Mechanical Property Testing of Hydrogenated Zirconium Irradiated with Electrons
Abstract
:1. Introduction
2. Materials and Preparation of Samples
3. Experimental Results and Discussion
3.1. Investigation of Gas Release from Zirconium Alloy Samples
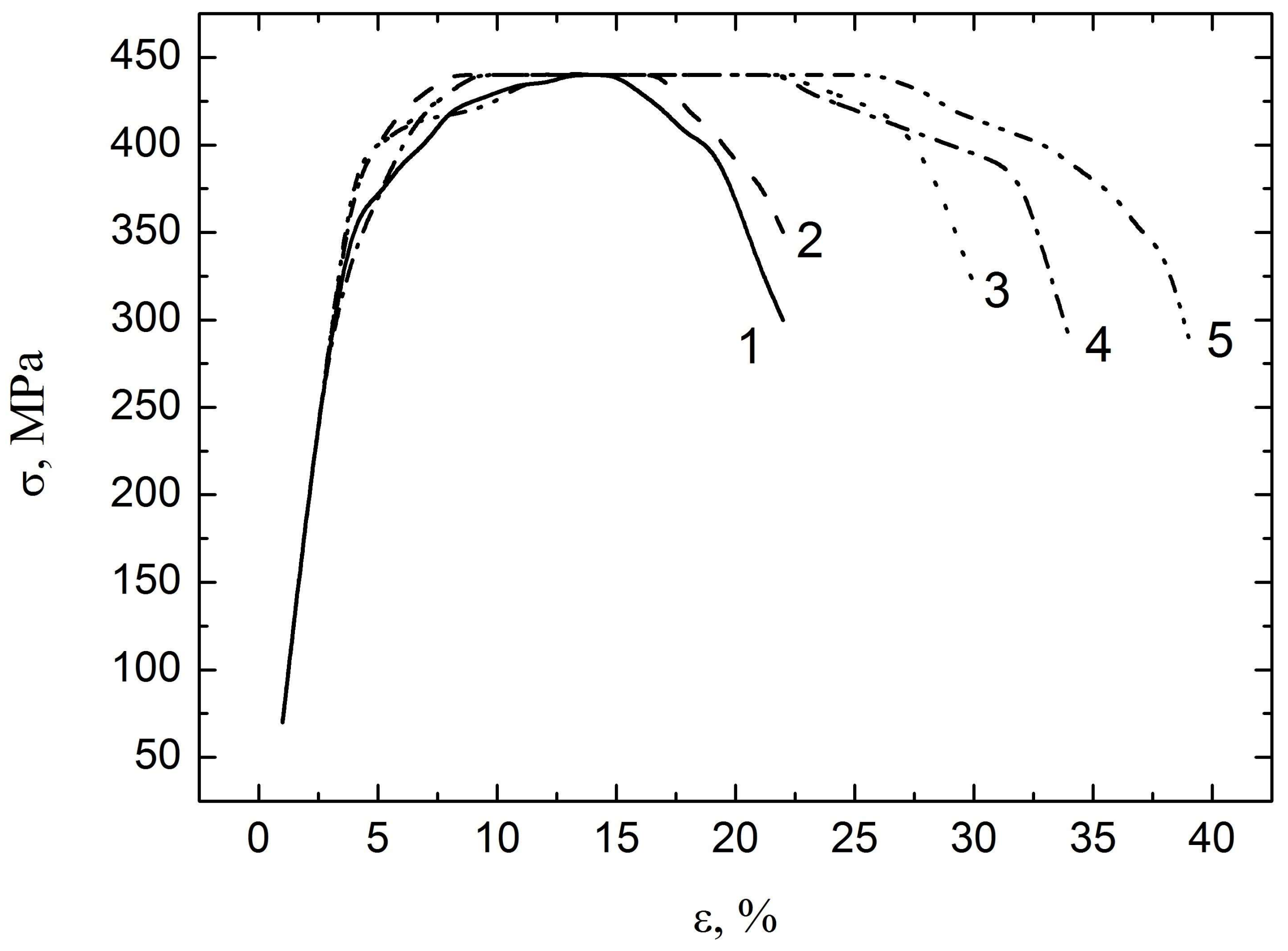
3.2. Mechanical Testing
4. Conclusions
- The results have shown that the strain of the zirconium alloy depends on the method for hydrogen removal from the alloy. Plasticity is slightly changed in the range of hydrogen concentrations from 0.035 to 0.07 wt %. After the thermal removal of hydrogen, plasticity is increased by 30–40%.
- Plasticity is increased by 70–80% when the hydrogen release is stimulated by electron beam irradiation, which is connected with the partial annealing of defects followed by the desorption of hydrogen.
- Hydrogenation of the alloy leads to the formation of traps, which was found by the peak position of gas release. The peak displacement between RSHR and from the TSHR is about 300 °C.
- The activation energy of hydrogen release was calculated for various heating rates of the hydrogenated zirconium samples. The calculations have shown that the activation energy varies from 1.3 eV to 2.5 eV.
- The microhardness of the zirconium alloy (the initial sample before hydrogenation) is 1300 MPa, hydrogenated from the gas phase for 30 min is 2400 MPa and 2700 MPa for 60 min. After electron beam irradiation conducted to remove hydrogen from the alloy, the microhardness is increased up to 3200 MPa. The microhardness is two times less after electrolytic saturation and is about 1500 MPa.
- The results of the investigation can be useful for a case in which there is a need for the removal of hydrogen and hardening of zirconium products using low-current electron beams, for example, small-scale betatron currents.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fukai, Y. The Metal-Hydrogen System; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; Volume 21. [Google Scholar]
- Puls Manfred, P. The Effect of Hydrogen and Hydrides on the Integrity of Zirconium Alloy Components: Delayed Hydride Cracking; MPP Consulting: Oakville, ON, Canada; Springer: London, UK, 2012; 451p. [Google Scholar]
- Pundt, A.; Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Zielinski, A.; Sobieszczyk, S. Hydrogen-enhanced degradation and oxide effects in zirconium alloys for nuclear applications. Int. J. Hydrogen Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Syrtanov, M.S.; Vilkhivskaya, O.V.; Pryamushko, T.S.; Kudiiarov, V.N.; Volesky, L. Effect of titanium ion implantation and deposition on hydrogenation behavior of Zr-1Nb alloy. Surf. Coat. Technol. 2016, 308, 2–9. [Google Scholar] [CrossRef]
- Azevedo, C.R.F. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Hallstadius, L.; Johnson, S.; Lahoda, E. Cladding for performance fuel. Progr. Nucl. Energy 2012, 57, 71–76. [Google Scholar] [CrossRef]
- Nagase, F. Hydride behavior in Zircaloy cladding tube during high-temperature transients. J. Nucl. Mater. 2011, 415, 117–122. [Google Scholar] [CrossRef]
- Lider, A.M.; Pushilina, N.S.; Kudiiarov, V.N.; Kroening, M. Investigation of hydrogen distribution from the surface to the depth in technically pure titanium alloy with the help of glow discharge optical emission spectroscopy. Appl. Mech. Mater. 2013, 302, 92–96. [Google Scholar] [CrossRef]
- Motta, A.T.; Chen, L.Q. Hydride formation in zirconium alloys. J. Miner. Met. Mater. Soc. 2012, 64, 1403–1408. [Google Scholar] [CrossRef]
- Nagase, F.; Fuketa, T. Investigation of hydride rim effect on failure of Zircaloy-4 cladding with tube burst test. J. Nucl. Sci. Technol. 2005, 42, 58–65. [Google Scholar] [CrossRef]
- Hanson, B.; Shimskey, R.; Lavender, C.; MacFarlan, P.; Eslinger, P. Hydride Rim Formation in Unirradiated Zircaloy. Available online: https://www.energy.gov/sites/prod/files/2013/08/f2/HydrideRimFormationZircaloy.pdf (accessed on 22 March 2018).
- Shimskey, R.; Hanson, B.; MacFarlan, P. Optimization of Hydride Rim Formation in Unirradiated Zr-4 Cladding. Available online: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-22835.pdf (accessed on 22 March 2018).
- Steinbruck, M. Hydrogen absorption by zirconium alloys at high temperatures. J. Nucl. Mater. 2004, 334, 58–64. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yeh, M.-S. Gaseous hydrogen embrittlement of a hydrided zirconium alloy. Metall. Mater. Trans. A 1998, 29, 1047–1056. [Google Scholar] [CrossRef]
- Terrani, K.A.; Balooch, M.; Wongsawaeng, D.; Jaiyen, S.; Olander, D.R. The kinetics of hydrogen desorption from and adsorption on zirconium hydride. J. Nucl. Mater. 2010, 397, 61–68. [Google Scholar] [CrossRef]
- Kammenzind, B.K.; Berquist, B.M.; Bajaj, R. The long-range migration of hydrogen through ziicaloy in response to tensile and compressive stress gradients. In Zirconium in the Nuclear Industry: Twelfth International Symposium; ASTM STP 1354; ASTM International: West Conshohocken, PA, USA, 2000; pp. 196–233. [Google Scholar]
- Shmakov, A.A.; Smirnov, E.A.; Bruchertseifer, H. Hydrogen diffusion and distribution in oxidized zirconium alloys by thermo-release method. Metallofiz. Noveishie Technol. 1999, 21, 35–39. (In Russian) [Google Scholar]
- Kim, Y.S.; Matvienko, Y.G.; Cheong, Y.M.; Kim, S.S.; Kwon, S.C. A model of the threshold stress intensity factor КiH for delayed hydride cracking of Zr-2,5Nb alloy. J. Nucl. Mater. 2000, 278, 251–257. [Google Scholar] [CrossRef]
- Shi, S.Q. Diffusion-controlled hydride growth near crack tip in zirconium under temperature transients. J. Nucl. Mater. 1999, 275, 318–323. [Google Scholar] [CrossRef]
- Sickafusa, K.E.; Matzkeb, H.J.; Hartmanna, T.H.; Yasudac, K.; Valdeza, J.A.; Chodak, P.; Nastasia, M.; Verralle, R.A. Radiation damage effects in zirconia. J. Nucl. Mater. 1999, 274, 66–77. [Google Scholar] [CrossRef]
- Puis, M.P. The effects of misfit and external stresses on terminal solid solubility in hydride-forming metals. Acta Metall. 1981, 29, 1961–1968. [Google Scholar] [CrossRef]
- Puis, M.P. Elastic and plastic accommodation effects on metal-hydride solubility. Acta Metall. 1984, 32, 1259–1269. [Google Scholar] [CrossRef]
- Dupin, N.I.; Ansara, I.; Servant, C.; Toffolon, C.; Lemaignan, C.; Brachet, J.C. A thermodynamic database for zirconium alloys. J. Nucl. Mater. 1999, 275, 287–295. [Google Scholar] [CrossRef]
- Ambler, J.F.R. Effect of direction of approach to temperature on the delayed hydrogen cracking behavior of cold-worked Zr-2,5Nb. In Zirconium in the Nuclear Industry; ASTM STP 824; ASTM International: West Conshohocken, PA, USA, 1984; pp. 653–674. [Google Scholar]
- Sagat, S.; Chow, C.K.; Puls, M.P.; Coleman, C.E. Delayed hydride cracking in zirconium alloys in a temperature gradient. J. Nucl. Mater. 2000, 279, 107–117. [Google Scholar] [CrossRef]
- Sawatzky, A.; Ells, C.E. Understanding hydrogen in zirconium. In Zirconium in the Nuclear Industry: Twelfth International Symposium; ASTM STP 1354; ASTM International: West Conshohocken, PA, USA, 2000; pp. 32–48. [Google Scholar]
- Wappling, D.; Massih, A.R.; Stable, P. A model for hydride induced embrittlement in zirconium-based alloys. J. Nucl. Mater. 1997, 249, 231–238. [Google Scholar] [CrossRef]
- Yamanakaa, S.; Yoshiokaa, K.; Unoa, M.; Katsuraa, M.; Anadab, H.; Matsudac, T.; Kobayashic, S. Thermal and mechanical properties of zirconium hydride. J. Alloys Compd. 1999, 293–295, 23–29. [Google Scholar] [CrossRef]
- Kaneda, A.; Yamamoto, M.; Naito, S.; Mabuchi, M.T.; Hashino, T. Electrical resistivity of zirconium hydrides and deuterides at high temperatures. J. Phys. Condens. Matter 1998, 10, 4645. [Google Scholar] [CrossRef]
- Tomiyasu, K.; Sugiyama, T.; Fuketa, T. Influence of cladding-peripheral hydride on mechanical fuel failure under reactivity-initiated accident conditions. J. Nucl. Sci. Technol. 2007, 44, 733–742. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Teshigawara, M.; Konara, K.; Nagata, S.; Shikama, T.; Yamawaki, M. Isotope Effects in Thermal Diffusivity and Electrical Resistivity of Zirconium Hydride and Deuteride. J. Nucl. Sci. Technol. 2002, 39, 402–406. [Google Scholar] [CrossRef]
- CSA. Technical Requirements for the In-Service Evaluation of Zirconium Alloy Pressure Tubes in CANDU Reactors; Nuclear Standard N 285.8-10; Canadian Standards Association: Mississauga, ON, Canada, 2010. [Google Scholar]
- Tyurin, Y.I.; Nikitenkov, N.N.; Sigfusson, I.T.; Hashhash, A.; Yaomin, V.; Dolgov, A.S.; Semkina, L.I. Diffusion and release of hydrogen from metals under the effect of ionizing radiation. Vacuum 2016, 131, 73–80. [Google Scholar] [CrossRef]
- Voskuilen, T.Y.; Zheng, Y.; Pourpoint, T. Development of a Sievert apparatus for characterization of high hydrogen sorption materials. Int. J. Hydrogen Energy 2010, 35, 10387–10395. [Google Scholar] [CrossRef]
- Tyurin, Y.I.; Larionov, V.V.; Nikitenkov, N.N. A laboratory device for measuring the diffusion coefficient of hydrogen in metals during their simultaneous hydrogenation and electron irradiation. Instrum. Expert Tech. 2016, 59, 772–774. [Google Scholar] [CrossRef]
- Kan, Q.; Yan, W.; Kang, G.; Sun, Q. Oliver–Pharr indentation method in determining elastic moduli of shape memory alloys—A phase transformable material. J. Mech. Phys. Solids 2013, 61, 2015–2033. [Google Scholar] [CrossRef]
- Woodrouff, D.P.; Delchar, T.A. Modern Techniques of Surface Science, 2nd ed.; Cambridge University Press: Hongkong, China, 1994. [Google Scholar]
- Bhushan, B. (Ed.) Springer Handbook of Nanotechnology; Springer: Berlin, Germany, 2007. [Google Scholar]
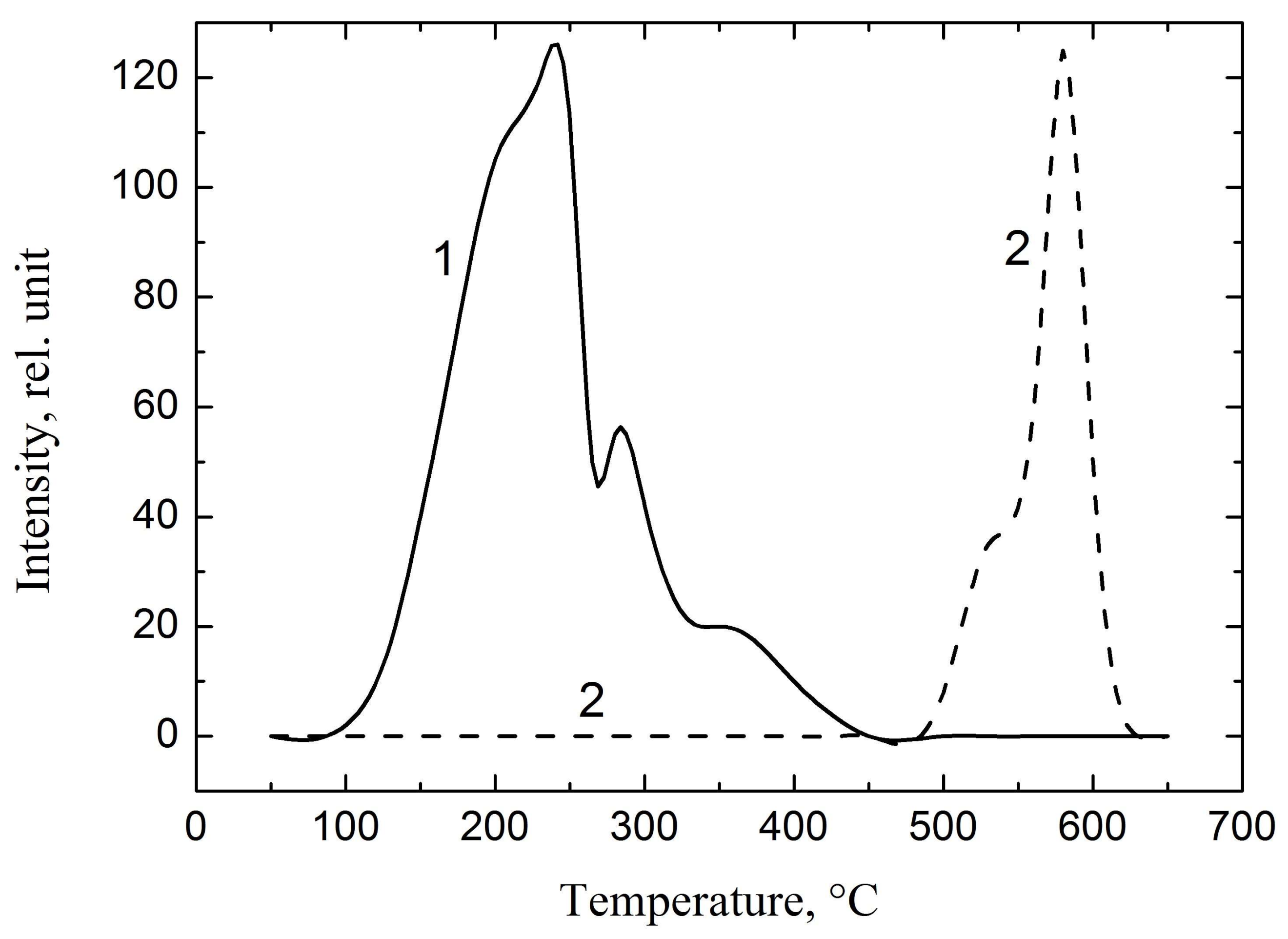


| Sample | (TSHR), K/s | (TSHR), K | (TSHR), eV |
|---|---|---|---|
| Zr-1Nb | 1 | 823 | 2.5 |
| (RSHR), K/s | (RSHR), K | (RSHR), eV | |
| 10 | 505 | 1.31 |
| Type of Treatment | No Irradiation | Electron Beam | ||||
|---|---|---|---|---|---|---|
| Time of treatment | initial | 30 min | 60 min | initial | 30 min | 60 min |
| Microhardness, MPa | 1300 | 2500 | 2700 | 2160 | 3100 | 3200 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudiiarov, V.N.; Larionov, V.V.; Tyurin, Y.I. Mechanical Property Testing of Hydrogenated Zirconium Irradiated with Electrons. Metals 2018, 8, 207. https://doi.org/10.3390/met8040207
Kudiiarov VN, Larionov VV, Tyurin YI. Mechanical Property Testing of Hydrogenated Zirconium Irradiated with Electrons. Metals. 2018; 8(4):207. https://doi.org/10.3390/met8040207
Chicago/Turabian StyleKudiiarov, Viktor N., Vitaliy V. Larionov, and Yuri I. Tyurin. 2018. "Mechanical Property Testing of Hydrogenated Zirconium Irradiated with Electrons" Metals 8, no. 4: 207. https://doi.org/10.3390/met8040207





