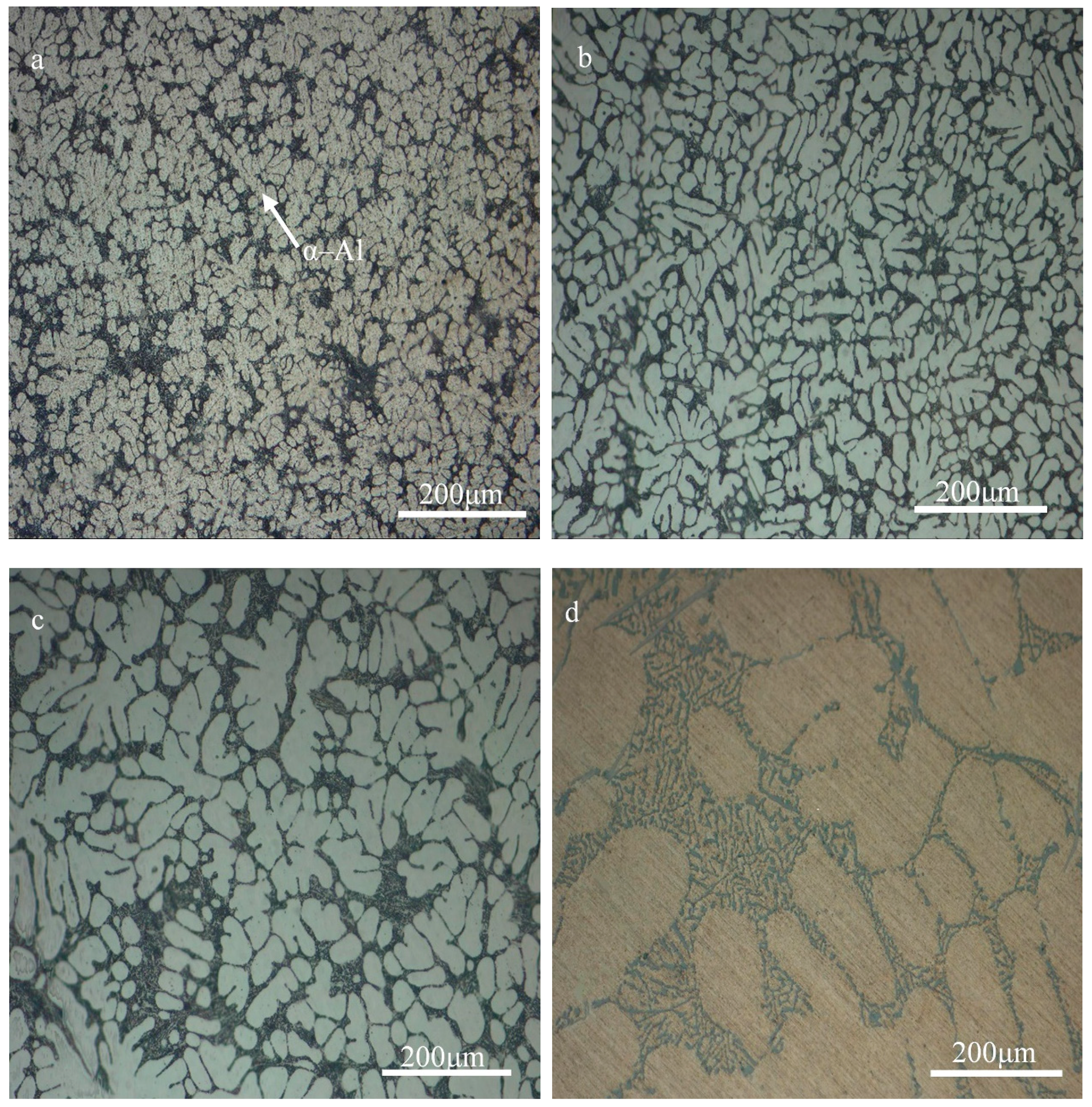
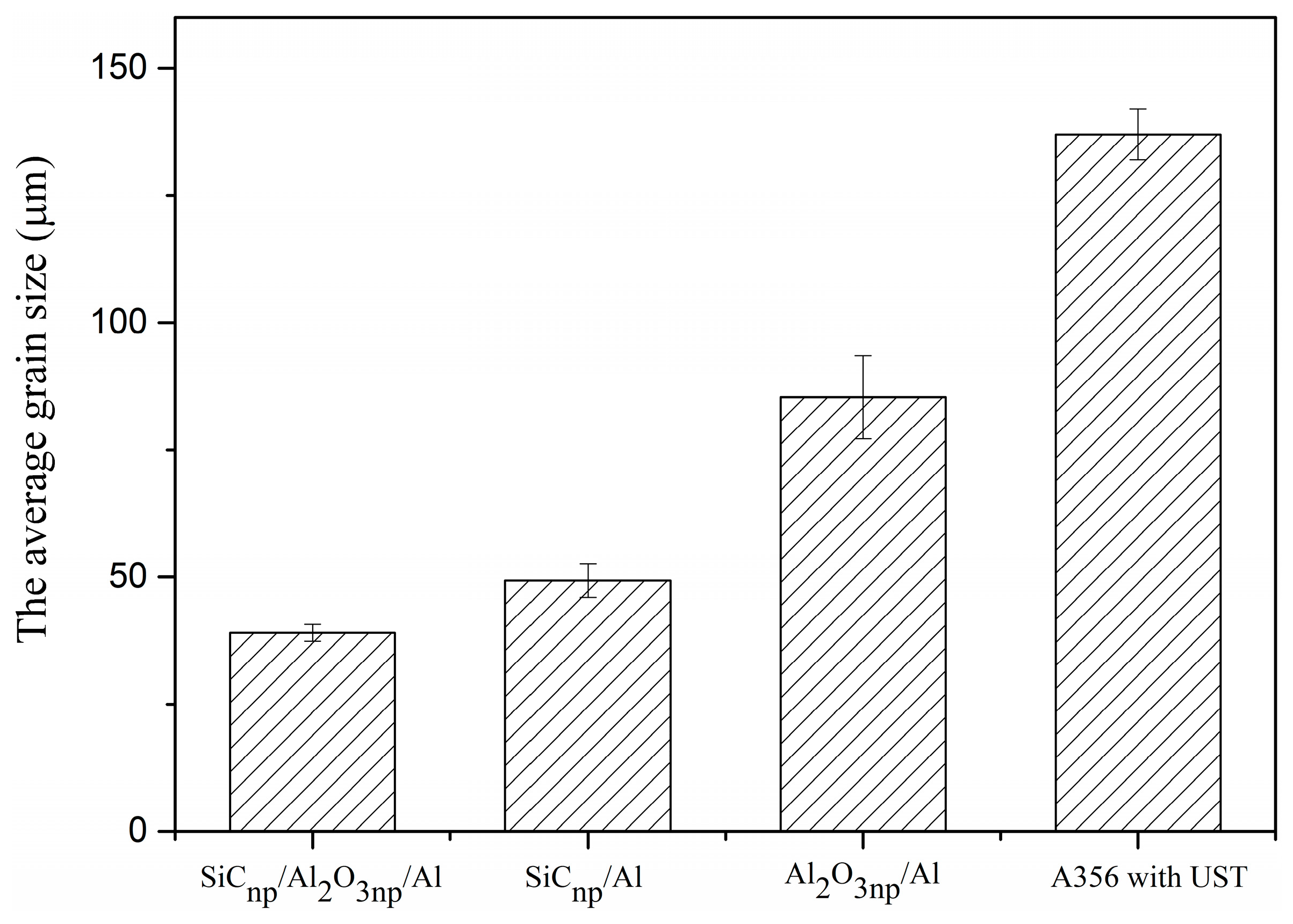
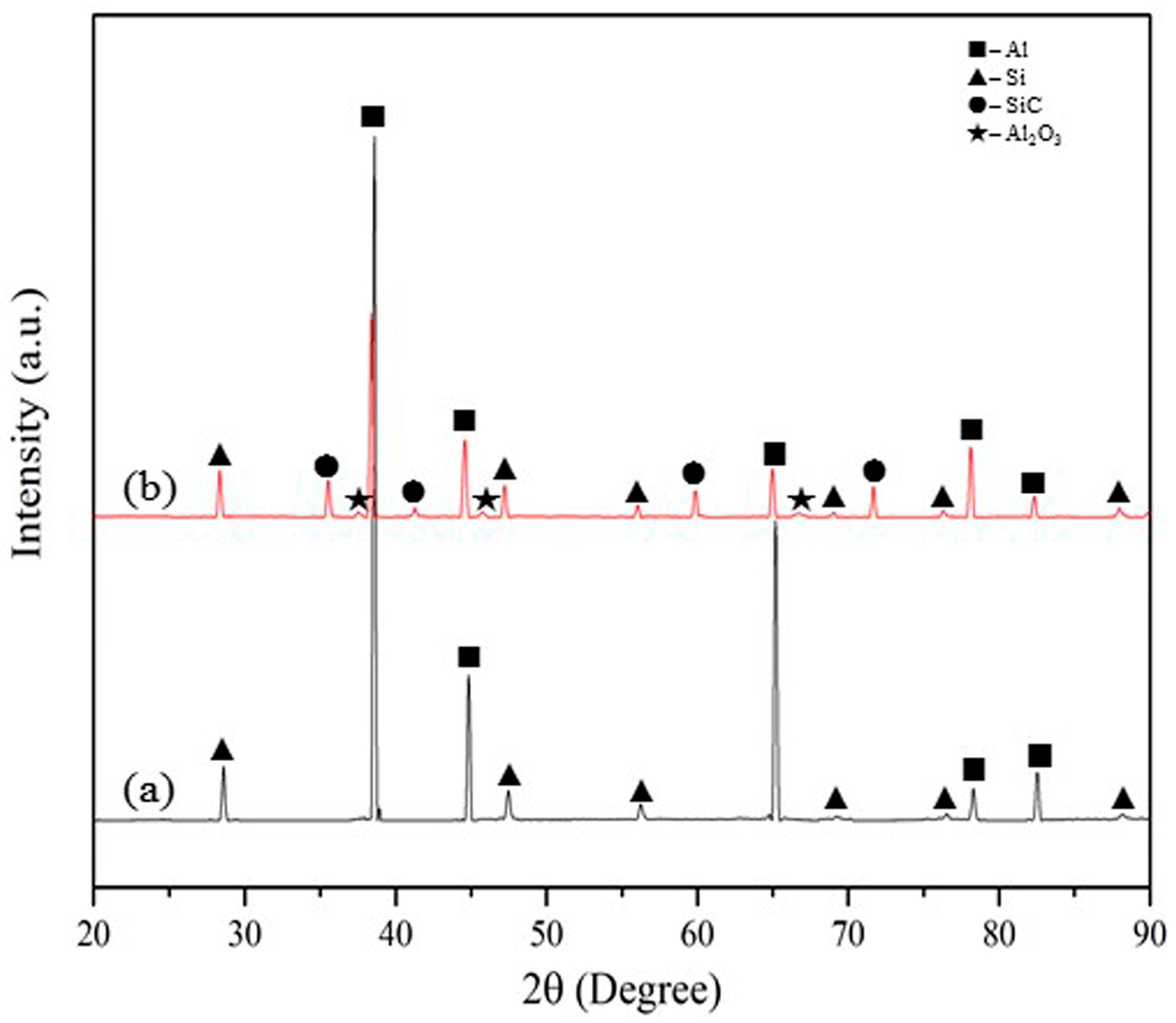
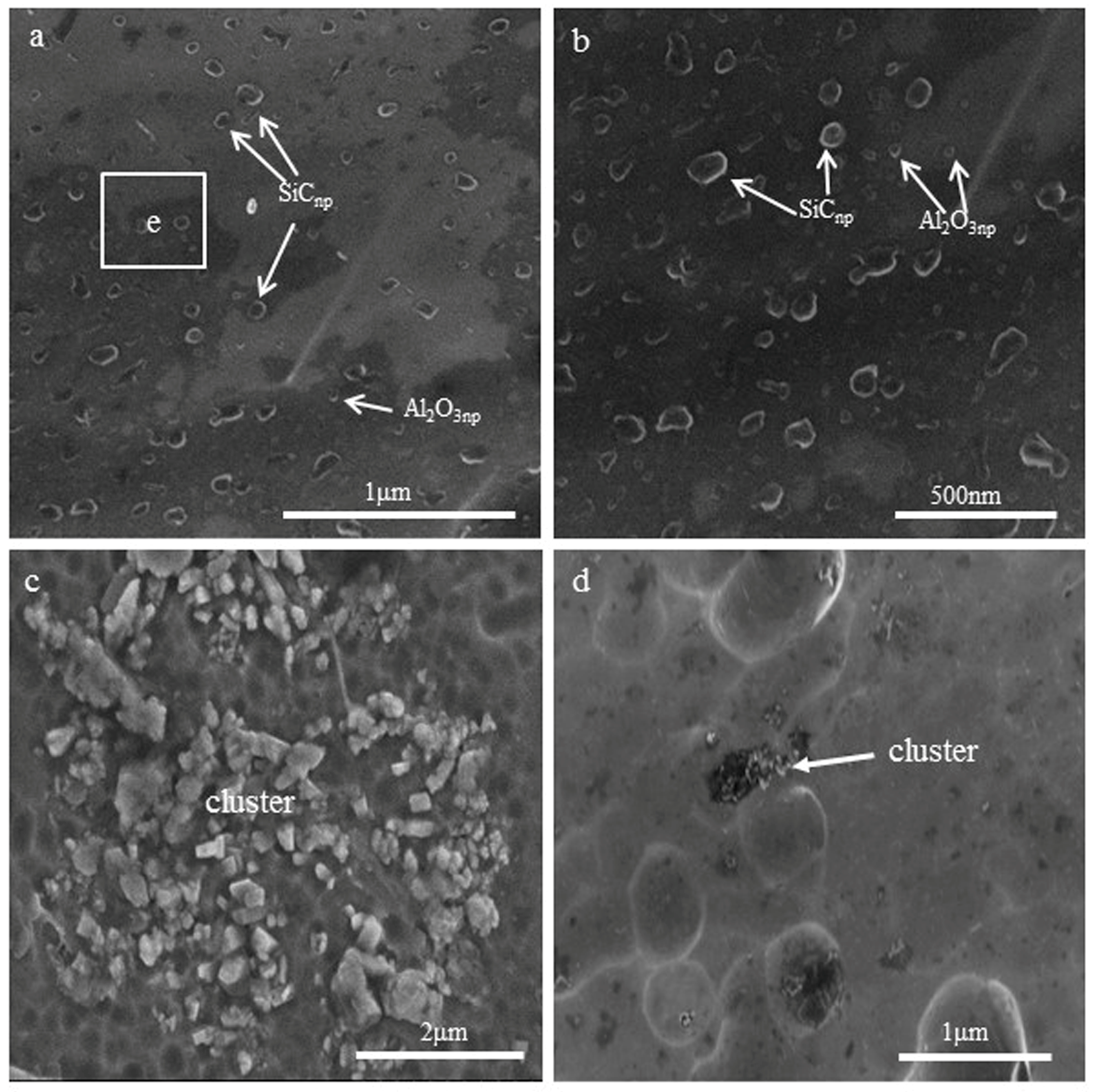
3.1. Microstructural Characteristics
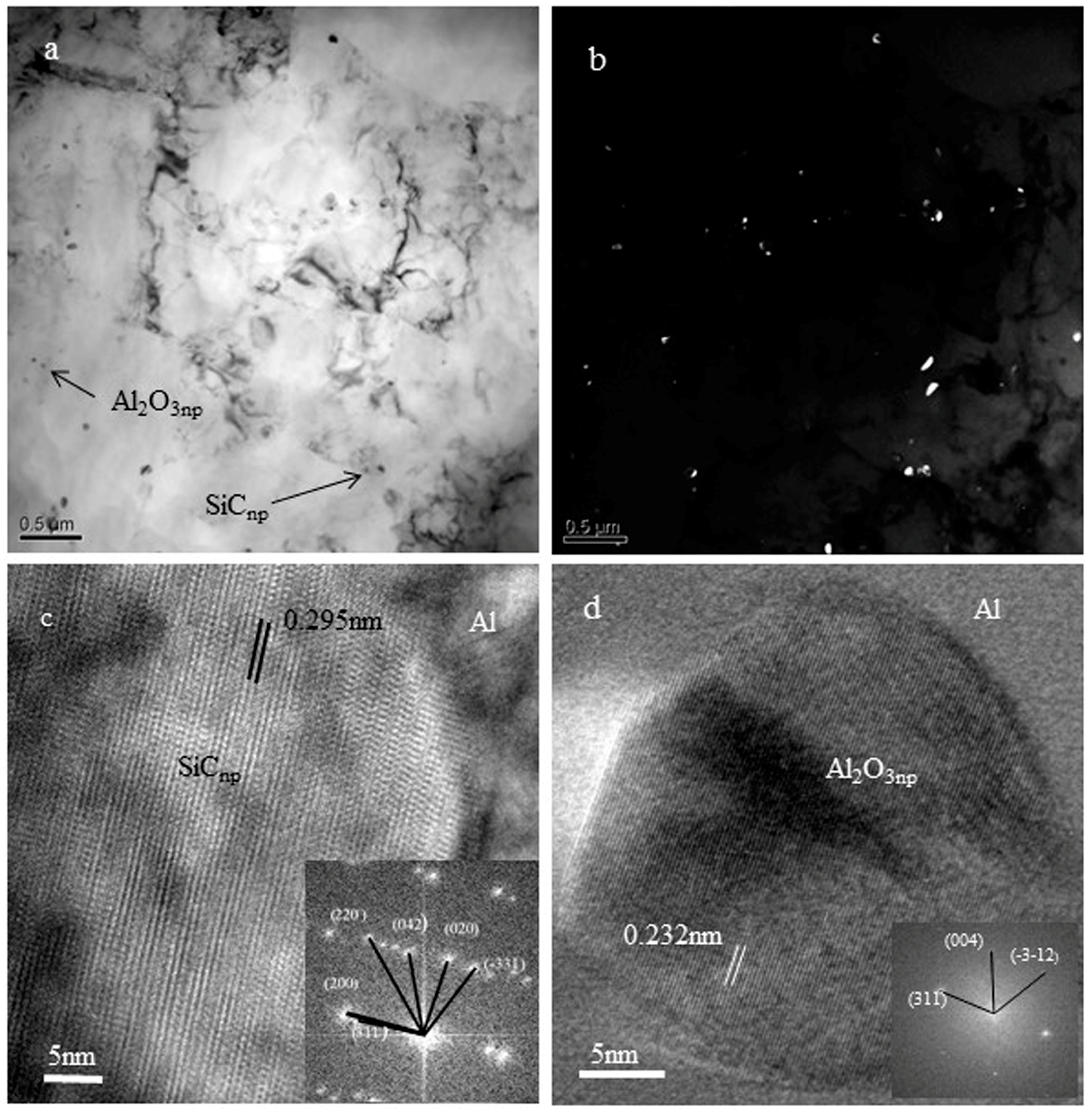
3.2. The Interface between Nanoparticles and Al
3.3. The Interaction between Nanoparticles with Solid–Liquid Interface
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paramsothy, M.; Hassan, S.F.; Srikanth, N.; Gupta, M. Simultaneously enhanced tensile and compressive response of AZ31-Nano Al2O3-AA5052 macrocomposite. J. Mater. Sci. 2009, 44, 4860–4873. [Google Scholar] [CrossRef]
- Zhou, D.; Qiu, F.; Jiang, Q. Simultaneously increasing the strength and ductility of nanosized TiN particle reinforced Al-Cu matrix composites. Mater. Sci. Eng. A 2014, 596, 98–102. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, J.Q.; Choi, H.; Pozuelo, M.; Ma, X.; Bhowmick, S.; Yang, J.-M.; Mathaudhu, S.; Li, X. Processing and properties of magnesium containing a dense uniform dispersion of nanoparticles. Nature 2015, 528, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Weiss, D.; Morrow, J. A novel manufacturing route for production of high-performance metal matrix nanocomposites. Manuf. Lett. 2013, 1, 62–65. [Google Scholar] [CrossRef]
- Choi, H.; Cho, W.H.; Konishi, H.; Kou, S.; Li, X. Nanoparticle-Induced Superior Hot Tearing Resistance of A206 Alloy. Metall. Mater. Trans. A 2013, 44, 1897–1907. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Lopez, H.; Kongshaug, D.; Schultz, B.; Rohatgi, P. Revised Orowan Strengthening: Effective Interparticle Spacing and Strain Field Considerations. Metall. Mater. Trans. A 2012, 43, 2110–2115. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Su, C.H.; Yang, Y.S.; Yeh, C.-S.; Tsai, C.-Y.; Wu, C.-L.; Wu, M.-T.; Shieh, D.-B. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 2005, 26, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Okumura, M.; Toshima, N. Stable dispersions of PVP-protected Au/Pt/Ag trimetallic nanoparticles as highly active colloidal catalysts for aerobic glucose oxidation. Phys. Chem. C 2011, 115, 14883–14891. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Garia-Rodriguez, S.; Puentes, J.; Li, X.C.; Osswald, T.A. Prediction of vortex height from mechanical mixing in metal matrix nanocomposite processing by means of dimensional analysis and scaling. J. Manuf. Process. 2014, 16, 212–217. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles-a review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; Li, X.C. Study on bulk aluminum matrix nano-composite fabricated by ultrasonic dispersion of nano-sized SiC particles in molten aluminum alloy. Mater. Sci. Eng. A 2004, 380, 378–383. [Google Scholar] [CrossRef]
- Chen, L.Y.; Peng, J.Y.; Xu, J.Q.; Choi, H.; Li, X.-C. Achieving uniform distribution and dispersion of a high percentage of nanoparticles in metal matrix nanocomposites by solidification processing. Scr. Mater. 2013, 69, 634–637. [Google Scholar] [CrossRef]
- Chen, L.Y.; Konishi, H.; Fehrenbacher, A.; Ma, C.; Xu, J.-Q.; Choi, H.; Xu, H.-F.; Pfefferkorn, F.-E.; Li, X.-C. Novel nanoprocessing route for bulk graphene nanoplatelets reinforced metal matrix nanocomposites. Scr. Mater. 2012, 67, 29–32. [Google Scholar] [CrossRef]
- Chen, L.Y.; Choi, H.; Fehrenbacher, A.; Xu, J.Q.; Ma, C.; Li, X.C. Uniform Dispersion of Nanoparticles in Metal Matrix Nanocomposites. TMS 2012, 1, 757–763. [Google Scholar]
- Jia, S.; Zhang, D.; Xuan, Y.; Nastac, L. An experimental and modeling investigation of aluminum-based alloys and nanocomposites processed by ultrasonic cavitation processing. Appl. Acoust. 2016, 103, 226–231. [Google Scholar] [CrossRef]
- Zhang, D.; Nastac, L. Numerical modeling of the dispersion of ceramic nanoparticles during ultrasonic processing of aluminum-based nanocomposites. J. Mater. Res. Technol. 2014, 3, 296–302. [Google Scholar] [CrossRef]
- Dehnavi, M.R.; Niroumand, B.; Ashrafizadeh, F.; Rohatgi, P.K. Effects of continuous and discontinuous ultrasonic treatments on mechanical properties and microstructural characteristics of cast Al413-SiC np nanocomposites. Mater. Sci. Eng. A 2014, 617, 73–83. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Schultz, B.F.; Rohatgi, P.K.; Kim, C.-S. Impact of Brownian motion on the particle settling in molten metals. Met. Mater. Int. 2014, 20, 747–755. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Kaptay, G.; Schultz, B.F.; Rohatgi, P.K.; Cho, K.; Kim, C.-S. Brownian motion effects on particle pushing and engulfment during solidification in metal-matrix composites. Metall. Mater. Trans. A 2014, 20, 4635–4645. [Google Scholar] [CrossRef]
- Ozsoy, I.B.; Li, G.; Choi, H.; Zhao, H. Shape effects on nanoparticle engulfment for metal matrix nanocomposites. J. Cryst. Growth 2015, 422, 62–68. [Google Scholar] [CrossRef]
- Choi, H.; Li, X. Refinement of primary Si and modification of eutectic Si for enhanced ductility of hypereutectic Al-20Si-4.5 Cu alloy with addition of Al2O3 nanoparticles. J. Mater. Sci. 2012, 47, 3096–3102. [Google Scholar] [CrossRef]
- Kim, W.J.; Yu, Y.J. The effect of the addition of multiwalled carbon nanotubes on the uniform distribution of TiC nanoparticles in aluminum nanocomposites. Scr. Mater. 2014, 72–73, 25–28. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.Y.; Jia, Y.W. Nanoparticle-inhibited growth of primary aluminum in Al–10Si alloys. Acta Mater. 2016, 103, 252–263. [Google Scholar] [CrossRef]
- De Cicco, M.P.; Turng, L.S.; Li, X.; Perepezko, J.H. Nucleation catalysis in aluminum alloy A356 using nanoscale inoculants. Metall. Mater. Trans. A 2011, 42, 2323–2330. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: New York, NY, USA, 2011. [Google Scholar]
- Médout-Marère, V.; Partyka, S.; Chauveteau, G.; Douillard, J.M. Surface heterogeneity of passively oxidized silicon carbide particles: Vapor adsorption isotherms. J. Colloid Interf. Sci. 2003, 262, 309–320. [Google Scholar] [CrossRef]
- Chen, X.; Levi, A.; Tosatti, E. Hamaker constant calculations and surface melting of metals. Surf. Sci. 1991, 251, 641–644. [Google Scholar] [CrossRef]
- Freitas, R. Nanomedicine; Southgate Publishers: Georgetown, TX, USA, 1999. [Google Scholar]
- Sinnott, S.B.; Dickey, E.C. Ceramic/metal interface structures and their relationship to atomic- and meso-scale properties. Mater. Sci. Eng. R 2003, 43, 1–59. [Google Scholar] [CrossRef]
- Saiz, E.; Cannon, R.M.; Tomsia, A.P. Energetics and atomic transport at liquid metal/Al2O3 interfaces. Acta Mater. 1999, 45, 4209–4220. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, H.; Wang, Z.; Li, Q.; Wu, J. Mechanical properties and corrosion behavior of Al/SiC composites. J. Alloy Compd. 2016, 678, 23–30. [Google Scholar] [CrossRef]
- Saboori, A.; Padovano, E.; Pavese, M.; Badini, M. Novel Magnesium Elektron21-AlN Nanocomposites Produced by Ultrasound-Assisted Casting; Microstructure, Thermal and Electrical Conductivity. Materials 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Yan, H. Solid–liquid interface dynamics during solidification of Al 7075–Al2O3np based metal matrix composites. Mater. Des. 2016, 94, 148–158. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Properties and deformation behaviour of Mg-Y2O3 nanocomposites. Acta Mater. 2007, 55, 5115–5121. [Google Scholar] [CrossRef]
- Loucif, A.; Figueiredo, R.B.; Baudin, T.; Brisset, F.; Chemam, R.; Langdon, T.G. Ultrafine grains and the Hall-Petch relationship in an Al-Mg-Si alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2012, 532, 139–145. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites: A model for predicting their yield strength. Scr. Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]
- Nardone, V.C.; Prewo, K.M. On the strength of discontinuous silicon carbide reinforced aluminum composites. Scr. Metall. 1986, 20, 43–48. [Google Scholar] [CrossRef]
- Sanaty-Zadeh, A. Comparison between current models for the strength of particulate-reinforced metal matrix nanocomposites with emphasis on consideration of Hall-Petch effect. Mater. Sci. Eng. A 2012, 531, 112–118. [Google Scholar] [CrossRef]
- Erman, A.; Groza, J.; Li, X.; Choi, H.; Cao, G. Nanoparticle effects in cast Mg-1wt% SiC nano-composites. Mater. Sci. Eng. A 2012, 558, 39–43. [Google Scholar] [CrossRef]
- El-Mahallawi, I.; Abdelkader, H.; Yousef, L.; Amer, A.; Mayer, J.; Schwedt, A. Influence of Al2O3 nano-dispersions on microstructure features and mechanical properties of cast and T6 heat-treated Al-Si hypoeutectic Alloys. Mater. Sci. Eng. A 2012, 556, 76–87. [Google Scholar] [CrossRef]
- Su, H.; Gao, W.; Feng, Z.; Lu, Z. Processing, microstructure and tensile properties of nano-sized Al2O3 particle reinforced aluminum matrix composites. Mater. Des. 2012, 36, 590–596. [Google Scholar] [CrossRef]

| Material | Alloy Composition (wt.%) | Impurity Content (≤wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Mg | Si | Ti | Fe | Mn | Cu | Zn | |
| A356 | BAL | 0.3–0.45 | 6.5–7.5 | 0.08–0.2 | 0.12 | 0.05 | 0.1 | 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Yu, J. Fabrication of Al2O3/SiC/Al Hybrid Nanocomposites Through Solidification Process for Improved Mechanical Properties. Metals 2018, 8, 572. https://doi.org/10.3390/met8080572
Jiang D, Yu J. Fabrication of Al2O3/SiC/Al Hybrid Nanocomposites Through Solidification Process for Improved Mechanical Properties. Metals. 2018; 8(8):572. https://doi.org/10.3390/met8080572
Chicago/Turabian StyleJiang, Dapeng, and Jiakang Yu. 2018. "Fabrication of Al2O3/SiC/Al Hybrid Nanocomposites Through Solidification Process for Improved Mechanical Properties" Metals 8, no. 8: 572. https://doi.org/10.3390/met8080572