Formation Criterion of Hydrogen-Induced Cracking in Steel Based on Fracture Mechanics
Abstract
:1. Introduction
2. Loading Effect of Hydrogen Molecules
2.1. HIC Generation Experiment
2.2. Effect of Hydrogen Pressure According to FE Simulation
3. Embrittlement Effect of Hydrogen Atom
3.1. Embrittlement Experiment
3.2. Embrittlement Mechanism According to MD Simulation
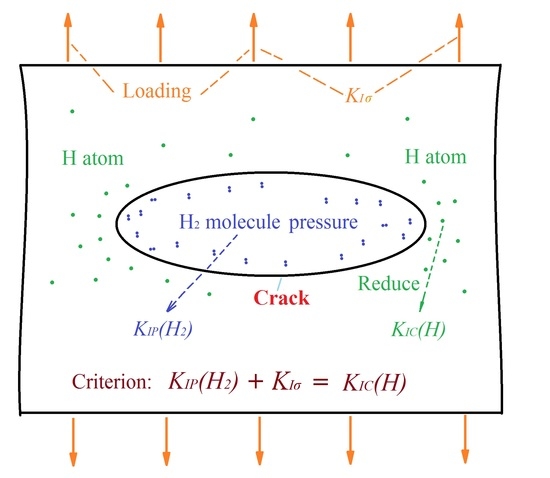
4. Theory of HIC Formation Criterion
4.1. Criterion of HIC Formation
4.2. Fracture Toughness with Hydrogen
4.3. Application Method of HIC Formation Criterion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koyama, M.; Tasan, C.T.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Hydrogen-assisted decohesion and localized plasticity in dual-phase steel. Acta Mater. 2014, 70, 174–187. [Google Scholar] [CrossRef]
- Geng, W.T.; Wang, V.; Li, J.X.; Ishikawa, N.; Kimizuka, H.; Tsuzaki, K.; Ogata, S. Hydrogen trapping in carbon supersaturated α-iron and its decohesion effect in martensitic steel. Scr. Mater. 2018, 149, 79–83. [Google Scholar] [CrossRef]
- Jambon, F.; Marchetti, L.; Sennour, M.; Jomard, F.; Chêne, J. SIMS and TEM investigation of hydrogen trapping on implantation defects in a nickel-based superalloy. J. Nucl. Mater. 2015, 466, 120–133. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The detrimental effect of hydrogen at dislocations on the hydrogen embrittlement susceptibility of Fe-C-X alloys: An experimental proof of the HELP mechanism. Int. J. Hydrogen Energy 2018, 43, 3050–3061. [Google Scholar] [CrossRef]
- Pezold, J.V.; Lymerakis, L.; Neugebeauer, J. Hydrogen-enhanced local plasticity at dilute bulk H concentrations: The role of H-H interactions and the formation of local hydrides. Acta Mater. 2011, 59, 2969–2980. [Google Scholar] [CrossRef]
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-enhanced-plasticity mediated decohesion for hydrogen-induced intergranular and “quasi-cleavage” fracture of lath martensitic steels. J. Mech. Phys. Solids 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Chatzidouros, E.V.; Traidia, A.; Devarapalli, R.S.; Pantelis, D.I.; Steriotis, T.A.; Jouiad, M. Effect of hydrogen on fracture toughness properties of a pipeline steel under simulated sour service conditions. Int. J. Hydrogen Energy 2018, 43, 5747–5759. [Google Scholar] [CrossRef]
- Thomas, R.L.S.; Scully, J.R.; Gangloff, P.G. Internal Hydrogen embrittlement of ultrahigh-strength AERMET 100 steel. Metall. Mater. Trans. A 2003, 34, 327–344. [Google Scholar] [CrossRef]
- Panasyuk, V.V.; Andreykiv, O.Y.; Gembara, O.V. Hydrogen degradation of materials under long-term operation of technological equipment. Int. J. Hydrogen Energy 2000, 25, 67–74. [Google Scholar] [CrossRef]
- Bilotta, G.; Henaff, G.; Halm, D.; Arzaghi, M. Experimental measurement of out-of-plane displacement in crack propagation under gaseous hydrogen. Int. J. Hydrogen Energy 2017, 42, 10568–10578. [Google Scholar] [CrossRef]
- Ronevich, J.A.; Somerday, B.P.; Feng, Z. Hydrogen accelerated fatigue crack growth of friction stir welded X52 steel pipe. Int. J. Hydrogen Energy 2017, 42, 4259–4268. [Google Scholar] [CrossRef]
- Skalskyi, V.; Andreikiv, O.; Dolinska, I. Assessment of subcritical crack growth in hydrogen-containing environment by the parameters of acoustic emission signals. Int. J. Hydrogen Energy 2018, 43, 5217–5224. [Google Scholar] [CrossRef]
- Toribio, J.; Kharin, V.; Vergara, D.; Lorenzo, M. Optimization of the simulation of stress-assisted hydrogen diffusion for studies of hydrogen embrittlement of notched bars. Mater. Sci. 2011, 46, 819–833. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Somerday, B.P.; Schembri, P.E.; Sofronis, P.; Foulk, J.W., III; Nibur, K.A.; Balch, D.K. On modeling hydrogen-induced crack propagation under sustained load. Miner. Met. Mater. Soc. 2014, 66, 1390–1398. [Google Scholar] [CrossRef]
- Panasyuk, V.V.; Ivanyts’kyi, Y.L.; Hembara, O.V.; Boiko, V.M. Influence of the stress-strain state on the distribution of hydrogen concentration in the process zone. Mater. Sci. 2014, 50, 315–323. [Google Scholar] [CrossRef]
- Sezgin, J.G.; Yamabe, J. Simulation of the impact of internal pressure on the integrity of a hydrogen-charged Type-316L stainless steel during slow strain rate tensile test. Int. J. Hydrogen Energy 2018, 43, 8558–8568. [Google Scholar] [CrossRef]
- Serra, E.; Benamati, G.; Ogorodnikova, O.V. Hydrogen isotopes transport parameters in fusion reactor materials. J. Nucl. Mater. 1998, 255, 105–115. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, H.; Lu, K.; Cao, W.; Sun, Y.; Fang, Y.; Xing, Y.; Du, Y.; Lu, M. Investigation of hydrogen concentration and hydrogen damage on API X80 steel surface under cathodic overprotection. Int. J. Hydrogen Energy 2017, 42, 29888–29896. [Google Scholar] [CrossRef]
- Toribio, J.; Lorenzo, M.; Vergara, D.; Kharin, V. Effects of manufacturing-induced residual stresses and strains on hydrogen embrittlement of cold drawn steels. Procedia Eng. 2011, 10, 3540–3545. [Google Scholar] [CrossRef]
- Dabah, E.; Kannengiesser, T.; Eliezer, D.; Boellinghaus, T. Hydrogen interaction with residual stresses in steel studied by synchrotron X-ray diffraction. Mater. Sci. Forum 2014, 772, 91–95. [Google Scholar] [CrossRef]
- Kamaludin, M.A.; Patel, Y.; Williams, J.G.; Blackman, B.R.K. A fracture mechanics approach to characterising the environmental stress cracking behaviour of thermoplastics. Theor. Appl. Fract. Mech. 2017, 92, 373–380. [Google Scholar] [CrossRef]
- Geng, W.T.; Wan, L.; Du, J.P.; Ishii, A.; Ishikawa, N.; Kimizuka, H.; Ogata, S. Hydrogen bubble nucleation in α-iron. Scr. Mater. 2017, 134, 105–109. [Google Scholar] [CrossRef]
- Chandran, P.; Bakshi, S.; Chatterjee, D. Study on the characteristics of hydrogen bubble formation and its transport during electrolysis of water. Chem. Eng. Sci. 2015, 138, 99–109. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, X.; Guan, X.; Wang, Q.; Fang, K.; Xu, X.; Lu, Y.; Gao, J.; Liu, Z.; Wang, T. Investigation of hydrogen bubbles behavior in tungsten by high-flux hydrogen implantation. J. Nucl. Mater. 2018, 503, 198–204. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D.; Tal-Gutelmacher, E. Hydrogen trapping in alloys studied by thermal desorption spectrometry. J. Alloys Compd. 2018, 747, 511–522. [Google Scholar] [CrossRef]
- Sezgin, J.G.; Bosch, C.; Montouchet, A.; Perrin, G.; Wolski, K. Modelling of hydrogen induced pressurization of internal cavities. Int. J. Hydrogen Energy 2017, 42, 15403–15414. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Qiao, L.J.; Qi, H.B.; Li, J.X.; He, J.Y.; Chu, W.Y. Hydrogen blistering and Hydrogen-induced cracking in amorphous Nickel phosphorus Coating. J. Non-Cryst. Solids 2007, 353, 4011–4014. [Google Scholar] [CrossRef]
- Zerbst, U.; Ainsworth, R.A.; Beier, H.T.; Pisarski, H.; Zhang, Z.L.; Nikbin, K.; Nitschke-Pagel, T.; Münstermann, S.; Kucharczyk, P.; Klingbeil, D. Review on fracture and crack propagation in weldments—A fracture mechanics perspective. Eng. Fract. Mech. 2014, 132, 200–276. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2012, 12, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ooi, E.T.; Natarajan, S. A review of the scaled boundary finite element method for two-dimensional linear elastic fracture mechanics. Eng. Fract. Mech. 2018, 187, 45–73. [Google Scholar] [CrossRef]
- Kulkov, S.S.; Bakulin, A.V.; Kulkvoa, S.E. Effect of boron on the hydrogen-induced grain boundary embrittlement in α-Fe. Int. J. Hydrogen Energy 2018, 43, 1909–1925. [Google Scholar] [CrossRef]
- Jokl, M.L.; Vitek, V.; McMahon, C.J., Jr. A microscopic theory of brittle fracture in deformable solids: A relation between ideal work to fracture and plastic work. Acta Metall. 1980, 28, 1479–1488. [Google Scholar] [CrossRef]
- Serebrinsky, S.; Carter, E.A.; Ortiz, M. A quantum-mechanically informed continuum model of hydrogen embrittlement. J. Mech. Phys. Solids 2004, 52, 2403–2430. [Google Scholar] [CrossRef]
- Rice, J.R.; Wang, J.S. Embrittlement of interfaces by solute segregation. Mater. Sci. Eng. A 1989, 107, 23–40. [Google Scholar] [CrossRef]
- Solanki, K.N.; Tschopp, M.A.; Bhatia, M.A.; Rhodes, N.R. Atomistic investigation of the role of grain boundary structure on hydrogen segregation and embrittlement in α-Fe. Metall. Mater. Trans. A 2013, 44A, 1365–1375. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Mater. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]

















| C | Mn | Si | Cr | Al | S | P | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.6 | 0.35 | 0.02 | 0.1 | 0.1 | 0.04 | 0.02 | Bal |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Fang, H. Formation Criterion of Hydrogen-Induced Cracking in Steel Based on Fracture Mechanics. Metals 2018, 8, 940. https://doi.org/10.3390/met8110940
Fu L, Fang H. Formation Criterion of Hydrogen-Induced Cracking in Steel Based on Fracture Mechanics. Metals. 2018; 8(11):940. https://doi.org/10.3390/met8110940
Chicago/Turabian StyleFu, Lei, and Hongyuan Fang. 2018. "Formation Criterion of Hydrogen-Induced Cracking in Steel Based on Fracture Mechanics" Metals 8, no. 11: 940. https://doi.org/10.3390/met8110940