Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation
Abstract
1. Introduction
2. Experimental
2.1. Plasma Immersion Implantation of Titanium
2.2. Characterization
3. Results and Discussion
3.1. Differential Scanning Calorimetry
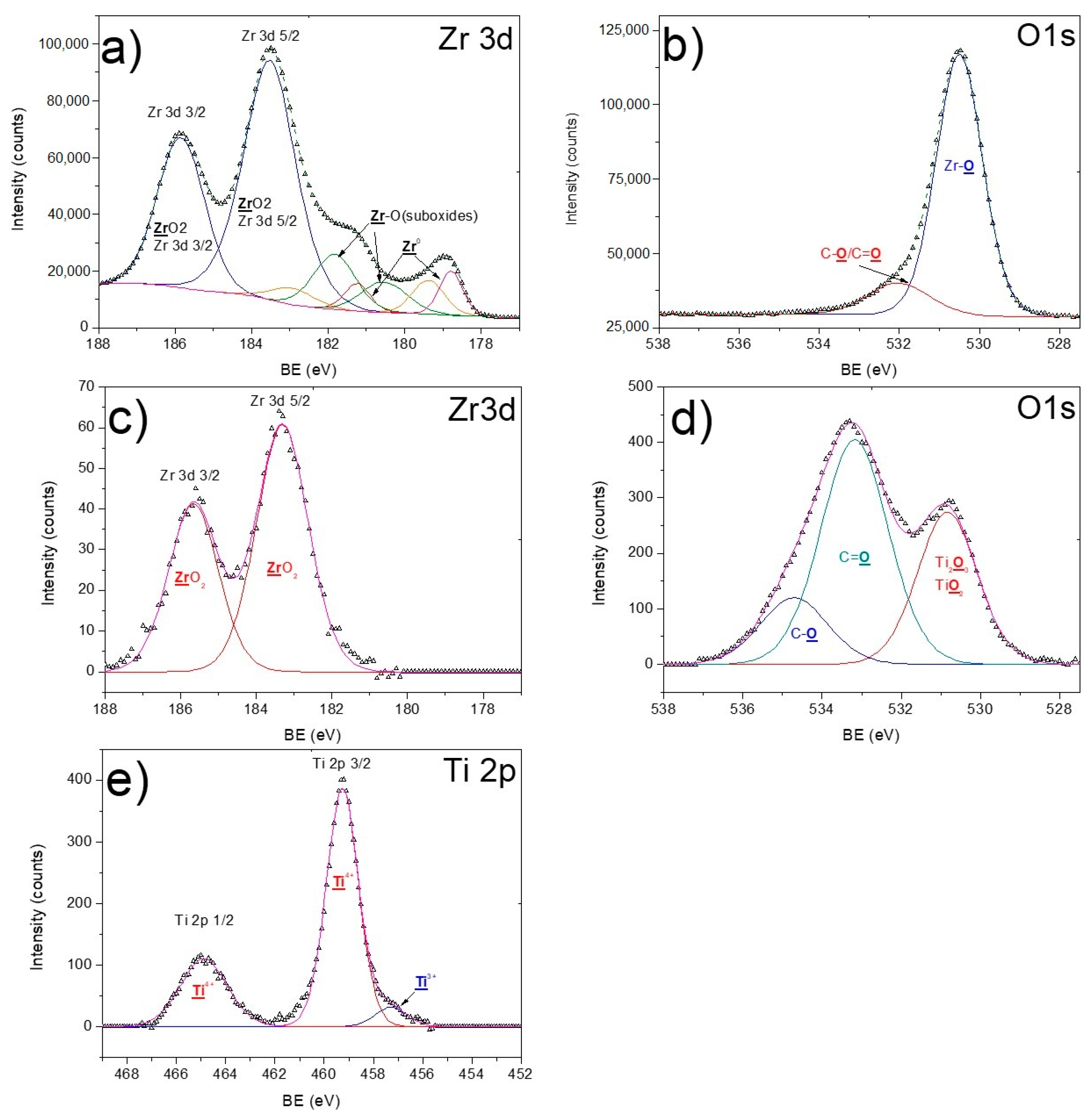
3.2. X-ray Photoelectron Spectroscopy
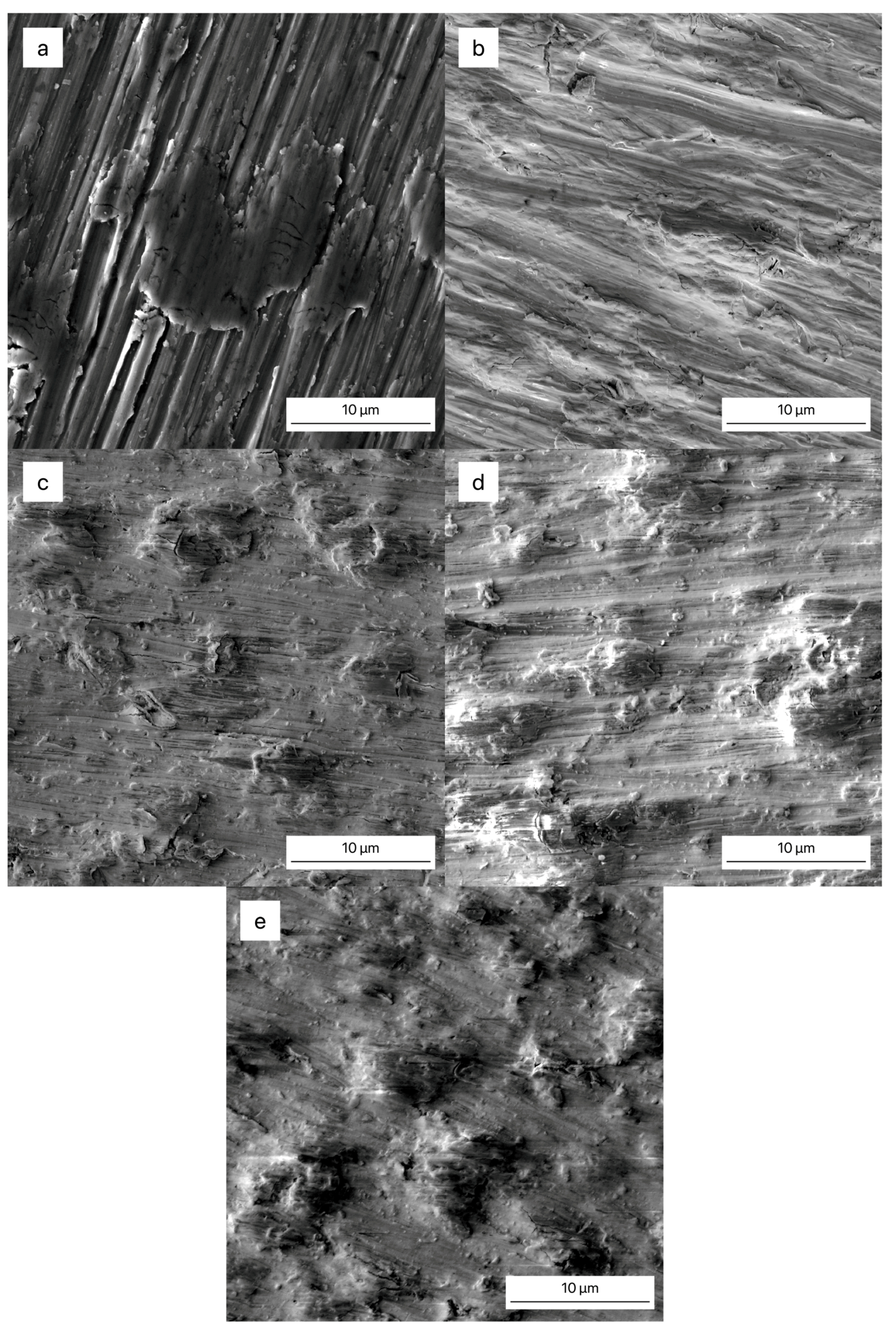
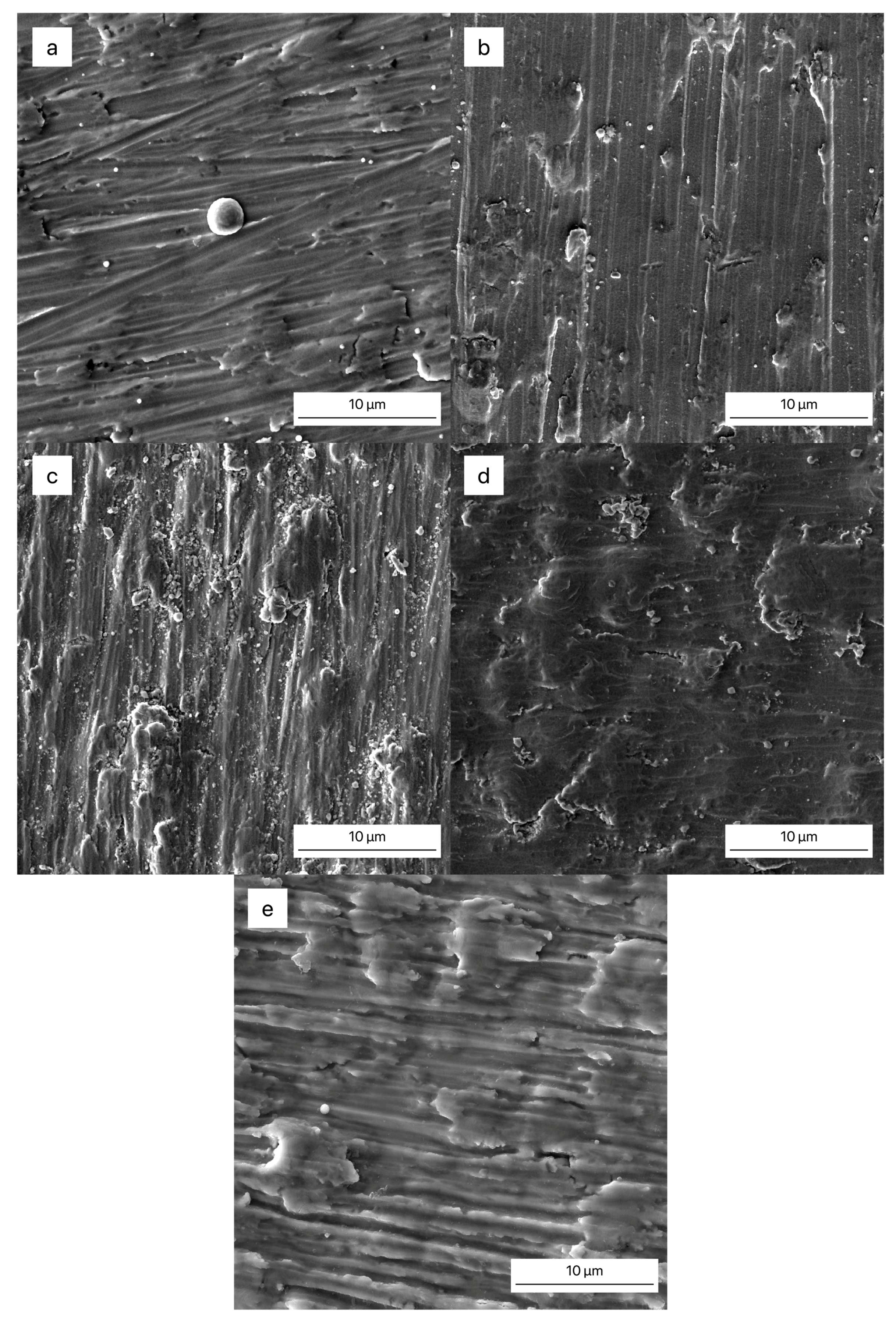
3.3. Surface Morphology
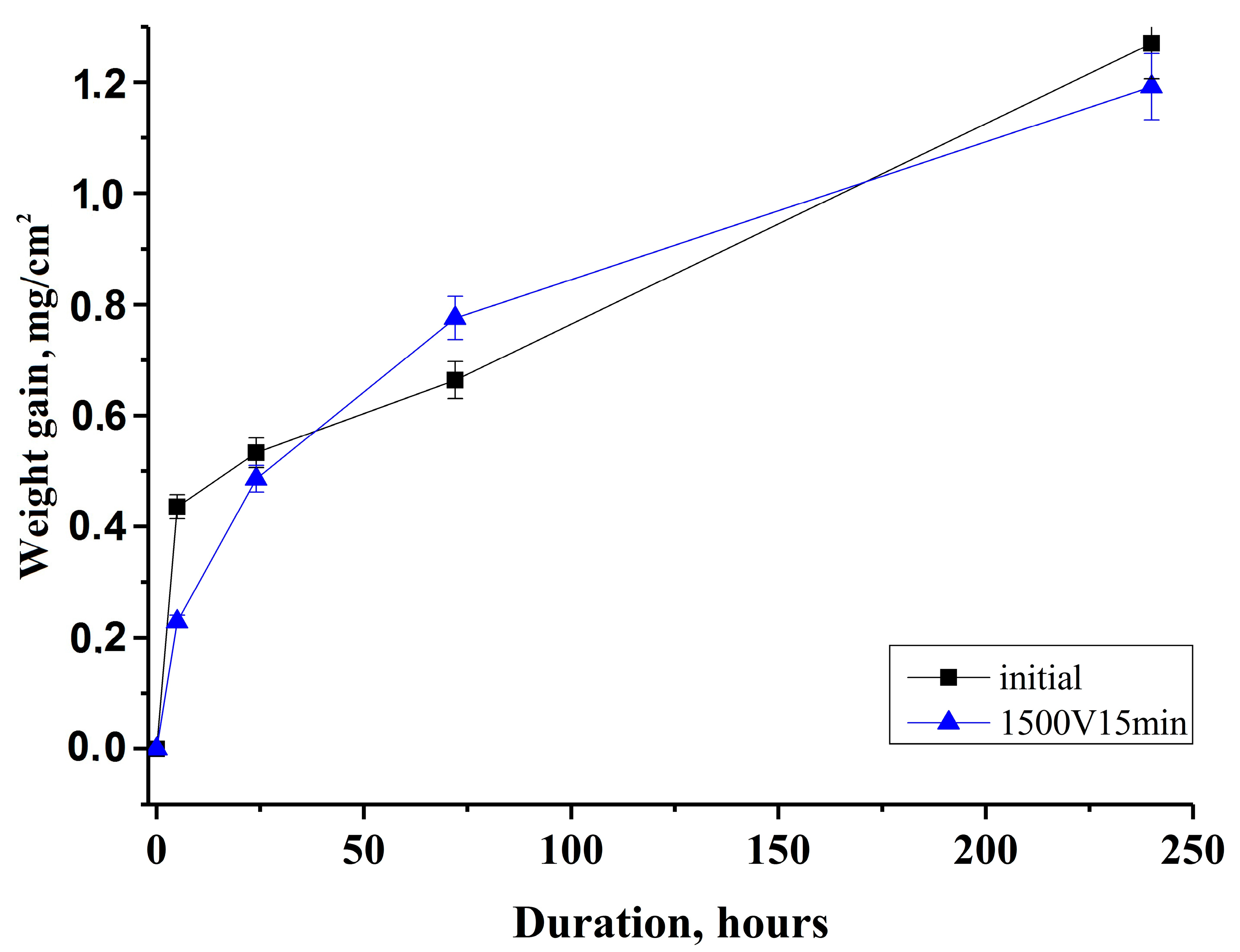
3.4. Weight-Gains
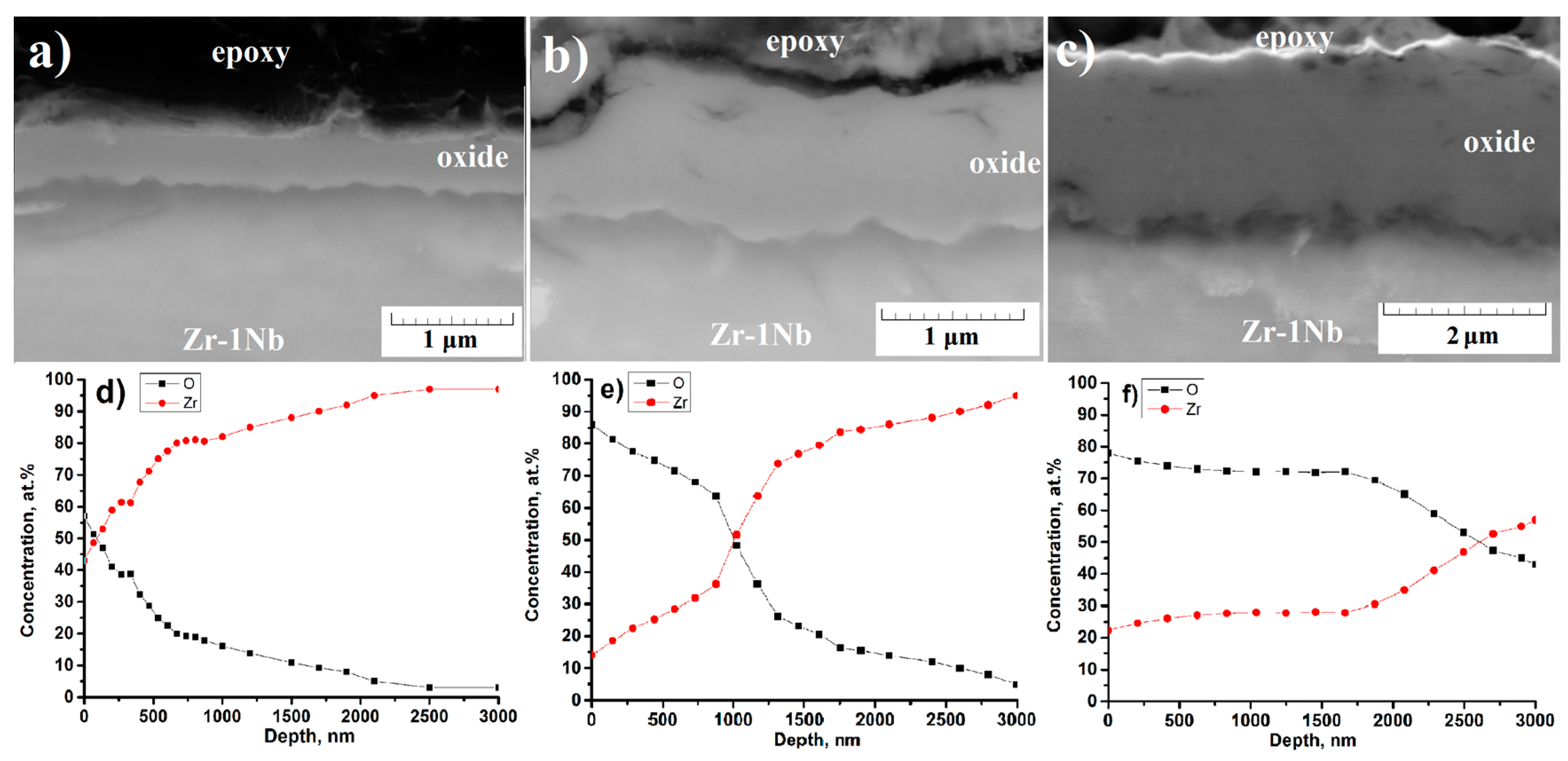
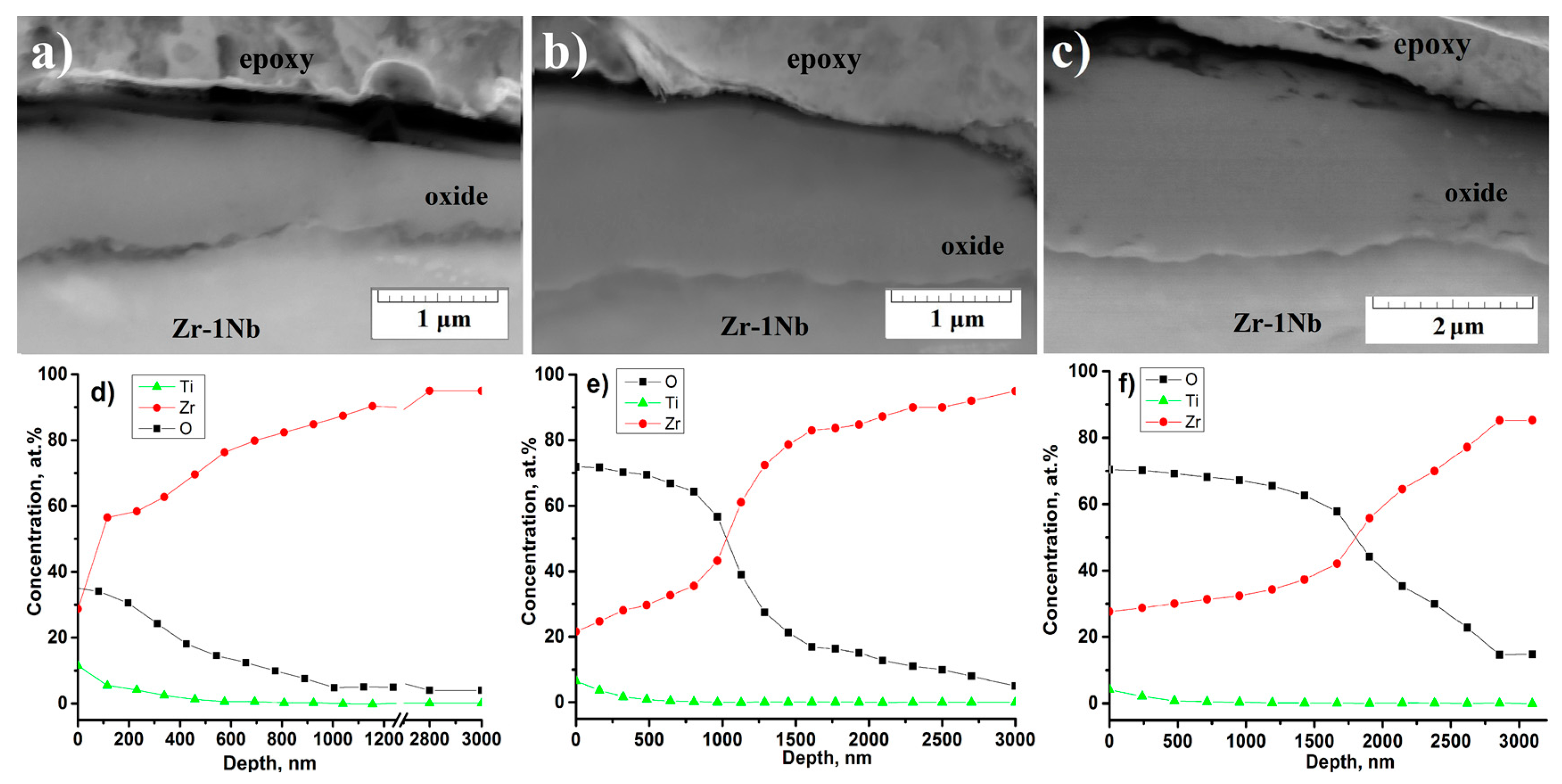
3.5. Microstructure of the Cross-Section of the Oxide Film before and after PIII of Ti
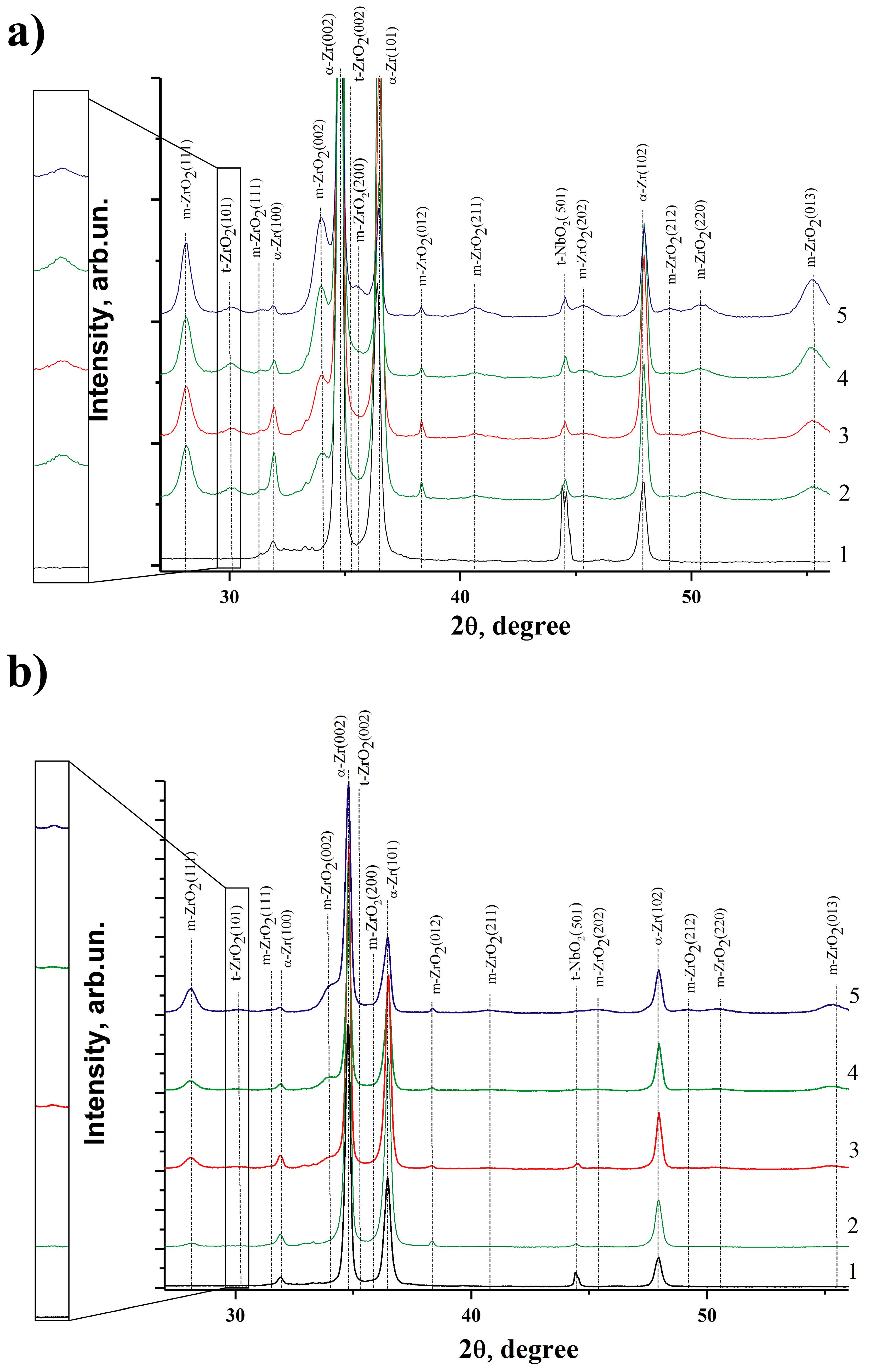
3.6. X-ray Diffraction Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Texture development and anisotropic deformation of zircaloys. Prog. Nucl. Energy 2006, 48, 325–359. [Google Scholar] [CrossRef]
- Kiran Kumar, M.; Aggarwal, S.; Kain, V.; Saario, T.; Bojinov, M. Effect of dissolved oxygen on oxidation and hydrogen pick up behaviour—Zircaloy vs. Zr–Nb alloys. Nucl. Eng. Des. 2010, 240, 985–994. [Google Scholar] [CrossRef]
- Ahmed, T.; Keys, L. The breakaway oxidation of zirconium and its alloys a review. J. Less Common Met. 1975, 39, 99–107. [Google Scholar] [CrossRef]
- Reddy, A.V.R.; Venugopal, V.; Sinha, R.K.; Banerjee, S. (Eds.) Proceedings of the International Conference on Peaceful Uses of Atomic Energy; Bhabha Atomic Research Centre: Mumbai, India, 2009; Volume 2, p. 384. [Google Scholar]
- McRae, G.; Coleman, C.; Leitch, B. The first step for delayed hydride cracking in zirconium alloys. J. Nucl. Mater. 2010, 396, 130–143. [Google Scholar] [CrossRef]
- Weatherly, G. Hydrogen Behaviour and Hydride Cracking Problems in Zirconium Alloys: Symposium on Zirconium Alloys in Service in Nuclear Reactors. Can. Metall. Q. 1985, 24, 225. [Google Scholar] [CrossRef]
- Pieroy, G. Mechanisms for the in-reactor creep of Zirconium alloys. J. Nucl. Mater. 1968, 26, 18–50. [Google Scholar] [CrossRef]
- Bespalov, I.; Datler, M.; Buhr, S.; Drachsel, W.; Rupprechter, G.; Suchorski, Y. Initial stages of oxide formation on the Zr surface at low oxygen pressure: An in situ FIM and XPS study. Ultramicroscopy 2015, 159, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.R.; Konings, R.J.M.; Motta, A.T. Corrosion of Zirconium Alloys. Compr. Nucl. Mater. 2012, 5, 49–68. [Google Scholar] [CrossRef]
- Motta, T.; Couet, A.; Comstock, R.J. Corrosion of Zirconium Alloys Used for Nuclear Fuel Cladding. Annu. Rev. Mater. Res. 2015, 45, 311–343. [Google Scholar] [CrossRef]
- Steinbrück, M.; Schaffer, S. High-Temperature Oxidation of Zircaloy-4 in Oxygen-Nitrogen Mixtures. Oxid. Met. 2016, 85, 245–262. [Google Scholar] [CrossRef]
- Abriata, J.P.; Bolcich, J.C. The Nb–Zr (Niobium–Zirconium) system. J. Phase Equilibria 1982, 3, 34–44. [Google Scholar] [CrossRef]
- Toffolon, C.; Brachet, J.C.; Servant, C.; Legras, L.; Charquet, D.; Barberis, P.; Mardon, J.P. Experimental study and preliminary thermodynamic calculations of the pseudo-ternary Zr-Nb-Fe-(O, Sn) system. In Zirconium in the Nuclear Industry: Thirteenth International Symposium; ASTM STP 1423; ASTM International: West Conshohocken, PA, USA, 2002; pp. 361–383. [Google Scholar]
- Lee, J.S. Operation and Maintenance of Spent Fuel Storage and Transportation Casks/Containers; IAEA: Vienna, Austria, 2007. [Google Scholar]
- Yegorova, L.; Lioutov, K.; Jouravkova, N.; Konobeev, A. Experimental Study of Embrittlement of Zr—1%Nb VVER Cladding under LOCA—Relevant Conditions; International Agreement Report; United States Nuclear Regulatory Commission: Washington, DC, USA, 2005.
- Batra, I.S.; Singh, R.N.; Sengupta, P.; Maji, B.C.; Madangopal, K.; Manikrishna, K.V.; Tewari, R.; Dey, G.K. Mitigation of hydride embrittlement of zirconium by yttrium. J. Nucl. Mater. 2009, 389, 500–503. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Gong, W.; Zhang, H. Effect of yttrium addition on microstructure and orientation of hydride precipitation in Zr-1Nb alloy. Int. J. Hydrogen Energy 2014, 39, 21116–21126. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Kim, I.; Khatkhatay, F.; Jiao, L.; Swadener, G.; Cole, J.I.; Gan, J.; Wang, H. TiN-based coatings on fuel cladding tubes for advanced nuclear reactors. J. Nucl. Mater. 2012, 429, 143–148. [Google Scholar] [CrossRef]
- Obrosov, A.; Sutygina, A.N.; Volinsky, A.A.; Manakhov, A.; Weiß, S.; Kashkarov, E.B. Effect of Hydrogen Exposure on Mechanical and Tribological Behavior of CrxN Coatings Deposited at Different Pressures on IN718. Materials 2017, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Xue, W.; Jia, X.; Du, J.; Wang, R.; Weng, L. Effect of voltage on properties of microarc oxidation films prepared in phosphate electrolyte on Zr-1Nb alloy. Surf. Coat. Technol. 2013, 222, 62–67. [Google Scholar] [CrossRef]
- Pushilina, N.S.; Kudiiarov, V.N.; Laptev, R.S.; Lider, A.M.; Teresov, A.D. Microstructure changes in Zr-1Nb alloy after pulsed electron beam surface modification and hydrogenation. Surf. Coat. Technol. 2015, 284, 63–68. [Google Scholar] [CrossRef]
- Terrani, K.A.; Parish, C.M.; Shin, D.; Pint, B.A. Protection of zirconium by alumina- and chromia-forming iron alloys under high-temperature steam exposure. J. Nucl. Mater. 2013, 438, 64–71. [Google Scholar] [CrossRef]
- Hui, R.; Cook, W.; Sun, C.; Xie, Y.; Yao, P.; Miles, J.; Olive, R.; Li, J.; Zheng, W.; Zhang, L. Deposition, characterization and performance evaluation of ceramic coatings on metallic substrates for supercritical water-cooled reactors. Surf. Coat. Technol. 2011, 205, 3512–3519. [Google Scholar] [CrossRef]
- Anders, A. Handbook of Plasma Immersion Ion Implantation and Deposition; Wiley-VCH Verl.: Weinheim, Germany, 2004. [Google Scholar]
- Ueda, M.; Silva, A.; Pillaca, E.; Mariano, S.; Rossi, J.; Oliveira, R.; Pichon, L.; Reuther, H. New possibilities of plasma immersion ion implantation (PIII) and deposition (PIII&D) in industrial components using metal tube fixtures. Surf. Coat. Technol. 2017, 312, 37–46. [Google Scholar] [CrossRef]
- Chu, P. Progress in direct-current plasma immersion ion implantation and recent applications of plasma immersion ion implantation and deposition. Surf. Coat. Technol. 2013, 229, 2–11. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Syrtanov, M.S.; Sutygina, A.N.; Shulepov, I.A.; Lider, A.M. Influence of plasma immersion titanium implantation on hydrogenation and mechanical properties of Zr-2.5Nb. Appl. Surf. Sci. 2016, 370, 142–148. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Syrtanov, M.S.; Vilkhivskaya, O.V.; Pryamushko, T.S.; Kudiiarov, V.N.; Volesky, L. Effect of titanium ion implantation and deposition on hydrogenation behavior of Zr-1Nb alloy. Surf. Coat. Technol. 2016, 308, 2–9. [Google Scholar] [CrossRef]
- Billone, M.; Yan, Y.; Burtseva, T.; Daum, R. Cladding Embrittlement during Postulated Loss-of-Coolant Accidents; NUREG/CR-6967, ANL07/04; Argonne National Laboratory: Lemont, IL, USA, 2008.
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers; John Wiley & Sons: Chichester, UK, 1992; ISBN 978-04-7193-592-6. [Google Scholar]
- Tezuka, Y.; Shin, S.; Ishii, T.; Ejima, T.; Suzuki, S.; Sato, S. Photoemission and Bremsstrahlung Isochromat Spectroscopy Studies of TiO2(Rutile) and SrTiO3. J. Phys. Soc. Jpn. 1994, 63, 347–357. [Google Scholar] [CrossRef]
- Beneš, O.; Van Uffelen, P.; van de Laar, J.; Győri, C.; Konings, R.J.M.; Hózer, Z. Kinetic studies of the α–β phase transition in the Zr1%Nb cladding for nuclear reactors. J. Nucl. Mater. 2011, 414, 88–91. [Google Scholar] [CrossRef]
- Canay, M.; Dan, C.A.; Arias, D. Phase transition temperature in the Zr-rich corner of Zr ± Nb ± Sn ± Fe alloys. J. Nucl. Mater. 2000, 280, 365–371. [Google Scholar] [CrossRef]
- Okamoto, H. Nb-Zr (niobium-zirconium). J. Phase Equilibria 1992, 13, 577. [Google Scholar] [CrossRef]
- Yang, H.L.; Kano, S.; Matsukawa, Y.; Li, Y.F.; Shen, J.J.; Li, F.; Zhao, Z.S.; Satoh, Y.; Abe, H. Effect of molybdenum on microstructures in Zr-1.2Nb alloys after β-quenching and subsequently 873 K annealing. Mater. Des. 2016, 104, 355–364. [Google Scholar] [CrossRef]
- Ivanov, O.S.; Grigorovich, V.K. Theoretical and experimental aspects of controlled nuclear fusion. In Proceedings of the Second UN International Conference on Peaceful Uses of Atomic Energy, Geneva, Switzerland, 1–13 September 1958; Volume 3, p. 34. [Google Scholar]
- Peng, D.; Bai, X.; Pan, F.; Sun, H.; Chen, B. Influence of titanium ions implantation on corrosion behavior of zirconium in 1 M H2SO4. Appl. Surf. Sci. 2006, 252, 2196–2203. [Google Scholar] [CrossRef]
- Guo, X. Hydrothermal degradation mechanism of tetragonal Zirconia. J. Mater. Sci. 2001, 36, 3737–3744. [Google Scholar] [CrossRef]
- Peng, D.; Bai, X.; Pan, F.; Sun, H.; Chen, B. Influence of aluminum ions implanted on oxidation behavior of ZIRLO alloy at 500 °C. Vacuum 2006, 80, 530–536. [Google Scholar] [CrossRef]
- Likhanskii, V.; Kolesnik, M. On the evolution of wave structure at the metal/oxide interface during oxidation of Zr alloys. Corros. Sci. 2014, 87, 416–420. [Google Scholar] [CrossRef]
- Molins, R.; Germidis, A.; Andrieu, E.; Newcomb, S.B.; Little, J.A. Microscopy of Oxidation 3. In Proceedings of the Third International Conference on the Microscopy of Oxidation, Cambridge, UK, 16–18 September 1996; Institute of Materials, Minerals and Mining: London, UK, 1996. [Google Scholar]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Yao, M.; Gao, C.; Huang, J.; Peng, J.; Liang, X.; Zhang, J.; Zhou, B.; Li, Q. Oxidation behavior of β-Nb precipitates in Zr–1Nb–0.2Bi alloy corroded in lithiated water at 360 °C. Corros. Sci. 2015, 100, 169–176. [Google Scholar] [CrossRef]
- Cox, B.; Kritsky, V.G.; Lemaignan, C.; Polley, V.; Ritchie, I.G.; Ruhmann, H.; Shishov, V.N.; Bibilashvili, Y.K.; Nikulina, A.V. Waterside Corrosion of Zirconium Alloys in Nuclear Power Plants; TECDOC-996; International Atomic Energy Agency: Vienna, Austria, 1998. [Google Scholar]
- Peng, D.; Bai, X.; Pan, F.; Sun, H.; Chen, B. Surface analysis of Zircaloy-2 implanted with carbon before and after oxidation in air at 500 °C. Mater. Charact. 2006, 56, 112–120. [Google Scholar] [CrossRef]
- Zhanga, X.Y.; Wan, Q.; Baib, X.D.; Jina, Z.X.; Liua, X.Y. Effect of Titanium ion implantation on the oxidation behaviour of zircalloy-4 at 500 °C. Vacuum 2006, 80, 1003–1006. [Google Scholar] [CrossRef]
- Xiong, L.B.; Li, J.L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: generation, properties and photocatalytic application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef]
- Ho, S.M. On the Structural Chemistry of Zirconium Oxide. Mater. Sci. Eng. 1982, 54, 23–29. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.W.; Penner-Hahn, J.E. X-ray-absorption studies of zirconia polymorphs. II. Effect of Y2O3 dopant on ZrO2 structure. Phys. Rev. B 1993, 48, 10074–10081. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Habazaki, H. Influences of structure and composition on the photoelectrochemical behaviour of anodic films on Zr and Zr-20 at.%Ti. Electrochim. Acta 2008, 53, 2272–2280. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization—An X-ray Absorption Study: III, Charge-Compensating Dopants. J. Am. Ceram. Soc. 1994, 77, 1289–1295. [Google Scholar] [CrossRef]
- Zschech, E.; Kountouros, P.N.; Petzow, G.; Behrens, P.; Lessmann, A.; Frahm, R. Synchrotron Radiation Ti-k XANES Study of TiO2–Y2O3-Stabilized Tetragonal Zirconia Polycrystals. J. Am. Ceram. Soc. 1993, 76, 197–201. [Google Scholar] [CrossRef]
- Traqueia, L.S.M.; Pagnier, T.; Marques, F.M.B. Structural and electrical characterization of titania-doped YSZ. J. Eur. Ceram. Soc. 1997, 17, 1019–1026. [Google Scholar] [CrossRef]
- Valigi, M.; Gazzoli, D.; Incocciati, E.; Dragone, R. Metastable phase formation in the TiO-ZrO, and Cd-ZrO. Solid State Ion. 1997, 101–103, 597–603. [Google Scholar] [CrossRef]
- Capel, F.; Moure, C.; Duran, P.; Gonzalez-Elipe, A.R.; Caballero, A. Titanium local environment and electrical conductivity of TiO2-doped stabilized tetragonal zirconia. J. Mater. Sci. 2000, 35, 345–352. [Google Scholar] [CrossRef]
- Hodgson, S.N.B.; Cawley, J. The effect of titanium oxide additions on the properties and behaviour of Y-TZP. J. Mater. Process. Technol. 2001, 119, 112–116. [Google Scholar] [CrossRef]
- Colomer, M.T.; Jurado, J.R. Structure, microstructure, and mixed conduction of [(ZrO2)0.92(Y2O3)0.08]0.9(TiO2)0.1. J. Solid State Chem. 2002, 165, 79–88. [Google Scholar] [CrossRef]
- Kashkarov, E.; Nikitenkov, N.; Sutygina, A.; Laptev, R.; Bordulev, Y.; Obrosov, A.; Liedke, M.; Wagner, A.; Zak, A.; Weiβ, S. Microstructure, defect structure and hydrogen trapping in zirconium alloy Zr-1Nb treated by plasma immersion Ti ion implantation and deposition. J. Alloys Compd. 2017, 732, 80–87. [Google Scholar] [CrossRef]


| Material | Arc Current, А | Pressure (Ar), Pа | Pulse Repetition Rate, kHz | Pulse Length, μs | Bias Voltage, В | Time of Implantation, min |
|---|---|---|---|---|---|---|
| Zr-1Nb | 70 | 0.15 | 100 | 5 | 1500 | 15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrosov, A.; Sutygina, A.N.; Manakhov, A.; Bolz, S.; Weiß, S.; Kashkarov, E.B. Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals 2018, 8, 27. https://doi.org/10.3390/met8010027
Obrosov A, Sutygina AN, Manakhov A, Bolz S, Weiß S, Kashkarov EB. Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals. 2018; 8(1):27. https://doi.org/10.3390/met8010027
Chicago/Turabian StyleObrosov, Aleksei, Alina N. Sutygina, Anton Manakhov, Sebastian Bolz, Sabine Weiß, and Egor B. Kashkarov. 2018. "Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation" Metals 8, no. 1: 27. https://doi.org/10.3390/met8010027
APA StyleObrosov, A., Sutygina, A. N., Manakhov, A., Bolz, S., Weiß, S., & Kashkarov, E. B. (2018). Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals, 8(1), 27. https://doi.org/10.3390/met8010027







