Hydrogen-Induced Phase Transformation and Microstructure Evolution for Ti-6Al-4V Parts Produced by Electron Beam Melting
Abstract
:1. Introduction
2. Materials and Experimental Procedure
2.1. Samples Preparation
2.2. Experimental Procedure
3. Results and Discussion
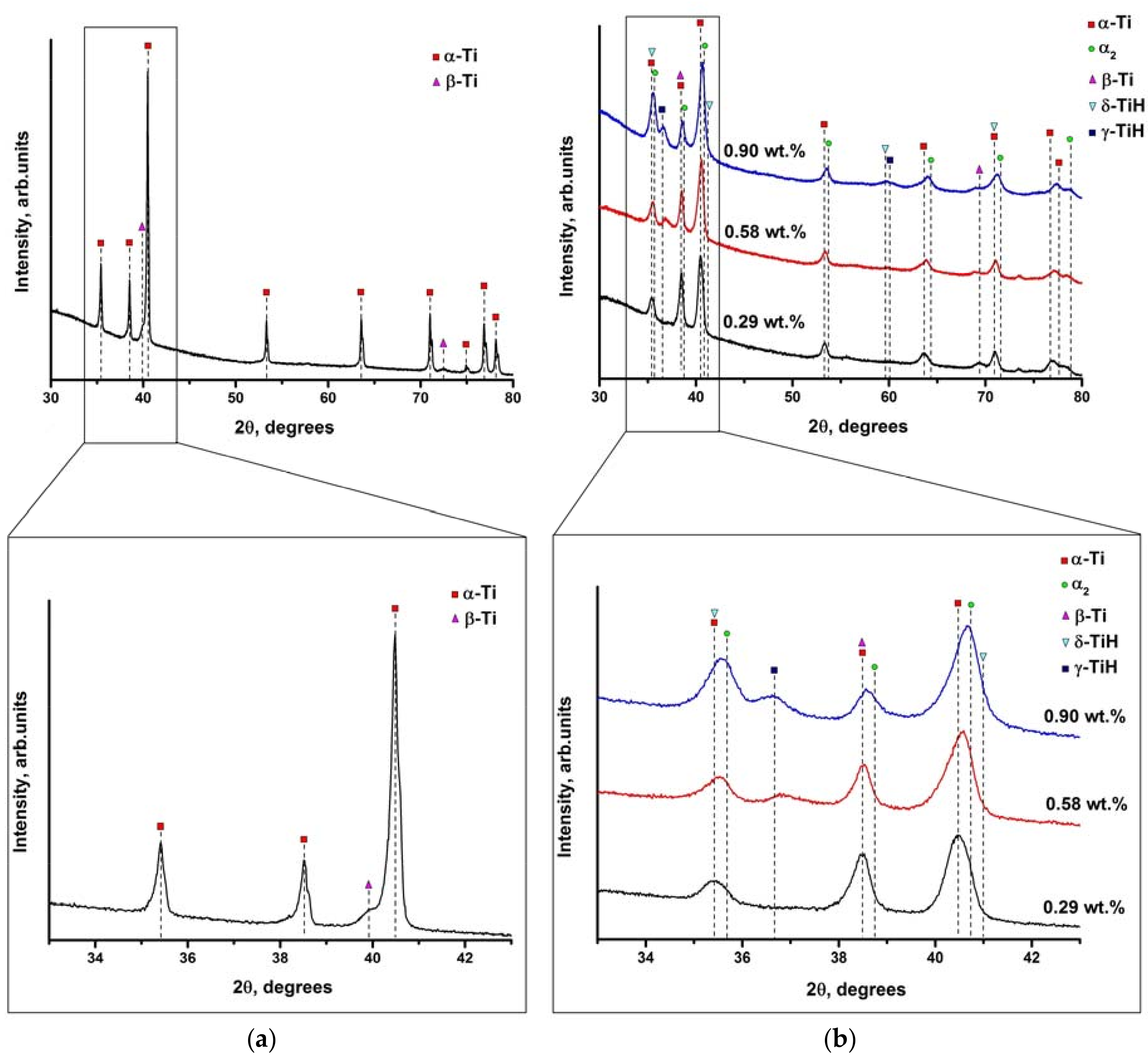
3.1. X-ray Diffraction
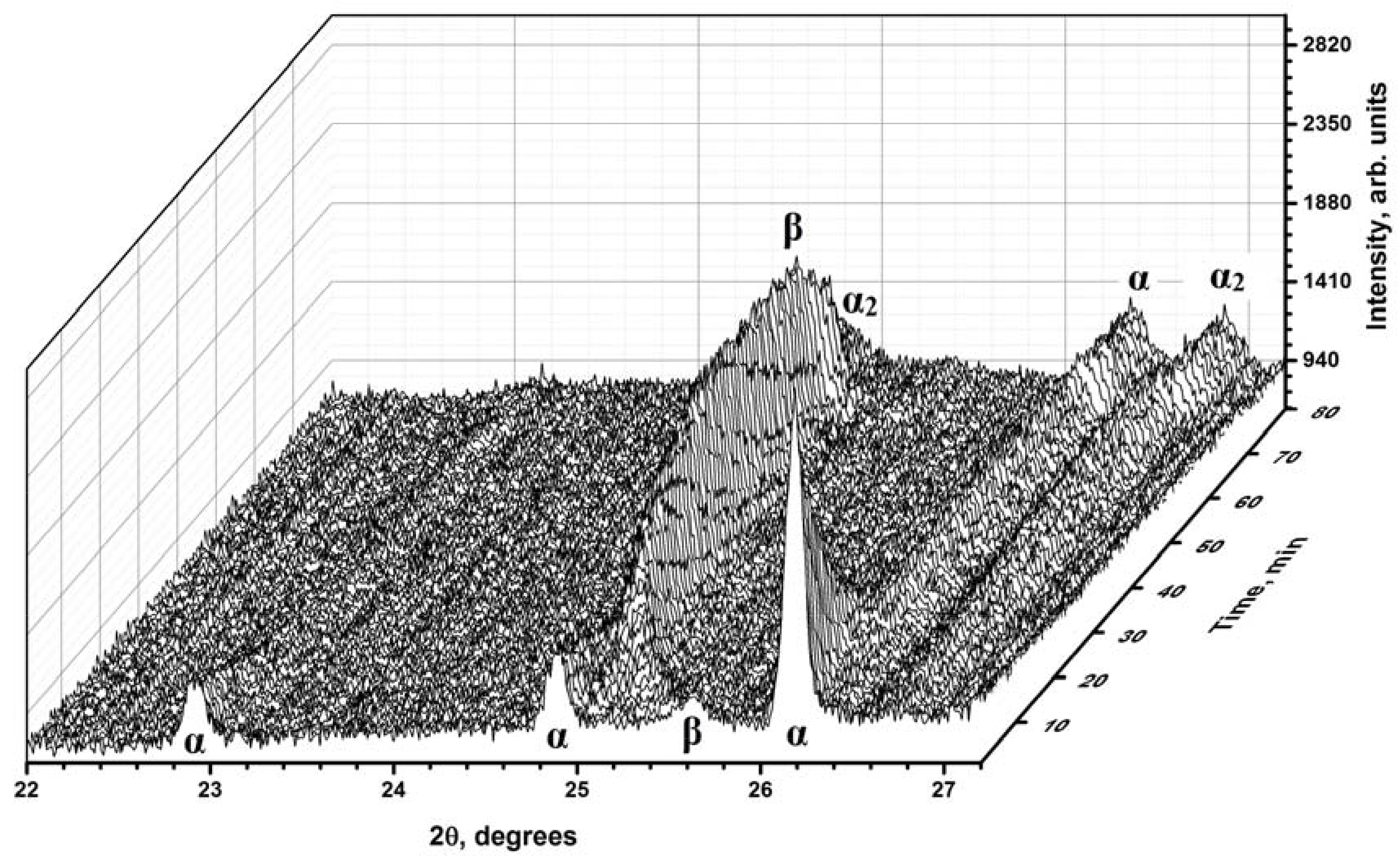
3.2. In-Situ XRD
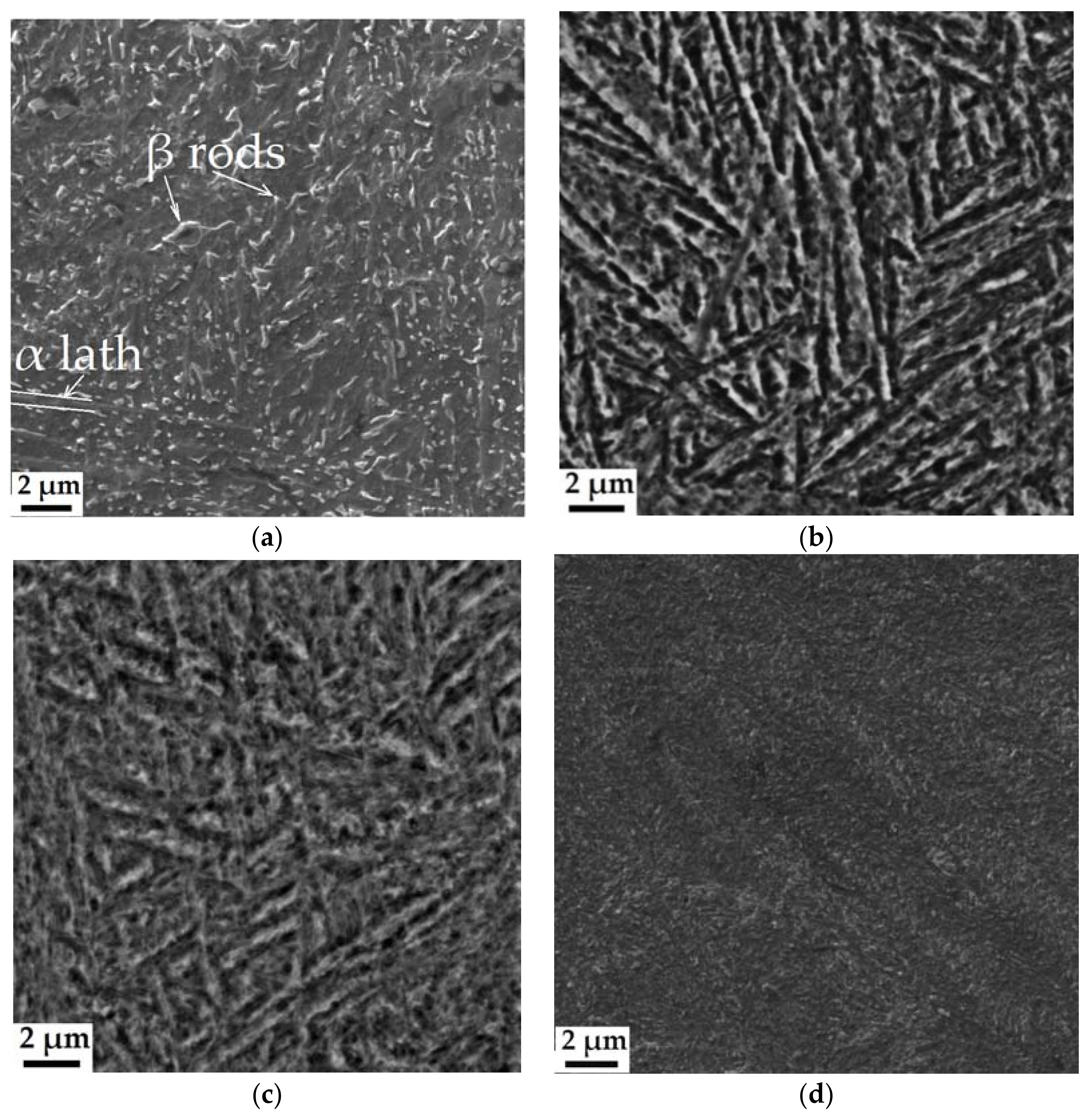
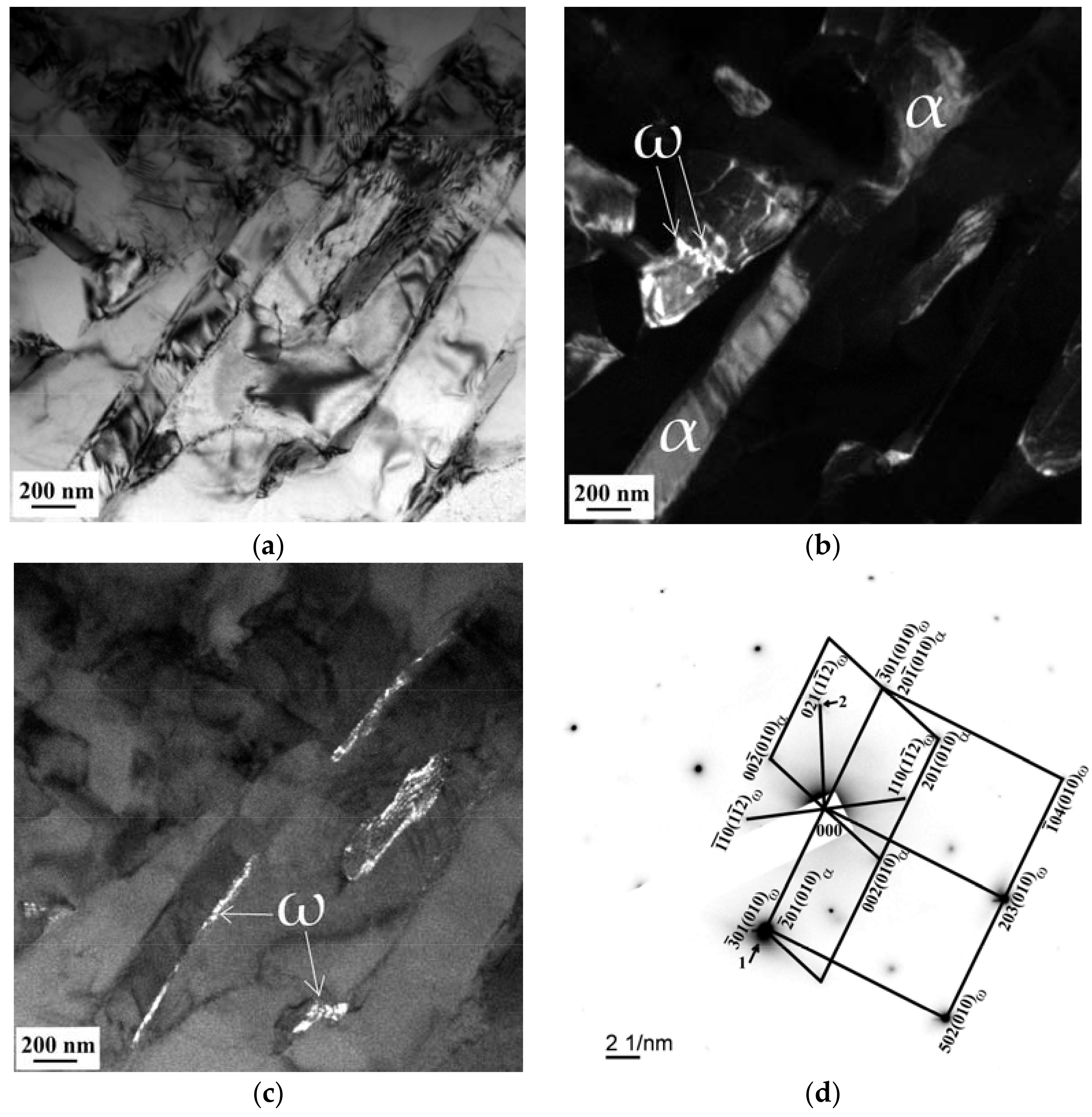
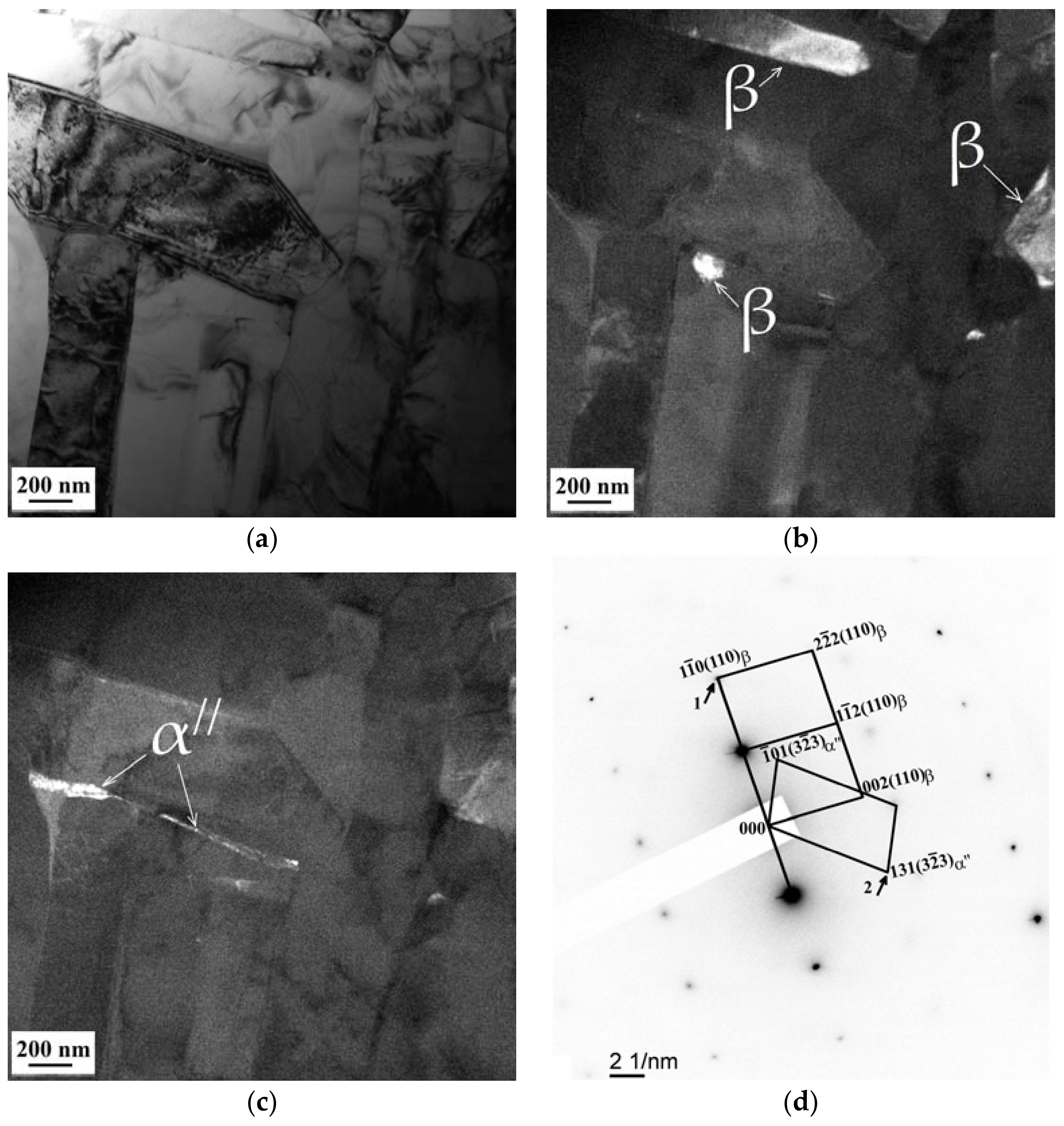
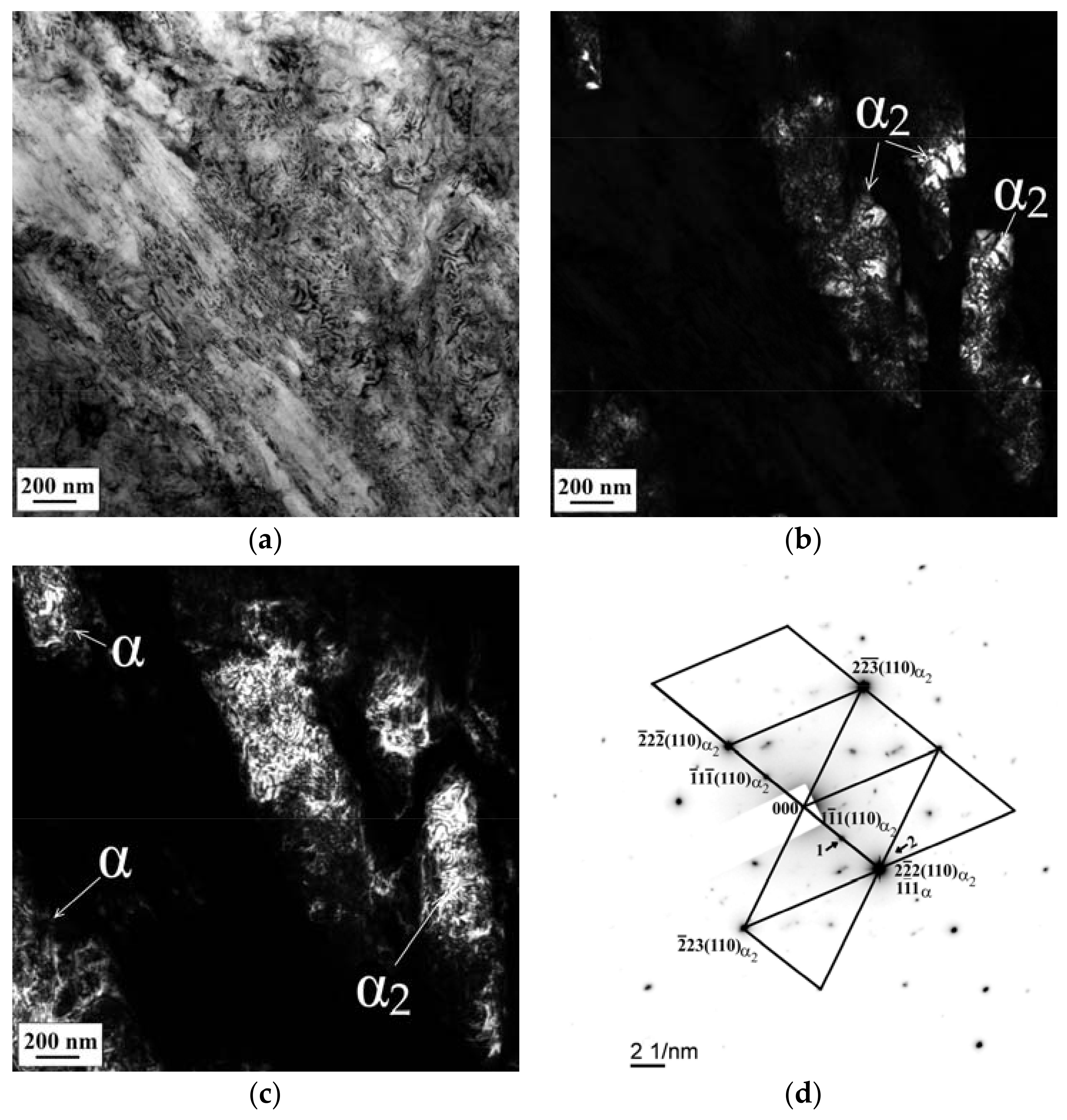
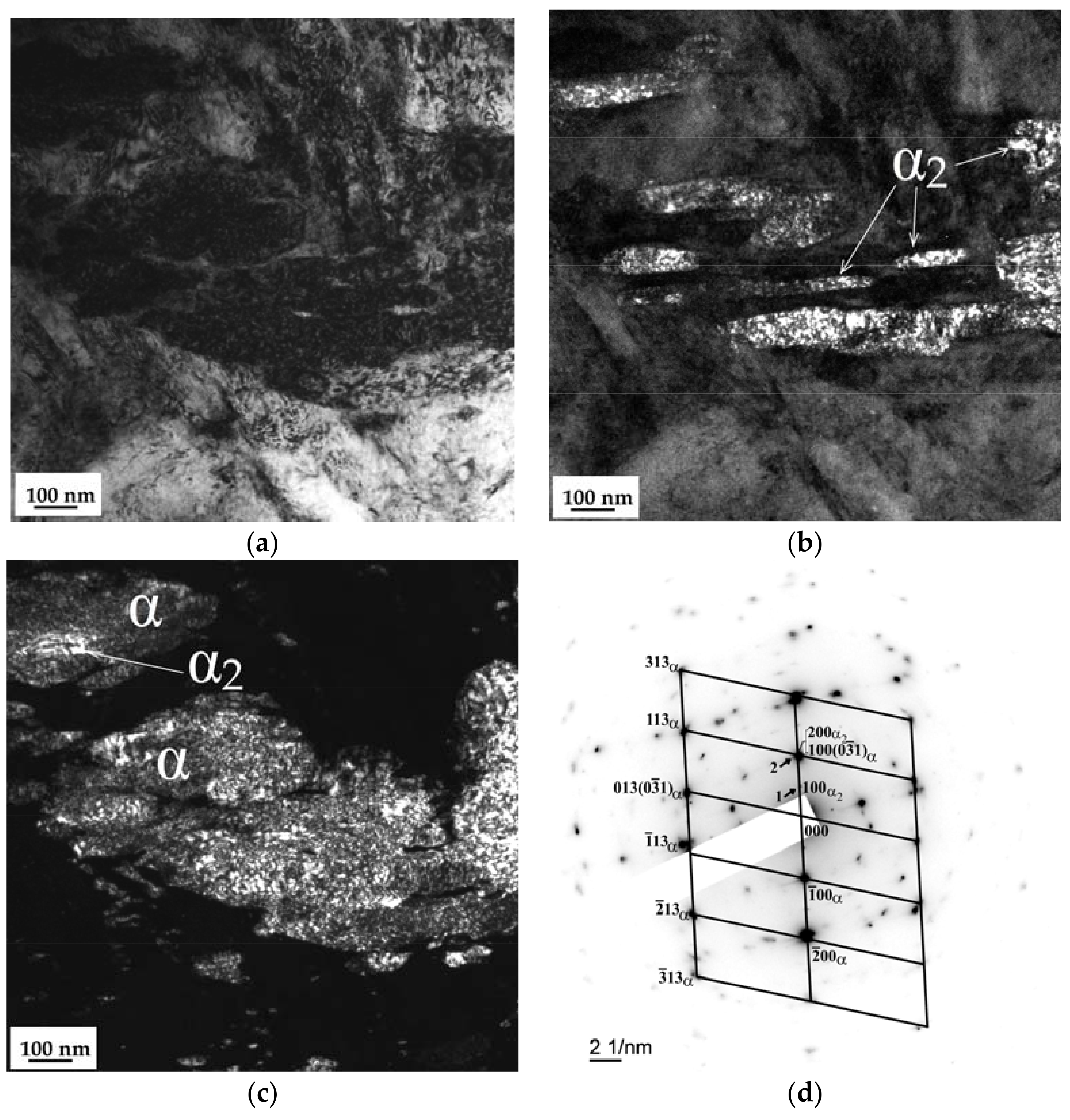
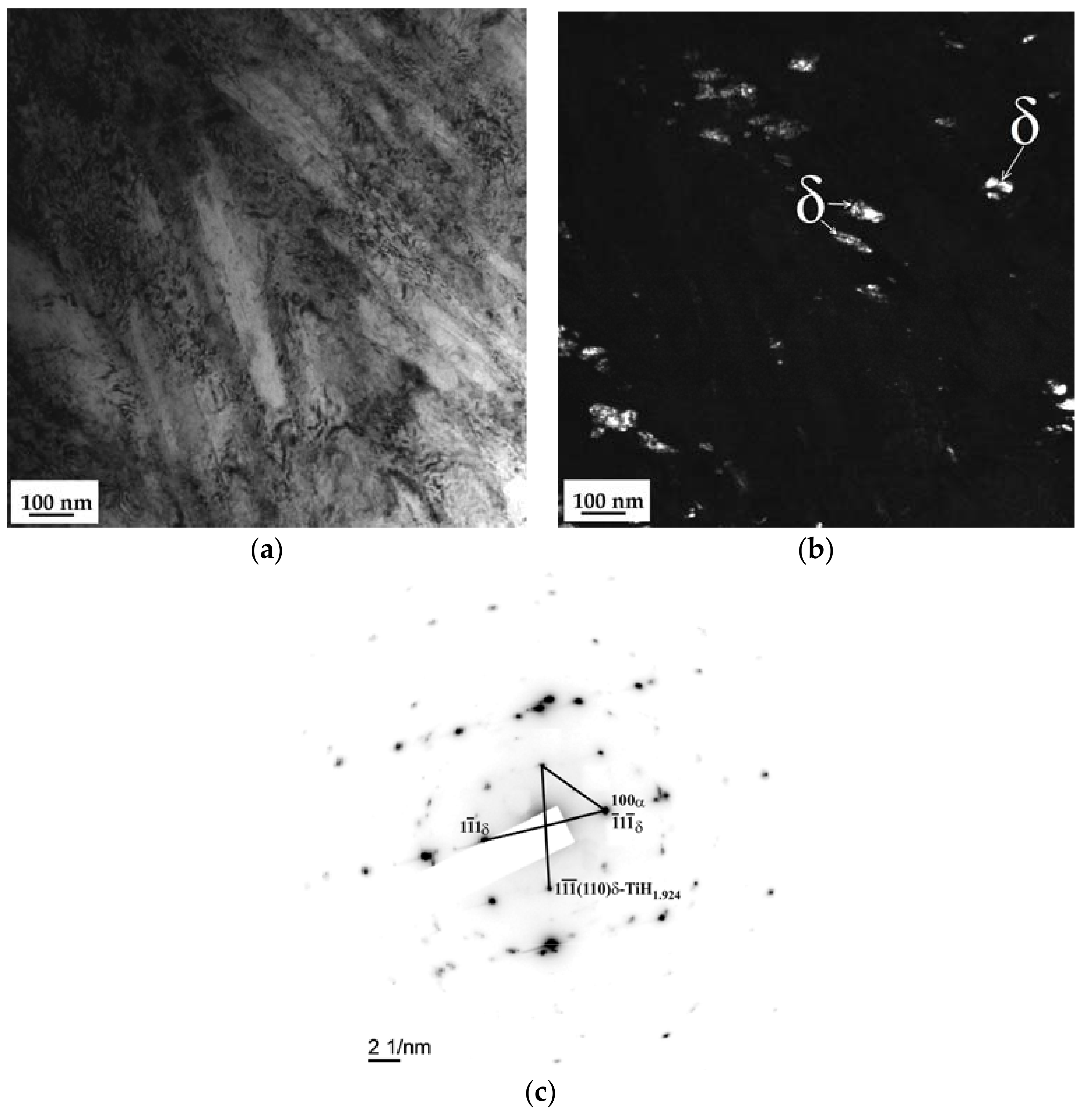
3.3. SEM and TEM Analysis
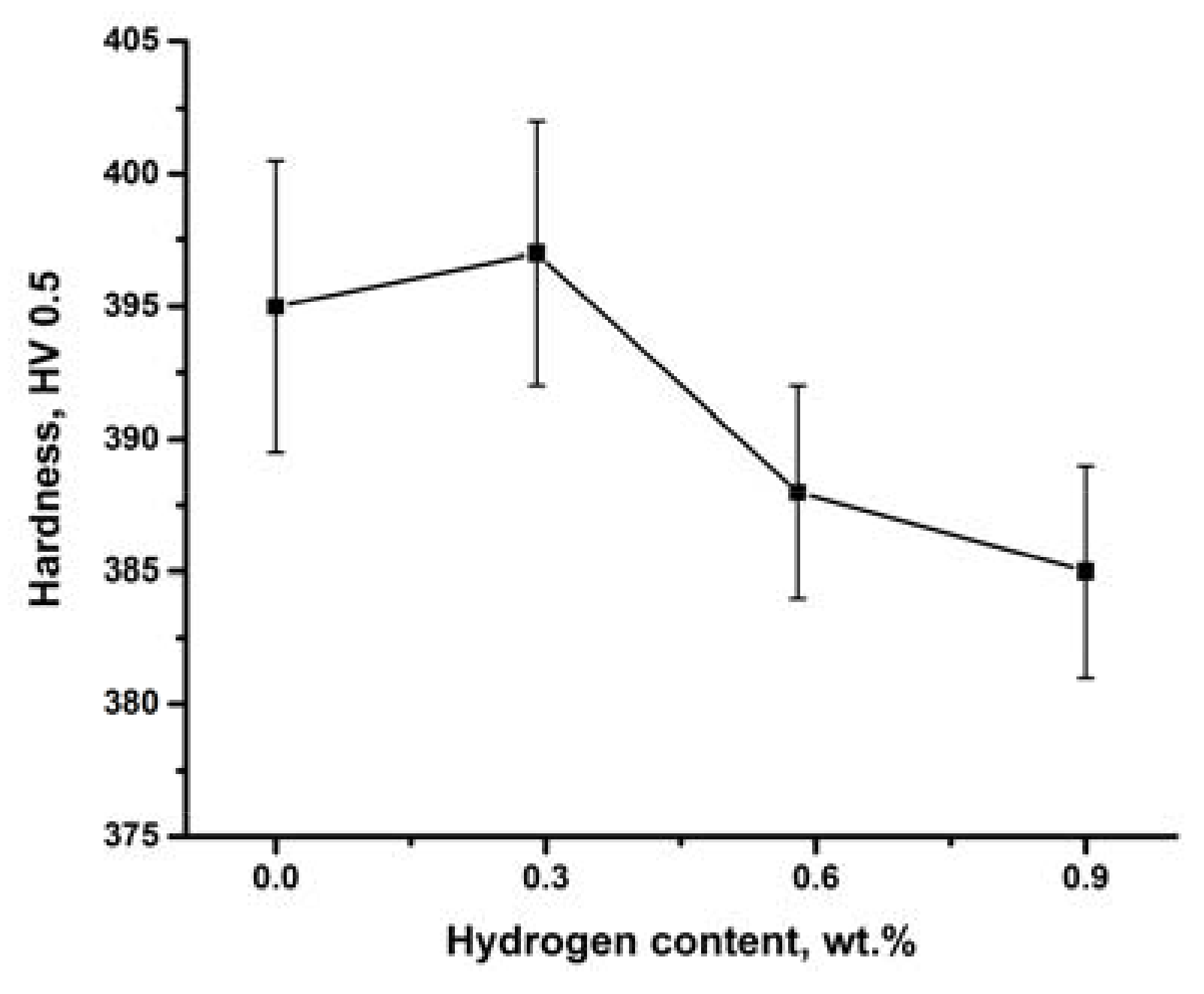
3.4. Microhardness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Hrabe, N.; Quinn, T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), Part 2: Energy input, orientation, and location. Mater. Sci. Eng. A 2013, 573, 271–277. [Google Scholar] [CrossRef]
- Hrabe, N.; Quinn, T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), Part 1: Distance from build plate and part size. Mater. Sci. Eng. A 2013, 573, 264–270. [Google Scholar] [CrossRef]
- Antonysamy, A.A.; Meyer, J.; Prangnell, P.B. Effect of build geometry on the β-grain structure and texture in additive manufacture of Ti6Al4V by selective electron beam melting. Mater. Charact. 2013, 84, 153–168. [Google Scholar] [CrossRef]
- Safdar, A.; Wei, L.-Y.; Snis, A.; Lai, Z. Evaluation of microstructural development in electron beam melted Ti-6Al-4V. Mater. Charact. 2012, 65, 8–15. [Google Scholar] [CrossRef]
- Tan, X.; Kok, Y.; Tan, Y.J.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Leong, K.F.; Chua, C.K. Graded microstructure and mechanical properties of additive manufactured Ti-6Al-4V via electron beam melting. Acta Mater. 2015, 97, 1–16. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D. Hydrogen cracking in titanium-based alloys. J. Alloys Compd. 2005, 404–406, 621–625. [Google Scholar] [CrossRef]
- Murr, L.E. Metallurgy of additive manufacturing: Examples from electron beam melting. Addit. Manuf. 2015, 5, 40–53. [Google Scholar] [CrossRef]
- Algardh, J.K.; Horn, T.; West, H.; Aman, R.; Snis, A.; Engqvist, H.; Lausmaa, J.; Harrysson, O. Thickness dependency of mechanical properties for thin-walled titanium parts manufactured by Electron Beam Melting (EBM)®. Addit. Manuf. 2016, 12, 45–50. [Google Scholar] [CrossRef]
- Al-Bermani, S.S.; Blackmore, M.L.; Zhang, W.; Todd, I. The Origin of Microstructural Diversity, Texture, and Mechanical Properties in Electron Beam Melted Ti-6Al-4V. Metall. Mater. Trans. A 2010, 41, 3422–3434. [Google Scholar] [CrossRef]
- Gil Mur, F.X.; Rodríguez, D.; Planell, J.A. Influence of tempering temperature and time on the α′-Ti-6Al-4V martensite. J. Alloys Compd. 1996, 234, 287–289. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F.H. The Additive Manufacturing (AM) of titanium alloys. Met. Powder Res. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D. High fugacity hydrogen effects at room temperature in titanium based alloys. J. Alloys Compd. 2005, 404–406, 613–616. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Pundt, A.; Kirchheim, R. The effect of residual hydrogen on hydrogenation behavior of titanium thin films. Scr. Mater. 2010, 62, 709–712. [Google Scholar] [CrossRef]
- Tao, J.; Hu, S.; Ji, L. Effect of trace solute hydrogen on the fatigue life of electron beam welded Ti-6Al-4V alloy joints. Mater. Sci. Eng. A 2017, 684, 542–551. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.Q.; Tao, C. Hydrogenation behavior of Ti-25Al-10Nb-3V-1Mo alloy and effect of hydrogen on its microstructure and hot deformability. Int. J. Hydrog. Energy 1997, 22, 125–129. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; He, P.; Feng, J.C. Effect of hydrogen on diffusion bonding of commercially pure titanium and hydrogenated Ti6Al4V alloys. Int. J. Hydrog. Energy 2009, 34, 1108–1113. [Google Scholar] [CrossRef]
- Luo, L.; Su, Y.; Guo, J.; Fu, H. Formation of titanium hydride in Ti-6Al-4V alloy. J. Alloys Compd. 2006, 425, 140–144. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, H.; Zhao, W.; Tian, X.; Hou, H.; Wang, Y. Influence of hydrogenation on microstructures and microhardness of Ti6Al4V alloy. Trans. Nonferr. Met. Soc. China 2008, 18, 506–511. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, H.; Zhong, Y.; Lee, C.S. Effect of thermo hydrogen treatment on lattice defects and microstructure refinement of Ti6Al4V alloy. Int. J. Hydrog. Energy 2010, 35, 6448–6454. [Google Scholar] [CrossRef]
- Shaoqing, Z.; Linruo, Z. Effect of hydrogen on the superplasticity and microstructure of Ti-6Al-4V alloy. J. Alloys Compd. 1995, 218, 233–236. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhou, L.; Liu, P.; Liu, Q.W. Microstructural evolution and hydride precipitation mechanism in hydrogenated Ti-6Al-4V alloy. Int. J. Hydrog. Energy 2009, 34, 9596–9602. [Google Scholar] [CrossRef]
- Mishin, I.P.; Grabovetskaya, G.P.; Zabudchenko, O.V.; Stepanova, E.N. Influence of Hydrogenation on Evolution of Submicrocrystalline Structure of Ti-6Al-4V Alloy upon Exposure to Temperature and Stress. Russ. Phys. J. 2014, 57, 423–428. [Google Scholar] [CrossRef]
- Skvortsova, S.V.; Panin, P.V.; Nochovnaya, N.A.; Grushin, I.A.; Mitropolskaya, N.G. The influence of hydrogen on phase and structural transformations in a titanium alloy VT6. Technol. Light Alloy 2011, 4, 35–40. (In Russian) [Google Scholar]
- Baek, S.-W.; Song, E.J.; Kim, J.H.; Jung, M.; Baek, U.B.; Nahm, S.H. Hydrogen embrittlement of 3-D printing manufactured austenitic stainless steel part for hydrogen service. Scr. Mater. 2017, 130, 87–90. [Google Scholar] [CrossRef]
- Bilgin, G.M.; Esen, Z.; Akın, Ş.K.; Dericioglu, A.F. Optimization of the mechanical properties of Ti-6Al-4V alloy fabricated by selective laser melting using thermohydrogen processes. Mater. Sci. Eng. A 2017, 700, 574–582. [Google Scholar] [CrossRef]
- ASTM F3001-14, Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium ELI (Extra Low Interstitial) with Powder Bed Fusion; ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Kudiiarov, V.N.; Syrtanov, M.S.; Bordulev, Y.S.; Babikhina, M.N.; Lider, A.M.; Gubin, V.E.; Murashkina, T.L. The hydrogen sorption and desorption behavior in spherical powder of pure titanium used for additive manufacturing. Int. J. Hydrog. Energy 2017, 42, 15283–15289. [Google Scholar] [CrossRef]
- Cheng, H.H.; Deng, X.X.; Li, S.L.; Chen, W.; Chen, D.M.; Yang, K. Design of PC based high pressure hydrogen absorption/desorption apparatus. Int. J. Hydrog. Energy 2007, 32, 3046–3053. [Google Scholar] [CrossRef]
- Sadykov, V.; Okhlupin, Y.; Yeremeev, N.; Vinokurov, Z.; Shmakov, A.; Belyaev, V.; Uvarov, N.; Mertens, J. In situ X-ray diffraction studies of Pr2−xNiO4+δ crystal structure relaxation caused by oxygen loss. Solid State Ion. 2014, 262, 918–922. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Pavlova, S.N.; Vinokurov, Z.S.; Shmakov, A.N.; Eremeev, N.F.; Fedorova, Y.E.; Yakimchuk, E.P.; Kriventsov, V.V.; Bolotov, V.A.; Tanashev, Y.Y.; et al. Application of SR Methods for the Study of Nanocomposite Materials for Hydrogen Energy. Phys. Procedia 2016, 84, 397–406. [Google Scholar] [CrossRef]
- Syrtanov, M.S.; Kudiiarov, V.N.; Kashkarov, E.B.; Shmakov, A.N.; Vinokurov, Z.S.; Babikhina, M.N.; Zolotarev, K.V. Application of Synchrotron Radiation for In Situ XRD Investigation of Zirconium Hydrides Formation at Gas-phase Hydrogenation. Phys. Procedia 2016, 84, 342–348. [Google Scholar] [CrossRef]
- Zhu, T.; Li, M. Effect of 0.770 wt% H addition on the microstructure of Ti-6Al-4V alloy and mechanism of δ hydride formation. J. Alloys Compd. 2009, 481, 480–485. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, H.-K.; Jung, W.-S.; Lee, B.-J. Atomistic modeling of the Ti-Al binary system. Comput. Mater. Sci. 2016, 119, 1–8. [Google Scholar] [CrossRef]
- Sun, P.; Fang, Z.Z.; Koopman, M.; Paramore, J.; Chandran, K.S.R.; Ren, Y.; Lu, J. An experimental study of the (Ti-6Al-4V)-xH phase diagram using in situ synchrotron XRD and TGA/DSC techniques. Acta Mater. 2015, 84, 29–41. [Google Scholar] [CrossRef]
- Perevalova, O.B.; Panin, A.V.; Kalashnikov, M.P.; Akulinkin, A.A.; Bozhko, I.A.; Sergeev, V.P. Elastic stresses and microstructure of TiAlN coatings. Inorg. Mater. Appl. Res. 2017, 8, 434–443. [Google Scholar] [CrossRef]
- Yingying, Z.; Shuhui, H.; Yingjuan, F.; Debin, S. Hydrogen induced softening mechanism in near alpha titanium alloy. J. Alloys Compd. 2012, 541, 60–64. [Google Scholar] [CrossRef]
- Kolachev, B.A.; Mamonova, F.S.; Lyasotskaya, V.S. Characteristics of the structure and properties of quenched titanium alloys. Met. Sci. Heat Treat. 1975, 17, 695–697. [Google Scholar] [CrossRef]
- Yan, M.; Yu, P. An Overview of Densification, Microstructure and Mechanical Property of Additively Manufactured Ti-6Al-4V—Comparison among Selective Laser Melting, Electron Beam Melting, Laser Metal Deposition and Selective Laser Sintering, and with Conventional Powder. In Sintering Techniques of Materials; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Johnson, H.H.; Morlet, J.G.; Troiano, A.R. Hydrogen crack initiation and delayed failure in steel. Trans. Metall. Soc. Am. Inst. Min. Metall. Pet. Eng. 1958, 212, 528–536. [Google Scholar]
- Van Leeuwen, H.P. A failure criterion for internal hydrogen embrittlement. Eng. Fract. Mech. 1977, 9, 291–296. [Google Scholar] [CrossRef]
- Baoguo, Y.; Yujie, W.; Yubin, Z.; Longqing, G. Hydrogenation Behavior of Ti6Al4V Alloy. Rare Met. Mater. Eng. 2017, 46, 1486–1490. [Google Scholar] [CrossRef]
- Bratanich, T.I.; Skorokhod, V.V.; Kopylova, L.I.; Kotko, A.V. Ti3Al destructive hydrogenation. Int. J. Hydrog. Energy 2011, 36, 1276–1286. [Google Scholar] [CrossRef]
- Straumal, B.; Baretzky, B. Grain Boundary Phase Transitions and their Influence on Properties of Polycrystals. Interface Sci. 2004, 12, 147–155. [Google Scholar] [CrossRef]
- Bokstein, B.S. Grain Boundary Diffusion, Stresses and Segregation. Defect Diffus. Forum 2012, 323–325, 31–40. [Google Scholar] [CrossRef]
- Fisher, D.J. Hydrogen Diffusion in Metals: A 30-Year Retrospective; Scitec Publications: Zurich-Uetikon, Switzerland, 1999; pp. 167–168. ISBN 9783908450429. [Google Scholar]
- Hirohata, Y.; Aihara, Y.; Hino, T.; Miki, N.; Nakagawa, S. Evaluation of hydrogen sorption and desorption for Ti-6A1-4V alloy as a vacuum vessel material. In Fusion Technology 1996; Elsevier: Amsterdam, The Netherlands, 1997; pp. 363–366. [Google Scholar]
- Miyoshi, T.; Naito, S.; Yamamoto, M.; Doi, M.; Kimura, M. Diffusion of hydrogen in titanium, Ti88Al12 and Ti3Al. J. Chem. Soc. Faraday Trans. 1996, 92, 483. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wang, C.-M. Effects of hydrogen loading and type of titanium hydride on grain refinement and mechanical properties of Ti-6Al-4V. J. Alloys Compd. 2014, 601, 274–279. [Google Scholar] [CrossRef]
- Shen, C.C.; Yu, C.Y.; Perng, T.P. Variation of structure and mechanical properties of Ti-6Al-4V with isothermal hydrogenation treatment. Acta Mater. 2009, 57, 868–874. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushilina, N.; Panin, A.; Syrtanov, M.; Kashkarov, E.; Kudiiarov, V.; Perevalova, O.; Laptev, R.; Lider, A.; Koptyug, A. Hydrogen-Induced Phase Transformation and Microstructure Evolution for Ti-6Al-4V Parts Produced by Electron Beam Melting. Metals 2018, 8, 301. https://doi.org/10.3390/met8050301
Pushilina N, Panin A, Syrtanov M, Kashkarov E, Kudiiarov V, Perevalova O, Laptev R, Lider A, Koptyug A. Hydrogen-Induced Phase Transformation and Microstructure Evolution for Ti-6Al-4V Parts Produced by Electron Beam Melting. Metals. 2018; 8(5):301. https://doi.org/10.3390/met8050301
Chicago/Turabian StylePushilina, Natalia, Alexey Panin, Maxim Syrtanov, Egor Kashkarov, Viktor Kudiiarov, Olga Perevalova, Roman Laptev, Andrey Lider, and Andrey Koptyug. 2018. "Hydrogen-Induced Phase Transformation and Microstructure Evolution for Ti-6Al-4V Parts Produced by Electron Beam Melting" Metals 8, no. 5: 301. https://doi.org/10.3390/met8050301
APA StylePushilina, N., Panin, A., Syrtanov, M., Kashkarov, E., Kudiiarov, V., Perevalova, O., Laptev, R., Lider, A., & Koptyug, A. (2018). Hydrogen-Induced Phase Transformation and Microstructure Evolution for Ti-6Al-4V Parts Produced by Electron Beam Melting. Metals, 8(5), 301. https://doi.org/10.3390/met8050301









