Surface Characterization and Secondary Electron Emission Properties of Alumina Containing MgO Film on Ag-Mg-Al Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. SEE Testing Arrangement
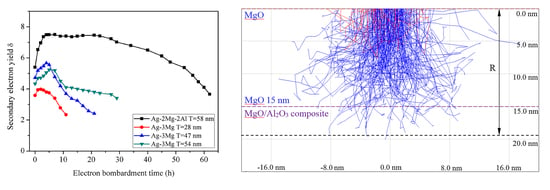
2.3. Surface Characterization and Secondary Electron Distribution Simulation
3. Results and Discussion
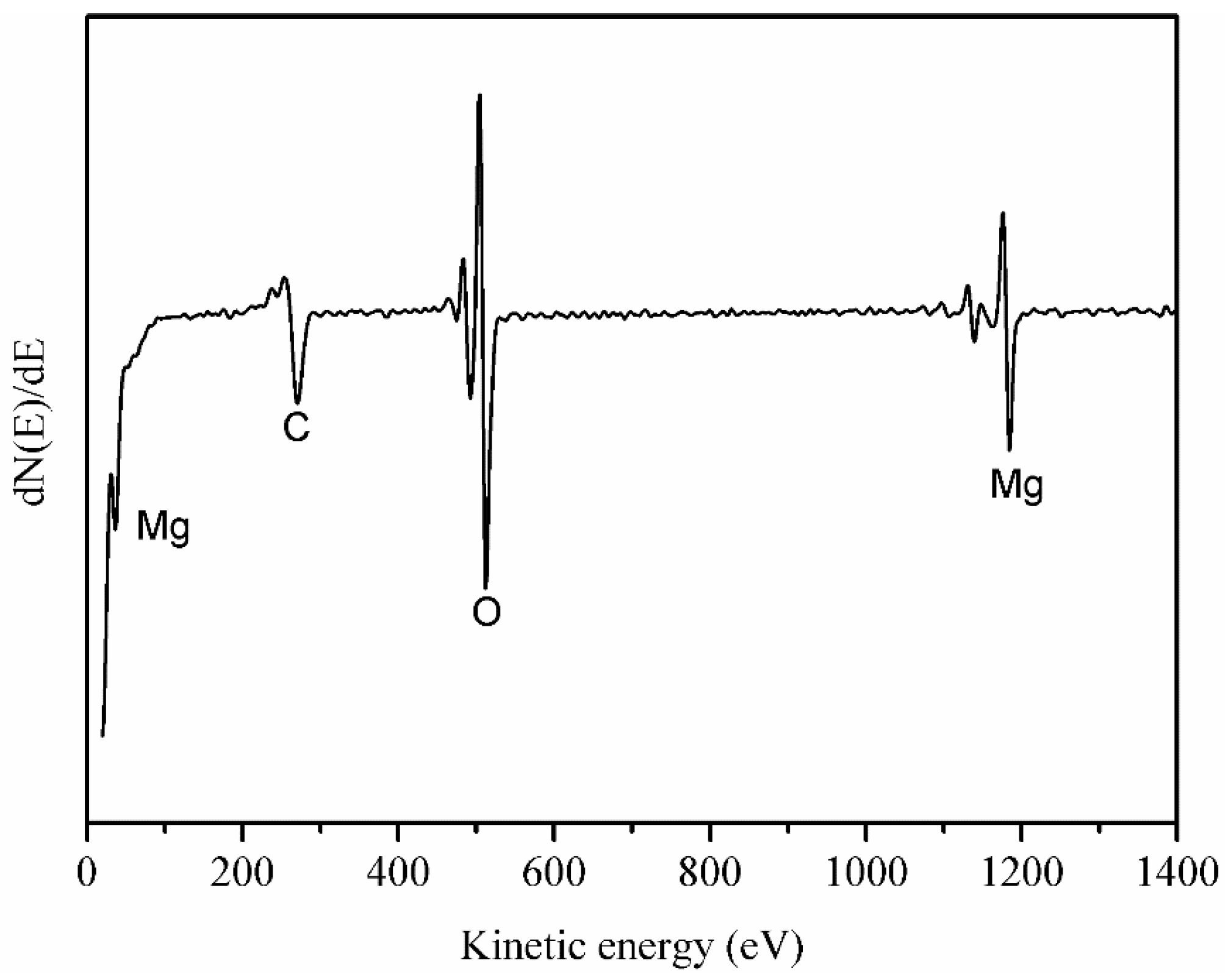
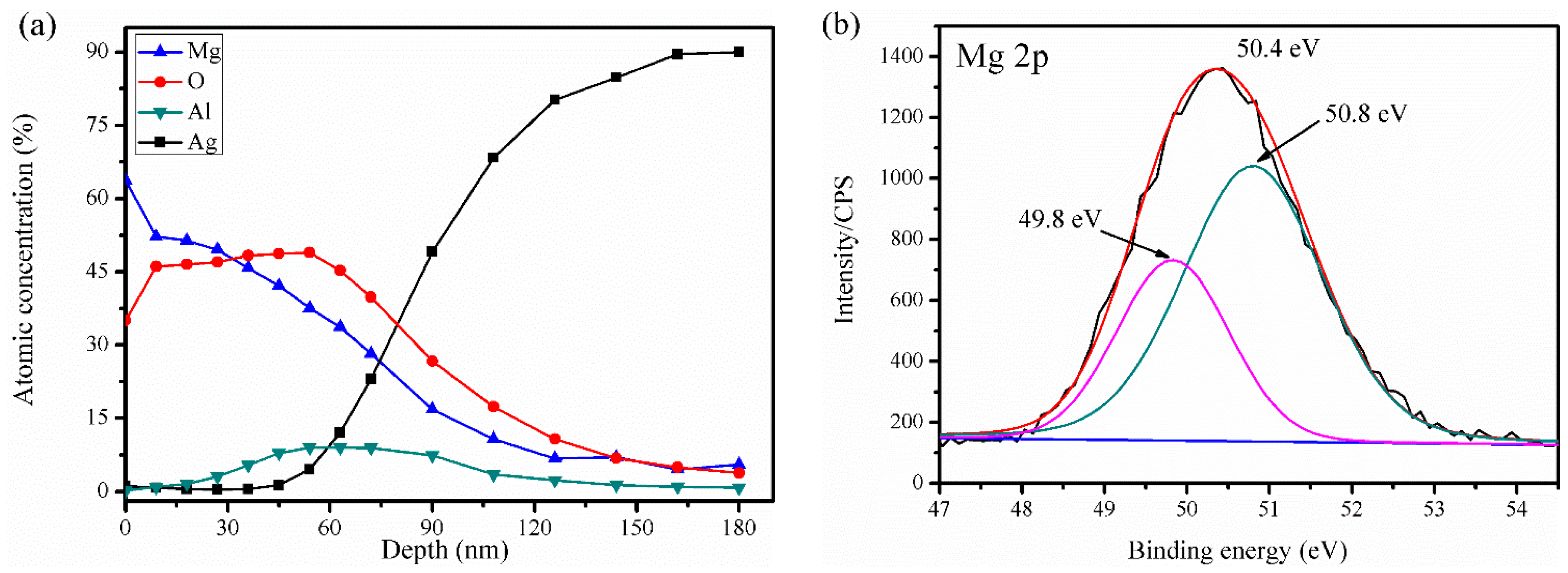
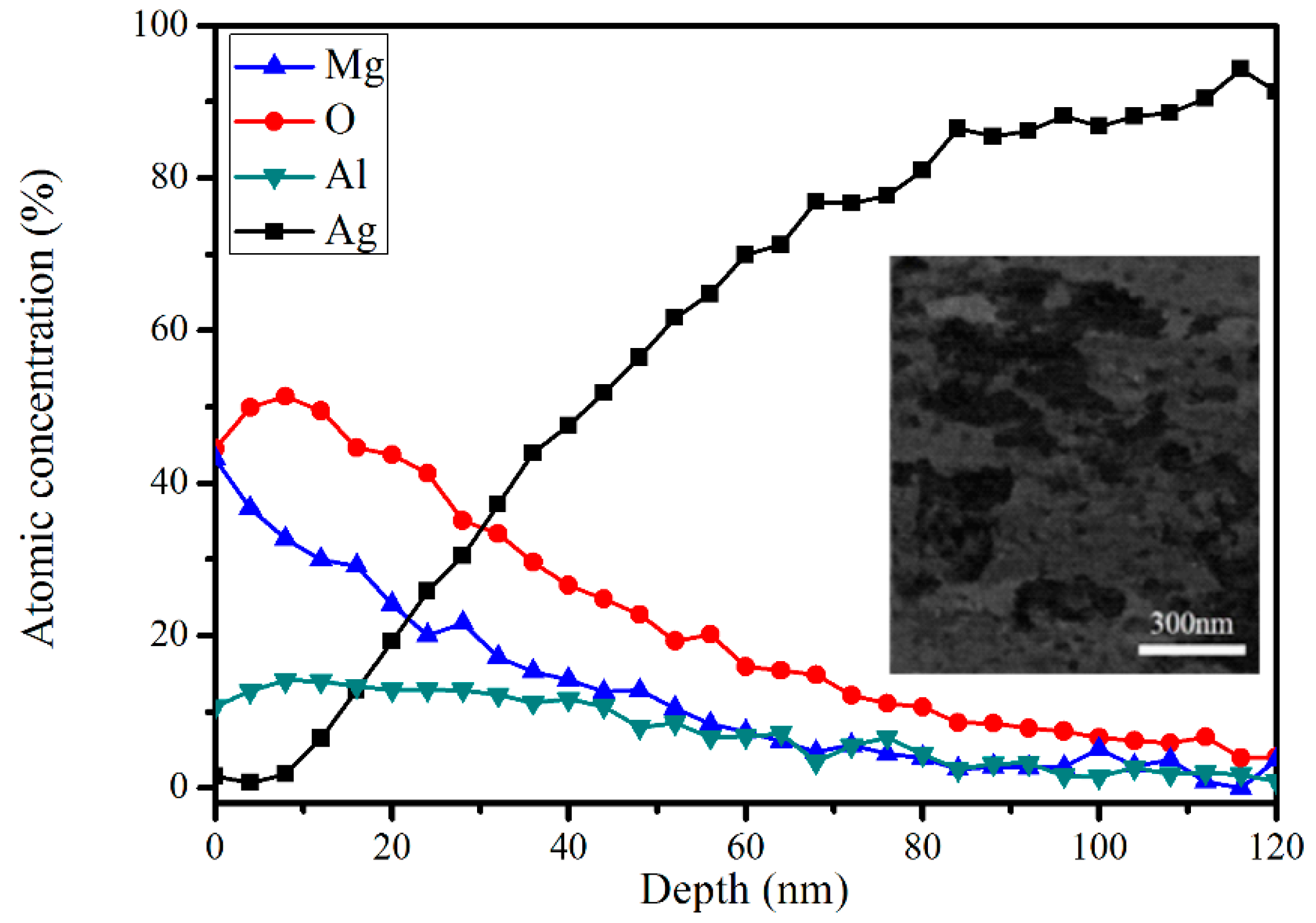
3.1. Surface Morphology and Composition
3.2. Oxidation Process during Thermal Activation
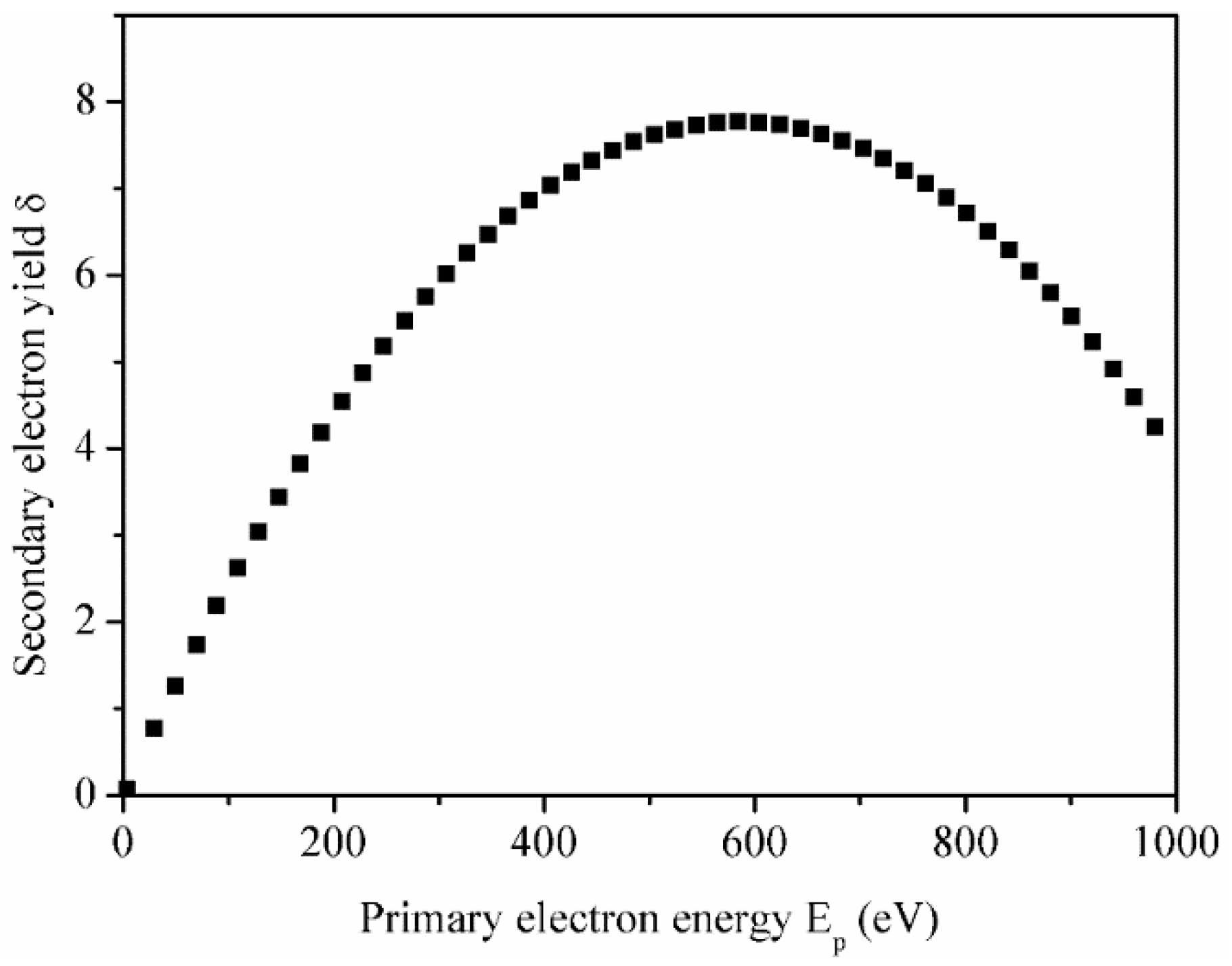
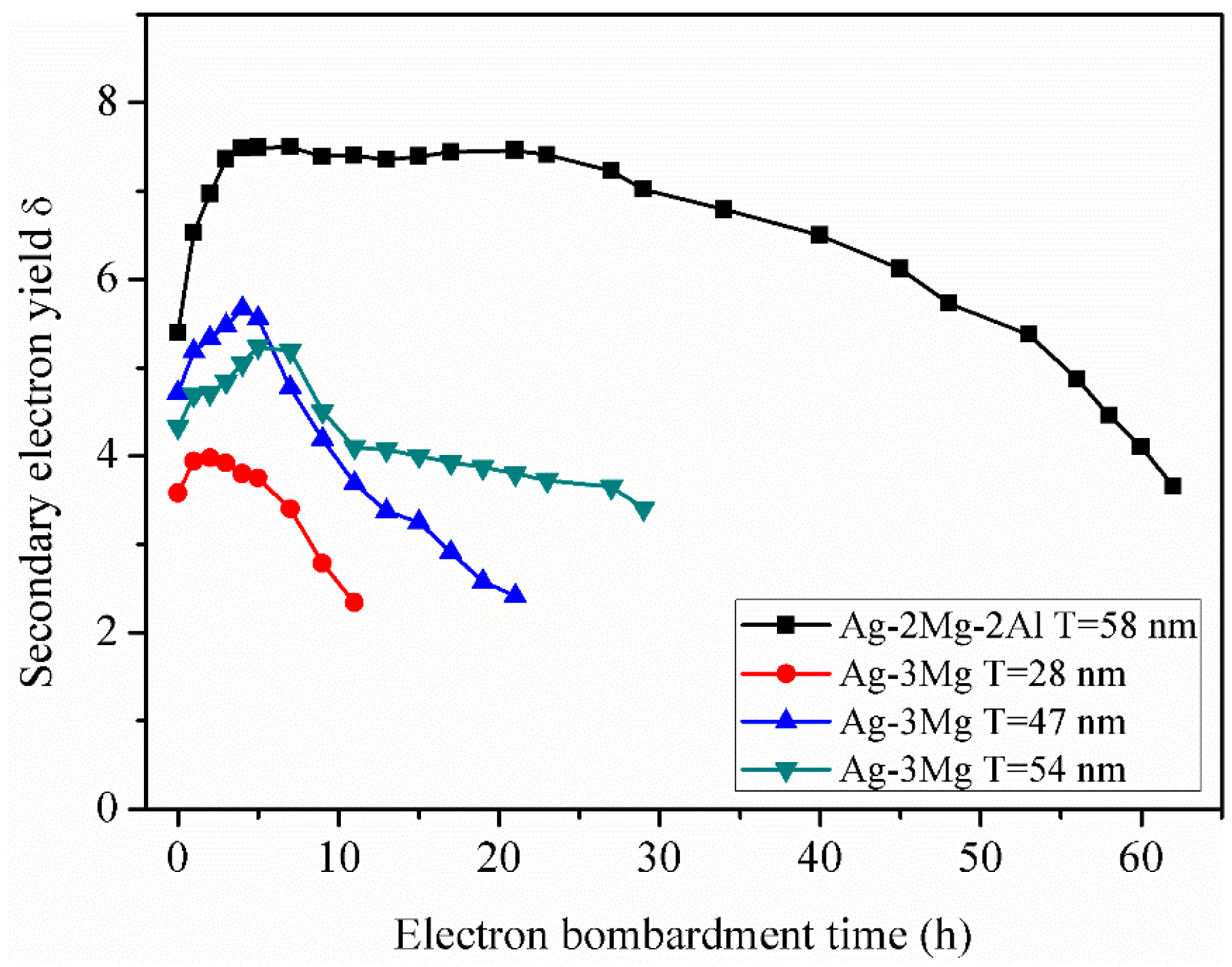
3.3. Secondary Electron Yield and Electron Bombardment Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schweda, H.S.; Thomann, P.; Busca, G. Characterization of a cesium beam detector. In Proceedings of the 2nd European Frequencey and Time Forum, Neuchatel, Switzerland, 16–18 March 1988; pp. 445–459. [Google Scholar]
- Yang, F.; Wang, J.; Liu, W.; Liu, X.; Zhou, M. Studies on the pressed yttrium oxide-tungsten matrix as a possible dispenser cathode material. Mater. Chem. Phys. 2015, 149–150, 288–294. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Ren, Z.; Yang, F.; Wang, Y.; Zhou, M. Secondary electron emission of Y2O3–Mo cermet cathode. Mater. Res. Bull. 2010, 45, 324–328. [Google Scholar] [CrossRef]
- Tamboli, S.H.; Singh, C.B.; Patil, R.B.; Puri, V.; Puri, R.K.; Singh, V.; Luo, M.F. Enhanced secondary electron emission yield of MgO thin films by vapor chopping technique for plasma display panels. J. Nanoelectron. Opt. 2011, 6, 156–161. [Google Scholar] [CrossRef]
- Tao, S.; Chan, H.; van der Graaf, H. Secondary electron emission materials for transmission dynodes in novel photomultipliers: a review. Materials 2016, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.; Yater, J.; Hor, C.; Abrams, R. Secondary electron emission studies. Appl. Surf. Sci. 1997, 111, 251–258. [Google Scholar] [CrossRef]
- Song, B.; Shen, W.; Mu, H.; Deng, J.; Hao, X.; Zhang, G. Measurements of secondary electron emission from dielectric window materials. IEEE Trans. Plasma Sci. 2013, 41, 2117–2122. [Google Scholar] [CrossRef]
- Wang, B.; Wu, E.; Wang, Y.; Xiong, L.; Liu, S. Activation treatment effects on characteristics of BeO layer and secondary electron emission properties of an activated Cu-Be alloy. Appl. Surf. Sci. 2015, 355, 19–25. [Google Scholar] [CrossRef]
- Wargo, P.; Haxby, B.V.; Shepherd, W.G. Preparation and properties of thin film MgO secondary emitters. J. Appl. Phys. 1956, 27, 1311–1316. [Google Scholar] [CrossRef]
- Choi, E.; Oh, H.; Kim, Y.; Ko, J.; Lim, J.; Kim, J.; Kim, D.; Cho, G.; Kang, S. Measurement of secondary electron emission coefficient (γ) of MgO protective layer with various crystallinities. JPN J. Appl. Phys. 1998, 37, 7015–7018. [Google Scholar] [CrossRef]
- Insepov, Z.; Ivanov, V.; Frisch, H. Comparison of candidate secondary electron emission materials. Nucl. Instrum. Meth. B 2010, 268, 3315–3320. [Google Scholar] [CrossRef]
- Lapington, J.S.; Thompson, D.P.; May, P.W.; Fox, N.A.; Howorth, J.; Milnes, J.; Taillandier, V. Investigation of the secondary emission characteristics of CVD diamond films for electron amplification. Nucl. Instrum. Meth. A 2009, 610, 253–257. [Google Scholar] [CrossRef]
- Rappaport, P. Methods of processing silver-magnesium secondary emitters for electron tubes. J. Appl. Phys. 1954, 25, 288–292. [Google Scholar] [CrossRef]
- Whetten, N.R.; Laponsky, A.B. Secondary electron emission from MgO thin films. J. Appl. Phys. 1959, 30, 432–435. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Zhou, F.; Liu, W.; Wang, F.; Lai, C. Influence of activation parameters on the thickness of MgO thin film on Ag-3Mg alloy and its secondary electron emission property. Mater. Res. Bull. 2017, 96, 35–39. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, T.; Yu, S.; Jin, S.; Heo, J.; Yi, W.; Jeon, D.; Kim, J.M. Thickness effect on secondary electron emission of MgO layers. Appl. Surf. Sci. 2001, 174, 62–69. [Google Scholar] [CrossRef]
- Singh, B.; Sarkar, S.; Singh, V.; Ram, S.K.; Kumar, S. Ion-induced secondary electrons emission measurement from MgO films deposited on multiwalled carbon nanotubes. Mater. Lett. 2012, 76, 131–134. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, J.; Hong, K.S.; Youn, H. Ion-induced secondary electron emission behavior of sol–gel-derived MgO thin films used for protective layers in alternating current plasma display panels. J. Appl. Phys. 2002, 92, 2855–2860. [Google Scholar] [CrossRef]
- Nakano, T.; Fujimoto, T.; Baba, S. Measurement of surface roughness and ion-induced secondary electron emission coefficient of MgO films prepared by high-pressure sputter deposition. Vacuum 2004, 74, 595–599. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Kupfer, H.; Richter, F.; Giegengack, H.; Hoyer, W. Structure and secondary electron emission properties of MgO films deposited by pulsed mid-frequency magnetron sputtering. J. Appl. Phys. 2003, 93, 1422–1427. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, F.; Wang, J.; Liu, W.; Zhang, Q.; Yin, Q. Characterization of MgO/Al2O3 composite film prepared by DC magnetron sputtering and its secondary electron emission properties. J. Electron. Mater. 2018, 47, 4116–4123. [Google Scholar] [CrossRef]
- Fan, J.C.C.; Henrich, V.E. Preparation and properties of sputtered MgO/Au, MgO/Ag, and MgO/Ni cermet films. J. Appl. Phys. 1974, 45, 3742–3748. [Google Scholar] [CrossRef]
- Henrich, V.E.; Fan, J.C.C. High-efficiency secondary-electron emission from sputtered MgO-Au cermets. Appl. Phys. Lett. 1973, 23, 7–8. [Google Scholar] [CrossRef]
- Ritz, V.H.; Shih, A.; Sobocinski, B. Performance of MgO/Au cermet films as secondary electron emission cold cathodes. Surf. Interface Anal. 1992, 18, 514–524. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Wei, Q.; Wu, S.; Hua, X.; Zhang, J. Electron-induced secondary electron emission properties of MgO/Au composite thin film prepared by magnetron sputtering. J. Electron. Mater. 2017, 46, 1466–1475. [Google Scholar] [CrossRef]
- Pan, Q. Mechanism of secondary electron emission of Cu-Al-Mg alloy. Rare Metals 1995, 14, 217–222. [Google Scholar]
- Li, Z.; Ma, J.; Feng, X.; Du, X.; Wang, W.; Wang, M. Effect of thermal annealing on the optical and structural properties of γ-Al2O3 films prepared on MgO substrates by MOCVD. Ceram. Int. 2016, 42, 551–558. [Google Scholar] [CrossRef]
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO V2.42—A fast and easy-to-use modeling tool for scanning electron microscopy and microanalysis users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yaws, C.L. Handbook of Vapor Pressure, Volume 4: Inorganic Compounds and Elements, 1st ed.; Gulf publishing: Houston, TX, USA, 1995. [Google Scholar]
- Zhou, X.; Thompson, G.E.; Skeldon, P.; Wood, G.C.; Shimizu, K.; Habazaki, H. Film formation and detachment during anodizing of Al-Mg alloys. Corros. Sci. 1999, 41, 1599–1613. [Google Scholar] [CrossRef]
- Godlstein, B.; Dresner, J. Growth of MgO films with high secondary electron emission on Al-Mg alloys. Surf. Sci. 1978, 71, 15–26. [Google Scholar]
- Fournier, V.; Marcus, P.; Olefjord, I. Oxidation of magnesium. Surf. Interface Anal. 2002, 34, 494–497. [Google Scholar] [CrossRef]
- Yao, H.B.; Li, Y.; Wee, A.T.S. An XPS investigation of the oxidationrcorrosion of melt-spun Mg. Appl. Surf. Sci. 2000, 158, 112–119. [Google Scholar] [CrossRef]
- Li, M.; Xu, M.; Zou, J.; Tao, H.; Wang, L.; Zhou, Z.; Peng, J. Realization of Al2O3/MgO laminated structure at low temperature for thin film encapsulation in organic light-emitting diodes. Nanotechnology 2016, 27, 494003. [Google Scholar] [CrossRef] [PubMed]
- Parry-Jones, A.C.; Weightman, P.; Andrews, P.T. The M4, 5N4, 5N4, 5 Auger spectra of Ag, Cd, In and Sn. J. Phys. C Solid State Phys. 1979, 12, 1587–1600. [Google Scholar] [CrossRef]
- Scholtz, J.J.; Dijkkamp, D.; Schmitz, R.W.A. Secondary electron emission properties. Philips J. Res. 1996, 50, 375–389. [Google Scholar] [CrossRef] [Green Version]
- Uhm, H.S.; Choi, J.H.; Cho, G.; Park, B.J.; Jung, R.J.; Choi, E.H. Characterization of electronic structure in dielectric materials by making use of the secondary electron emission. Curr. Appl. Phys. 2013, 13, 396–402. [Google Scholar] [CrossRef]
- Young, J.R. Dissipation of Energy by 2.5–10 kev Electrons in Al2O3. J. Appl. Phys. 1957, 28, 524–525. [Google Scholar] [CrossRef]
- Rajopadhye, N.R.; Bhoraskar, S.V. Secondary electron emission of sputtered alumina films. Pramana—J. Phys. 1985, 25, 327–334. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, J. Bond dissociation energies. In CRC Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 1567–1571. [Google Scholar]
- Dresner, J.; Goldstein, B. Dissociation of MgO films under heat and electron bombardment and its effects on secondary emission. J. Appl. Phys. 1976, 47, 1038–1043. [Google Scholar] [CrossRef]


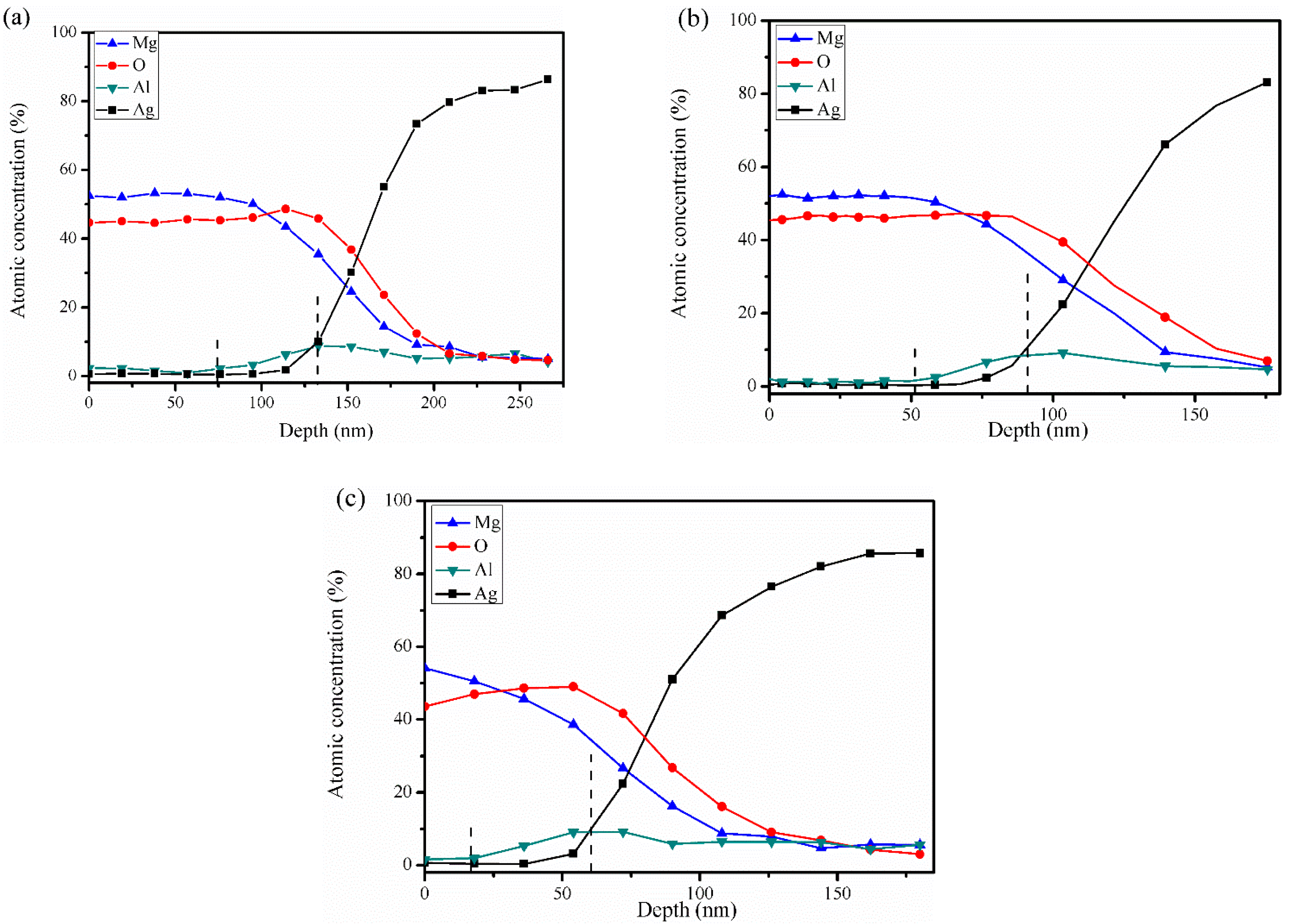


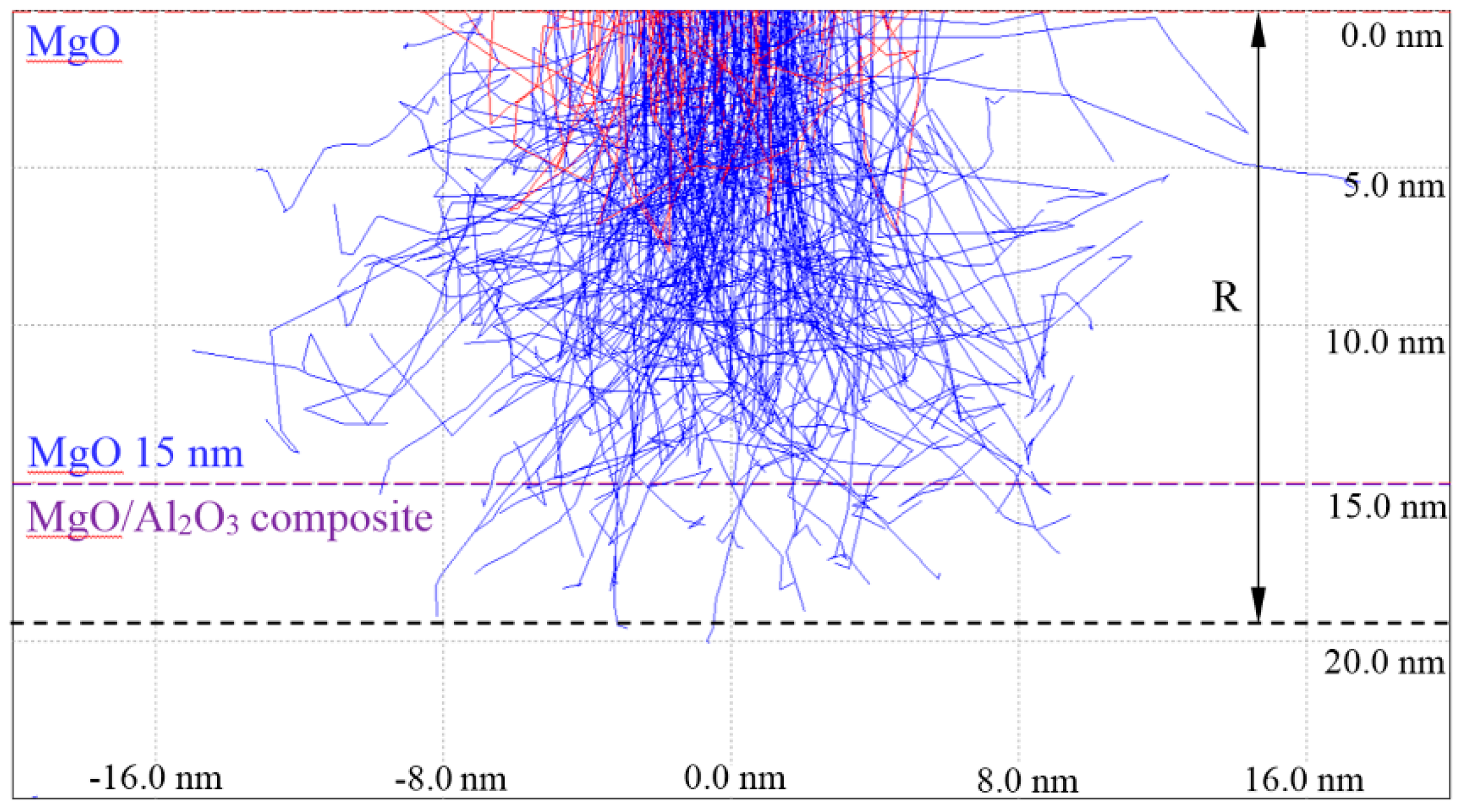
| Activation Parameters | TMgO (nm) | Originating Depth of Al (nm) | ||
|---|---|---|---|---|
| Temperature (°C) | Duration Time (min) | PO2 (Pa) | ||
| 500 | 30 | 20.0 | 60 | 15 |
| 600 | 30 | 15.0 | 133 | 75 |
| 600 | 30 | 20.0 | 90 | 51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Zhang, Q.; Wang, F.; Wang, J.; Yang, Y.; Lai, C.; Liu, W.; Wang, J. Surface Characterization and Secondary Electron Emission Properties of Alumina Containing MgO Film on Ag-Mg-Al Alloy. Metals 2018, 8, 570. https://doi.org/10.3390/met8080570
Zhou F, Zhang Q, Wang F, Wang J, Yang Y, Lai C, Liu W, Wang J. Surface Characterization and Secondary Electron Emission Properties of Alumina Containing MgO Film on Ag-Mg-Al Alloy. Metals. 2018; 8(8):570. https://doi.org/10.3390/met8080570
Chicago/Turabian StyleZhou, Fan, Quan Zhang, Feifei Wang, Jing Wang, Yunfei Yang, Chen Lai, Wei Liu, and Jinshu Wang. 2018. "Surface Characterization and Secondary Electron Emission Properties of Alumina Containing MgO Film on Ag-Mg-Al Alloy" Metals 8, no. 8: 570. https://doi.org/10.3390/met8080570