Effect of Tempering Temperature on the Low Temperature Impact Toughness of 42CrMo4-V Steel
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
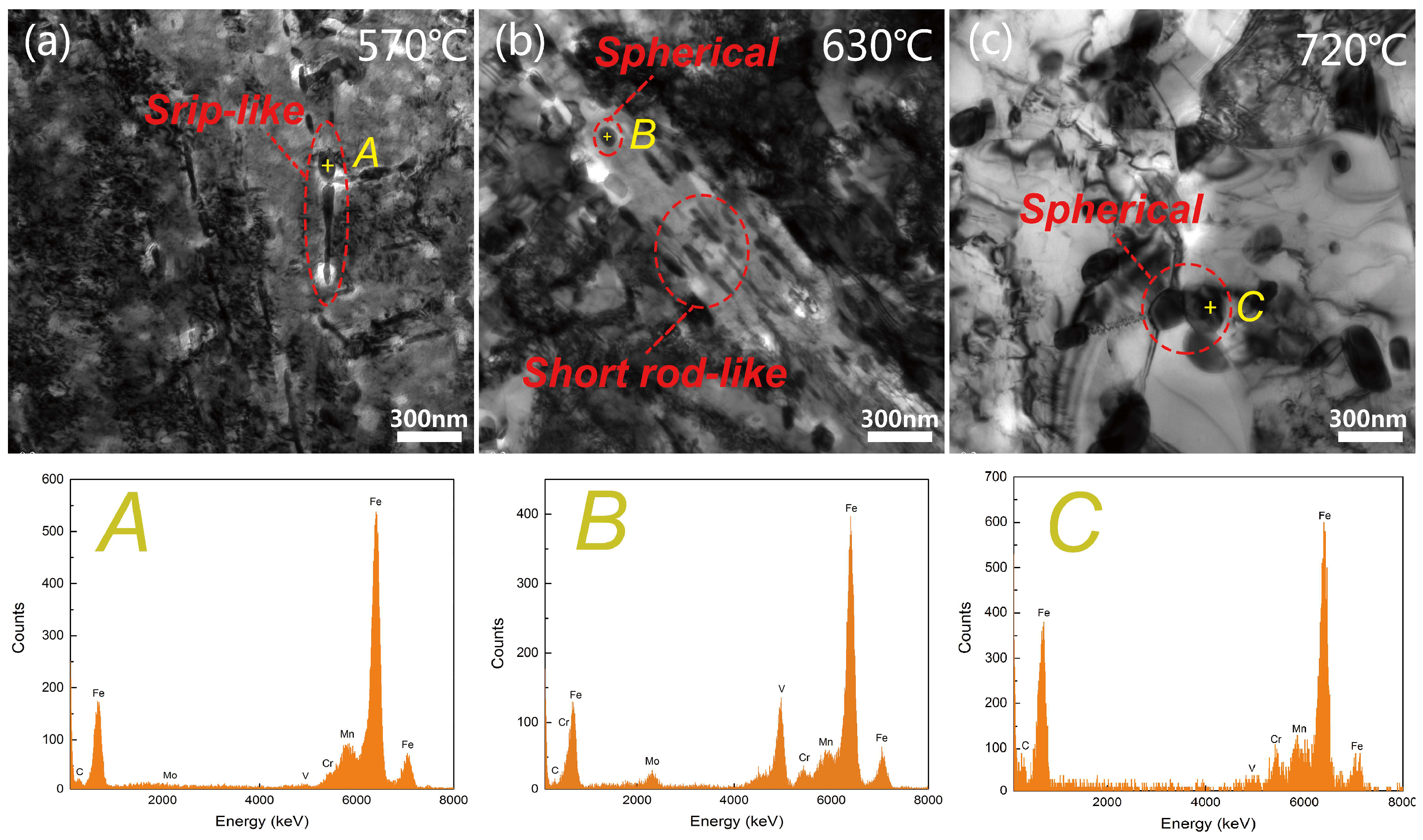
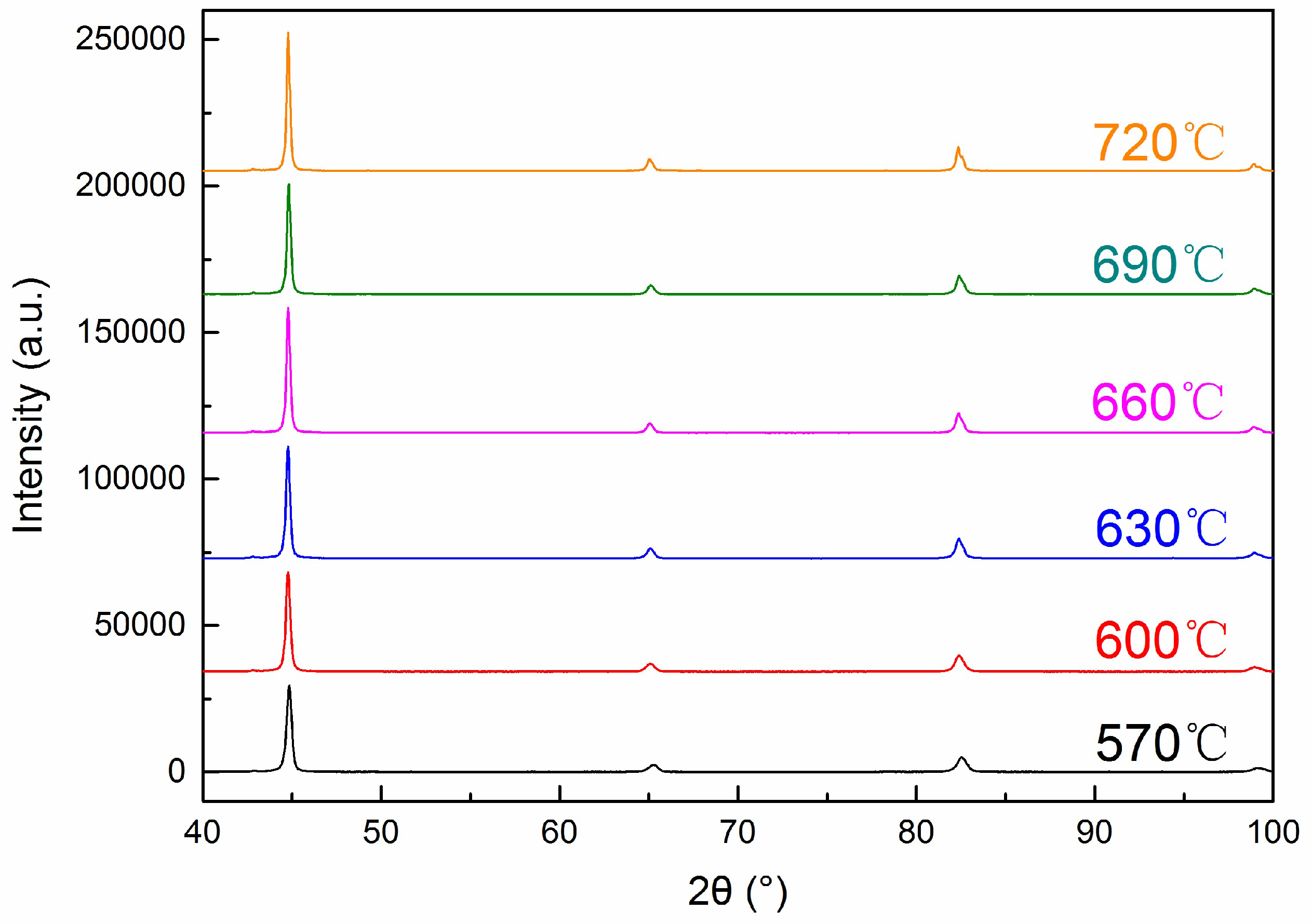
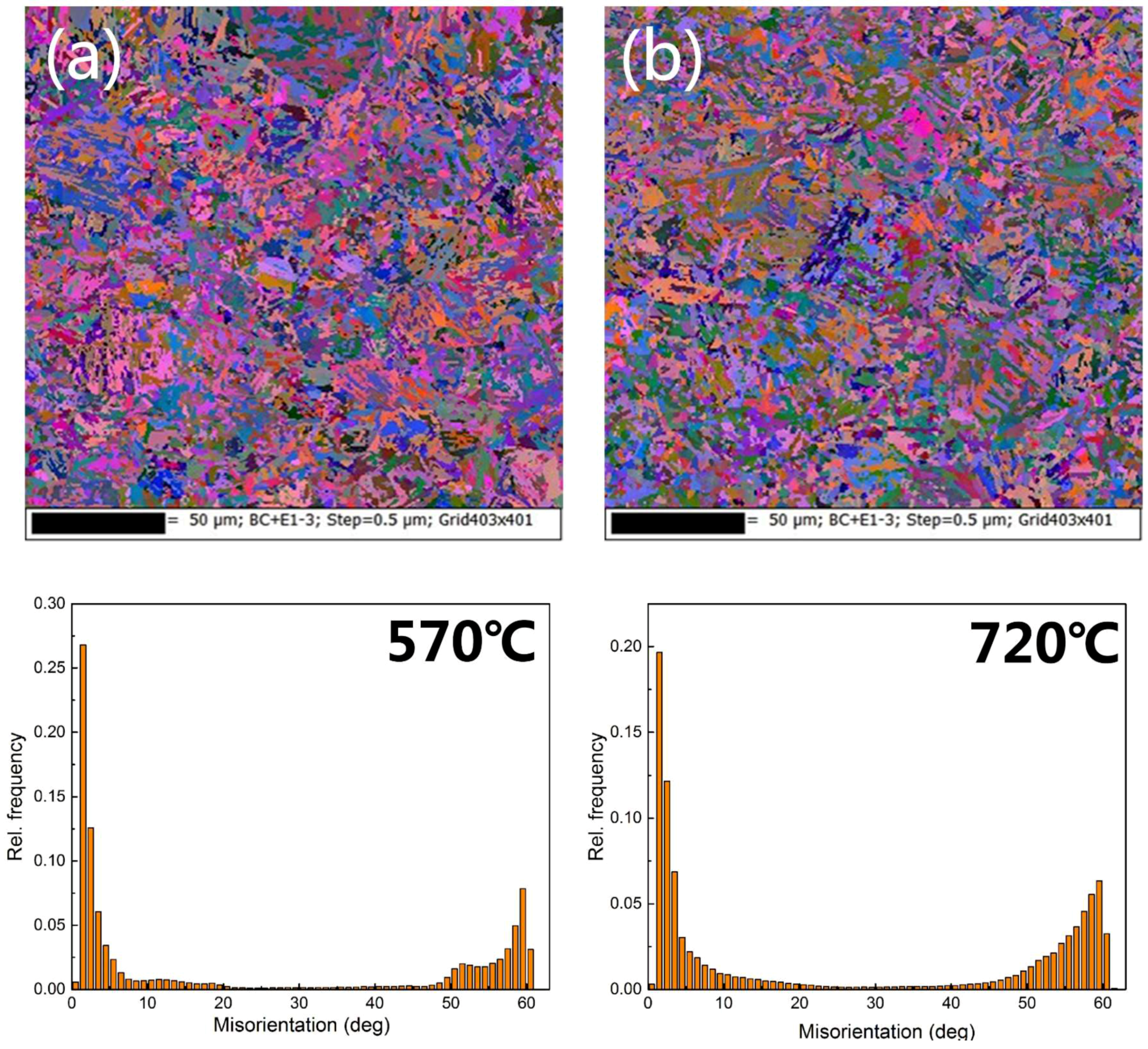
3.1. Microstructures
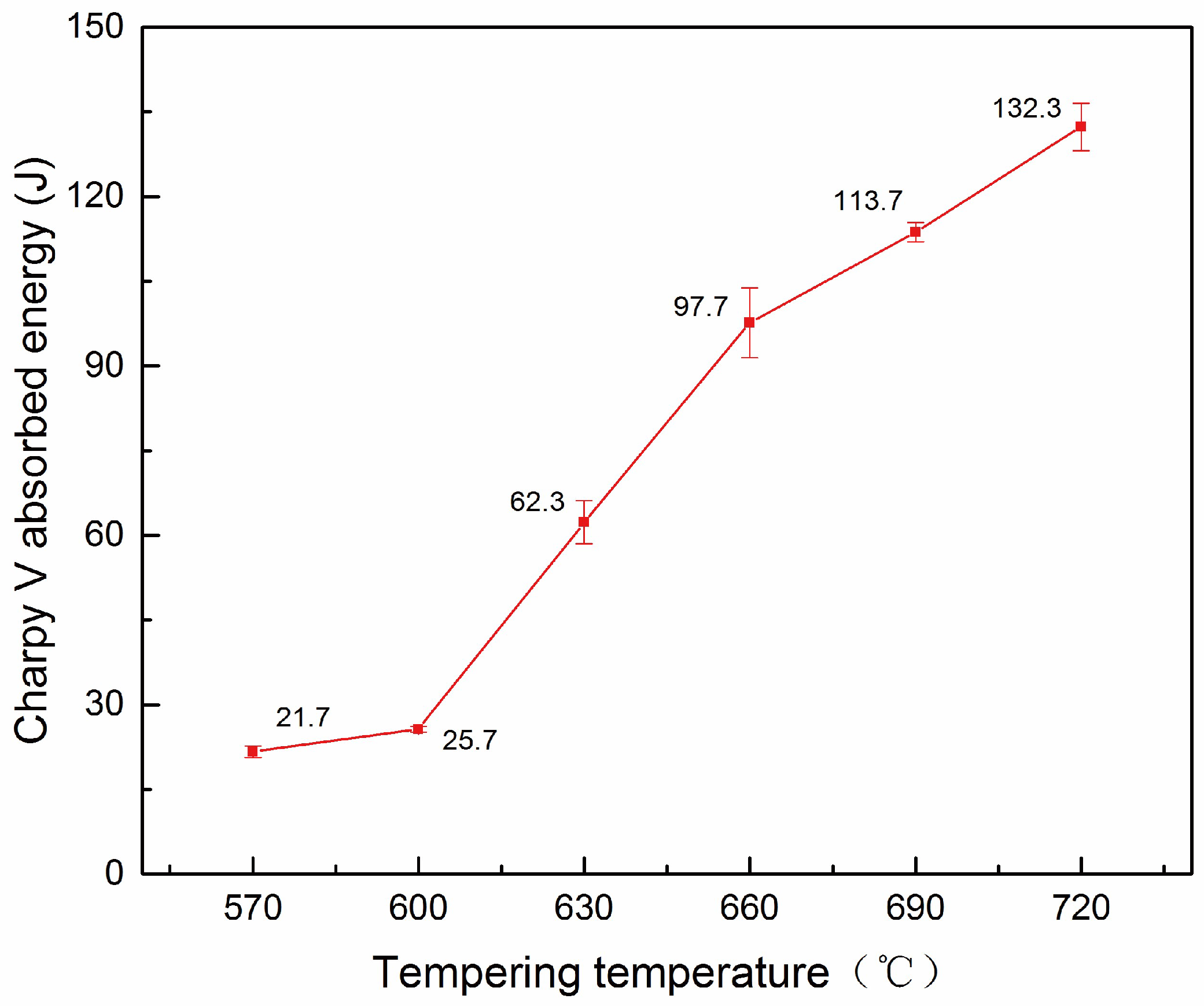
3.2. Impact Energy
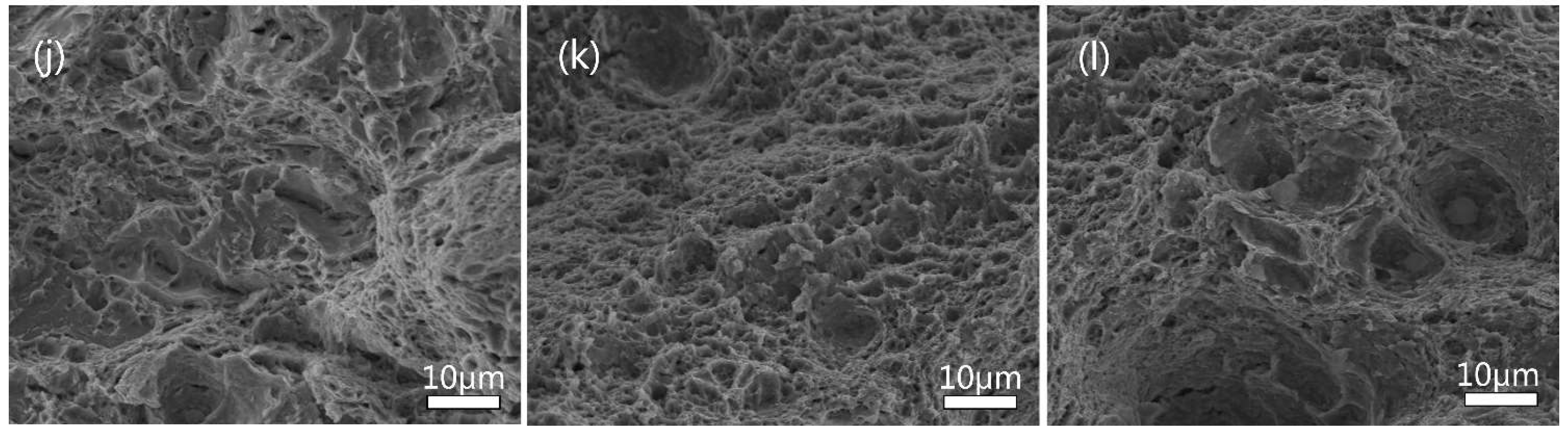
3.3. Mechanisms
4. Conclusions
- The addition of V to 42CrMo4 steel leads to the appearance of M8C7 type carbides, and the carbide precipitation sequence of 42CrMo4-V steel is M8C7 → M3C. As the tempering temperature increases, the spheroidizing process of M3C carbides can be divided into two stages and it has a significant effect on the low temperature impact toughness.
- With the increase of tempering temperature from 570 °C to 720 °C, the low temperature impact energy of 42CrMo4-V steel increases greatly from 26 J to 132 J. The changes of toughness are attributed to the evolution of microstructure, including the softening of matrix metal, the evolution of carbide precipitates and grain structures.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quan, G.Z.; Li, G.S.; Chen, T.; Wang, Y.X.; Zhang, Y.W.; Zhou, J. Dynamic recrystallization kinetics of 42CrMo steel during compression at different temperatures and strain rates. Mater. Sci. Eng. A 2011, 528, 4643–4651. [Google Scholar] [CrossRef]
- Demir, T.; Übeyli, M.; Yıldırım, R.O. Investigation on the ballistic impact behavior of various alloys against 7.62 mm armor piercing projectile. Mater. Des. 2008, 29, 2009–2016. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, L.; Wen, X.; Zhang, C.L.; Liu, Y.Z. Heat Treatment Properties of 42CrMo Steel for Bearing Ring of Varisized Shield Tunneling Machine. Acta Metall. Sin. 2014, 27, 383–388. [Google Scholar] [CrossRef]
- Ilhan, E.; Findik, F.; Aslanlar, S. An investigation of the factors affecting the design of drum dryers. Mater. Des. 2003, 24, 503–507. [Google Scholar] [CrossRef]
- Chaouch, D.; Guessasma, S.; Sadok, A. Finite Element simulation coupled to optimisation stochastic process to assess the effect of heat treatment on the mechanical properties of 42CrMo4 steel. Mater. Des. 2012, 34, 679–684. [Google Scholar] [CrossRef]
- Nouari, M.; Molinari, A. Experimental verification of a diffusion tool wear model using a 42CrMo4 steel with an uncoated cemented tungsten carbide at various cutting speeds. Wear 2005, 259, 1151–1159. [Google Scholar] [CrossRef]
- Starke, P.; Walther, F.; Eifler, D. New fatigue life calculation method for quenched and tempered steel SAE 4140. Mater. Sci. Eng. A 2009, 523, 246–252. [Google Scholar] [CrossRef]
- Klocke, F.; Schneider, S.; Ehle, L.; Meyer, H.; Hensgen, L.; Klink, A. Investigations on Surface Integrity of Heat Treated 42CrMo4 (AISI 4140) Processed by Sinking EDM. Procedia CIRP 2016, 42, 580–585. [Google Scholar] [CrossRef]
- Kreethi, R.; Mondal, A.K.; Dutta, K. Ratcheting fatigue behaviour of 42CrMo4 steel under different heat treatment conditions. Mater. Sci. Eng. A 2017, 679, 66–74. [Google Scholar] [CrossRef]
- Nikitin, I.; Besel, M. Correlation between residual stress and plastic strain amplitude during low cycle fatigue of mechanically surface treated austenitic stainless steel AISI 304 and ferritic–pearlitic steel SAE 1045. Mater. Sci. Eng. A 2008, 491, 297–303. [Google Scholar] [CrossRef]
- Nagarajan, V.; Putatunda, S.; Boileau, J. Fatigue Crack Growth Behavior of Austempered AISI 4140 Steel with Dissolved Hydrogen. Metals 2017, 7, 466. [Google Scholar] [CrossRef]
- Ulutan, M.; Celik, O.N.; Gasan, H.; Er, U. Effect of Different Surface Treatment Methods on the Friction and Wear Behavior of AISI 4140 Steel. J. Mater. Sci. Technol. 2010, 26, 251–257. [Google Scholar] [CrossRef]
- Miokovic, T.; Schulze, V.; Vohringer, O.; Löhe, D. Influence of cyclic temperature changes on the microstructure of AISI 4140 after laser surface hardening. Acta Mater. 2007, 55, 589–599. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, Z.; Han, T.; Tan, A.C.C. Fracture analysis of wind turbine main shaft. Eng. Fail. Anal. 2013, 34, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zeng, X.; Li, J.; Yang, X.; Wang, H.J. A review on recent advancements of substructures for offshore wind turbines. Energy Convers. Manag. 2018, 158, 103–119. [Google Scholar] [CrossRef]
- Staśko, R.; Adrian, H.; Adrian, A. Effect of nitrogen and vanadium on austenite grain growth kinetics of a low alloy steel. Mater. Charact. 2006, 56, 340–347. [Google Scholar] [CrossRef]
- Scott, C.P.; Fazeli, F.; Amirkhiz, B.S.; Pushkareva, I.; Allain, S.Y.P. Structure-properties relationship of ultra-fine grained V-microalloyed dual phase steels. Mater. Sci. Eng. A 2017, 703, 293–303. [Google Scholar] [CrossRef]
- Di Schino, A.; Guarnachelli, C. Microstructure and cleavage resistance of high strength steels. Mater. Sci. Forum 2010, 638–642, 3188–3193. [Google Scholar] [CrossRef]
- Ceschini, L.; Marconi, A.; Martini, C.; Morri, A.; Di Schino, A. Tensile and impact behaviour of a microalloyed medium carbon steel: Effect of the cooling condition and corresponding microstructure. Mater. Des. 2013, 45, 171–178. [Google Scholar] [CrossRef]
- Chen, J.D.; Mo, W.L.; Wang, P.; Lu, S.P. Effects of tempering temperature on the impact toughness of steel 42CrMo. Acta Metall. Sin. 2012, 48, 1186–1193. [Google Scholar] [CrossRef]
- Furukawa, S.; Ihara, H.; Murata, Y.; Tsukada, Y.; Koyama, T. Simulation of dislocation recovery in lath martensite steels using the phase-field method. Comput. Mater. Sci. 2016, 119, 108–113. [Google Scholar] [CrossRef]
- Liu, H.H.; Fu, P.X.; Liu, H.W.; Sun, C.; Ma, X.P.; Li, D.Z. Microstructure evolution and mechanical properties in 718H pre-hardened mold steel during tempering. Mater. Sci. Eng. A 2018, 709, 181–192. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Bao, H.; Weng, Y.Q.; Liu, W. Effect of tempering temperature on the toughness of 9Cr–3W–3Co martensitic heat resistant steel. Mater. Des. 2014, 54, 874–879. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Liu, Y.; Li, C.; Liu, C.X.; Guo, Q.Y. Carbide precipitation in Nb-V-Ti microalloyed ultra-high strength steel during tempering. Mater. Sci. Eng. A 2017, 683, 215–226. [Google Scholar] [CrossRef]
- Janovec, J.; Svoboda, M.; Výrostková, A.; Kroupa, A. Time–temperature–precipitation diagrams of carbide evolution in low alloy steels. Mater. Sci. Eng. A 2005, 402, 288–293. [Google Scholar] [CrossRef]
- Ning, A.; Mao, W.; Chen, X.; Guo, H.J.; Guo, J. Precipitation Behavior of Carbides in H13 Hot Work Die Steel and Its Strengthening during Tempering. Metals 2017, 7, 70. [Google Scholar] [CrossRef]
- Ye, D.L.; Hu, J.H. Practical Inorganic Thermodynamic Data Manual, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Chen, J.X. Steelmaking Common Chart Data Manual, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Yong, Q.L. The Second Phase of the Steel Materials; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Chen, J.D. Microstructure and Defect Research of 42CrMo Steel Forgings for Nuclear Polar Crane. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, May 2013. (In Chinese). [Google Scholar]
- Chao, Y.J.; Ward, J.D., Jr.; Sands, R.G. Charpy impact energy, fracture toughness and ductile-brittle transition temperature of dual-phase 590 steel. Mater. Des. 2007, 28, 551–557. [Google Scholar] [CrossRef]
- Zhong, Q.P.; Zhao, Z.H. Fractography; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Hao, L.H.; Xiao, N.M.; Zheng, C.W.; Li, D.Z. Mechanical Properties and Temper Resistance of Deformation Induced Ferrite in a Low Carbon Steel. J. Mater. Sci. Technol. 2010, 26, 1107–1113. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Jiang, B.; Zhou, L.Y.; Liu, Y.Z. Effect of large-size M23C6-type carbides on the low-temperature toughness of martensitic heat-resistant steels. J. Alloys Compd. 2016, 685, 248–257. [Google Scholar] [CrossRef]
- Cui, Y.X.; Wang, C.L. Metal Fracture Analysis; Harbin Industrial University Press: Harbin, China, 1998. [Google Scholar]
- Liu, J.; Yu, H.; Zhou, T.; Song, C.H.; Zhang, K. Effect of double quenching and tempering heat treatment on the microstructure and mechanical properties of a novel 5Cr steel processed by electro-slag casting. Mater. Sci. Eng. A 2014, 619, 212–220. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Shi, J.; Hui, W.J.; Dong, H. Effect of microstructural refinement on the toughness of low carbon martensitic steel. Scr. Mater. 2008, 58, 492–495. [Google Scholar] [CrossRef]






| Alloy | C | Si | Mn | Cr | Mo | S | P | Ni | V |
|---|---|---|---|---|---|---|---|---|---|
| 42CrMo4-V | 0.40 | 0.25 | 0.73 | 1.12 | 0.26 | 0.0001 | 0.010 | 0.53 | 0.19 |
| 42CrMo4 | 0.38–0.45 | 0.17–0.37 | 0.50–0.80 | 0.90–1.20 | 0.15–0.25 | ≤0.035 | ≤0.035 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Fu, P.-X.; Liu, H.-W.; Liu, H.-H.; Du, N.-Y. Effect of Tempering Temperature on the Low Temperature Impact Toughness of 42CrMo4-V Steel. Metals 2018, 8, 232. https://doi.org/10.3390/met8040232
Sun C, Fu P-X, Liu H-W, Liu H-H, Du N-Y. Effect of Tempering Temperature on the Low Temperature Impact Toughness of 42CrMo4-V Steel. Metals. 2018; 8(4):232. https://doi.org/10.3390/met8040232
Chicago/Turabian StyleSun, Chen, Pai-Xian Fu, Hong-Wei Liu, Hang-Hang Liu, and Ning-Yu Du. 2018. "Effect of Tempering Temperature on the Low Temperature Impact Toughness of 42CrMo4-V Steel" Metals 8, no. 4: 232. https://doi.org/10.3390/met8040232




