Analysis of the Microstructure and Selected Properties of the Aluminium Alloys Used in Automotive Air-Conditioning Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructure Investigations
3.2. Thermal Stability Investigations
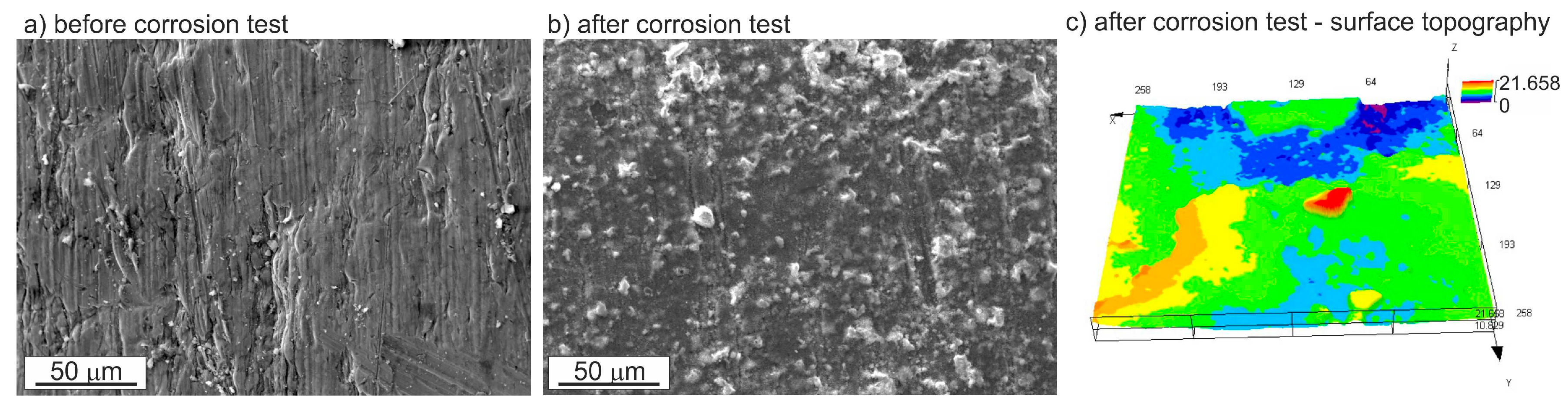
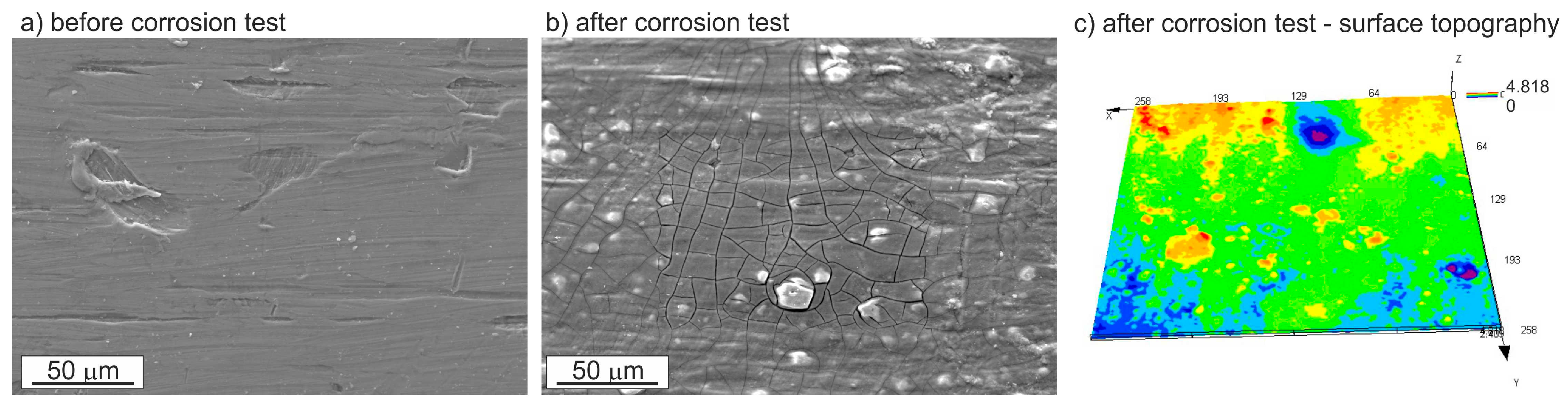
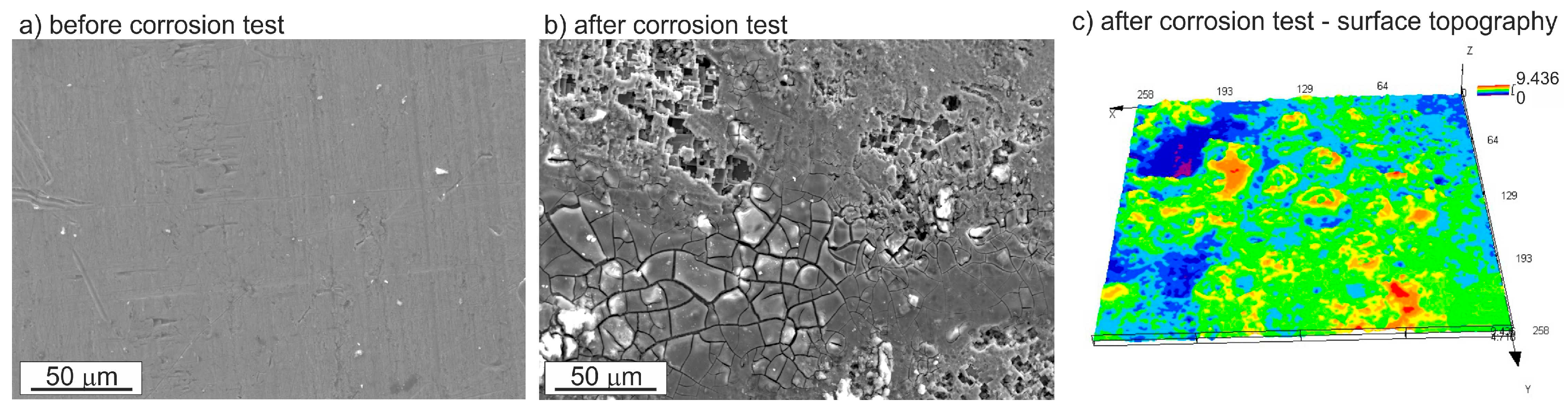
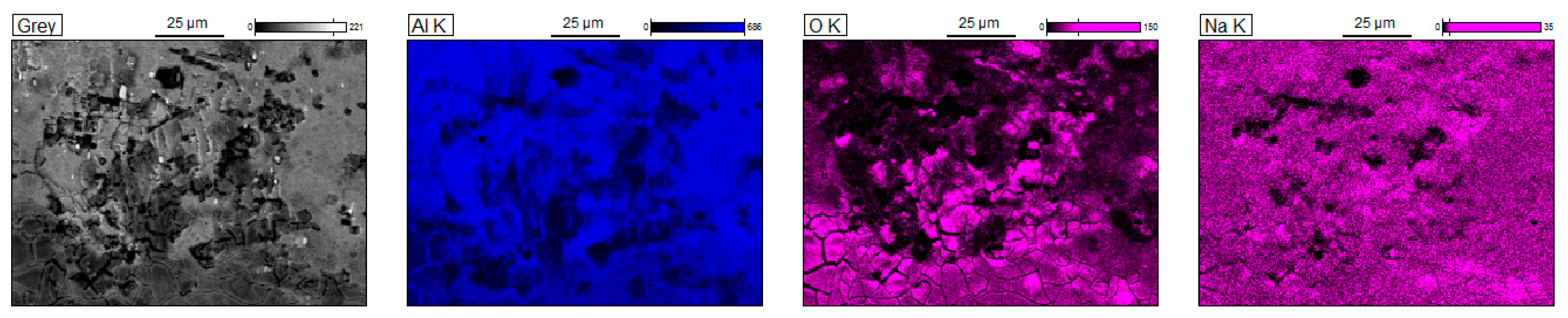
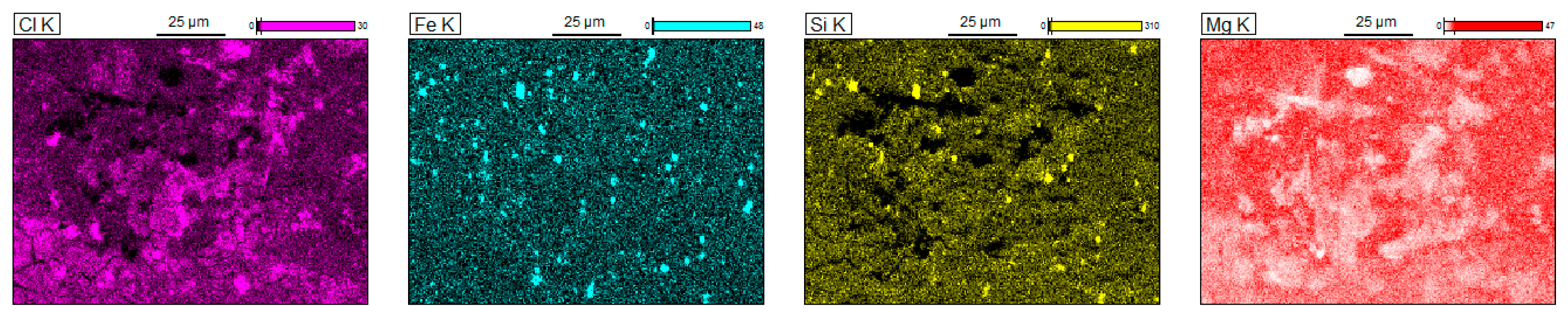
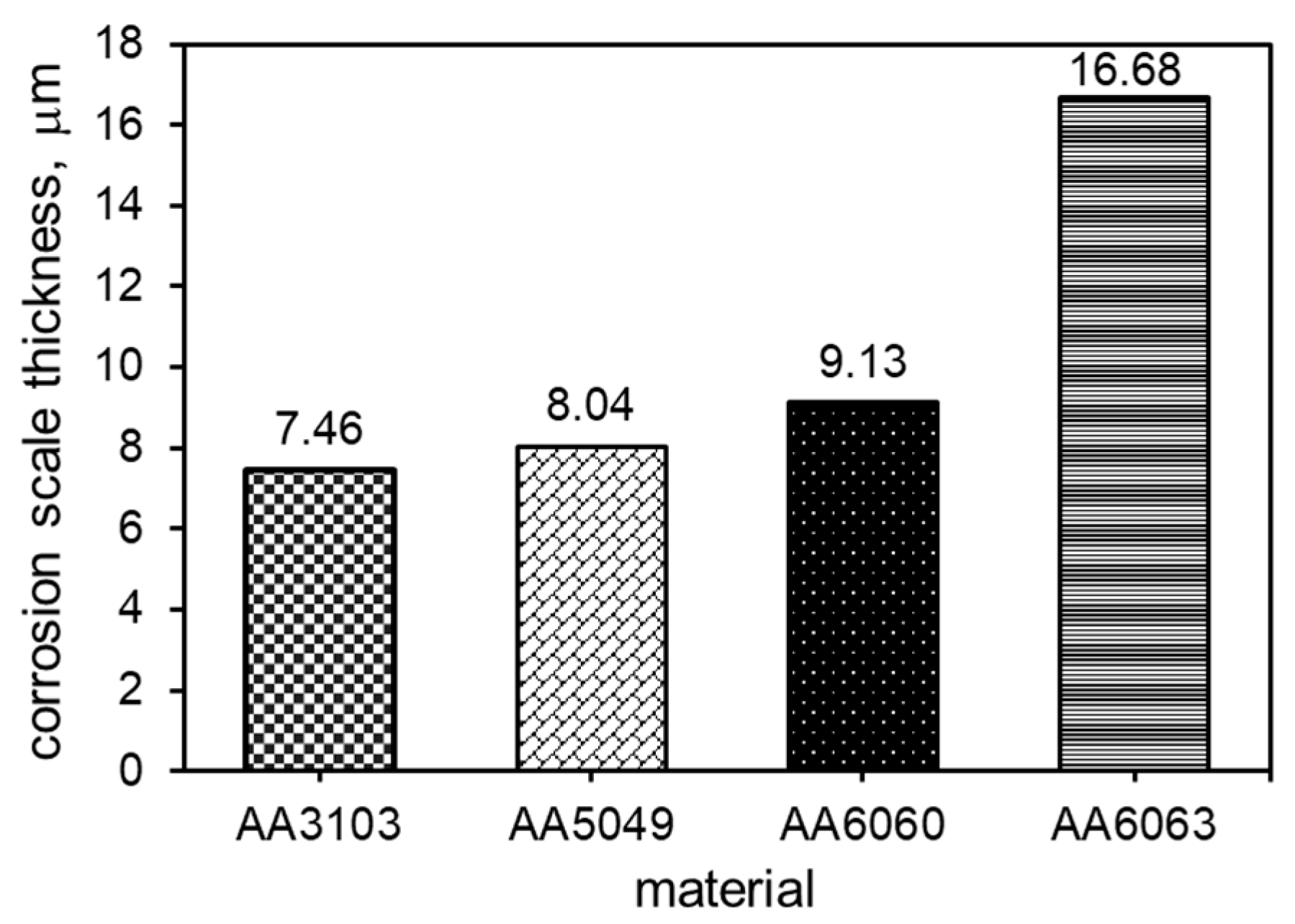
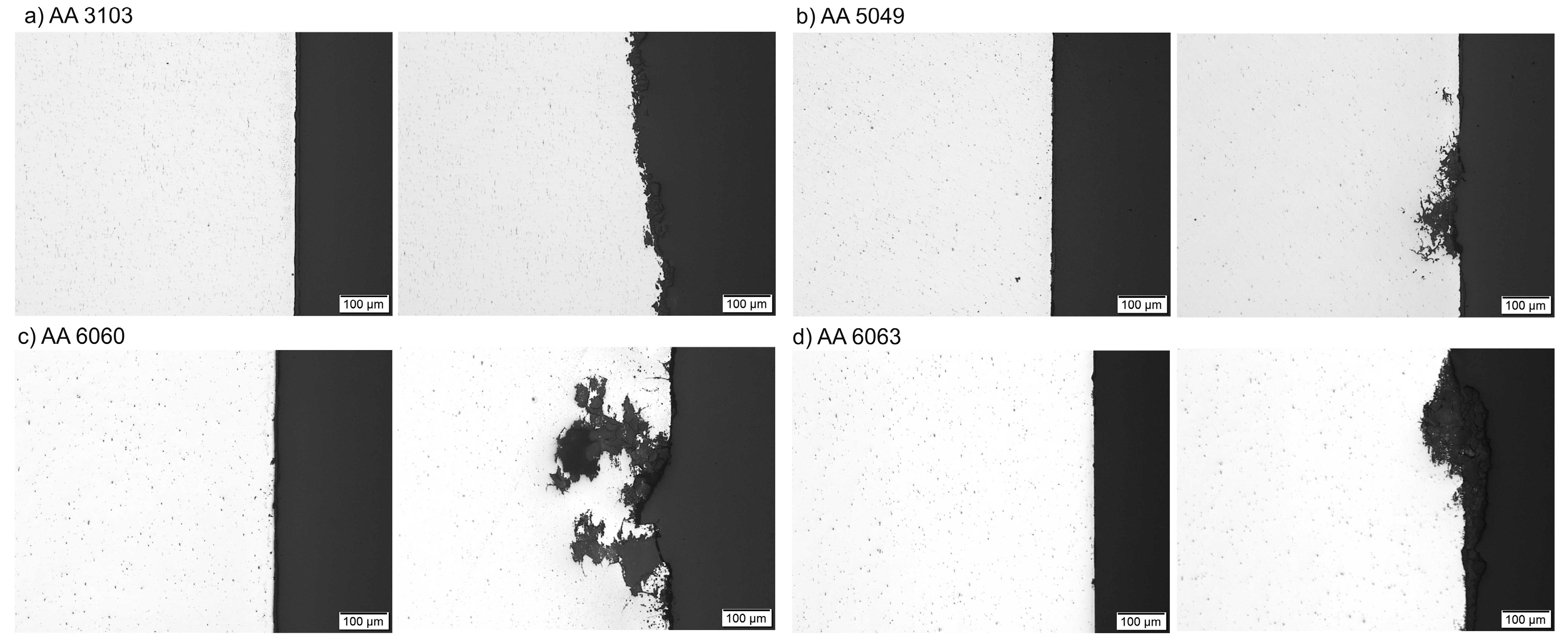
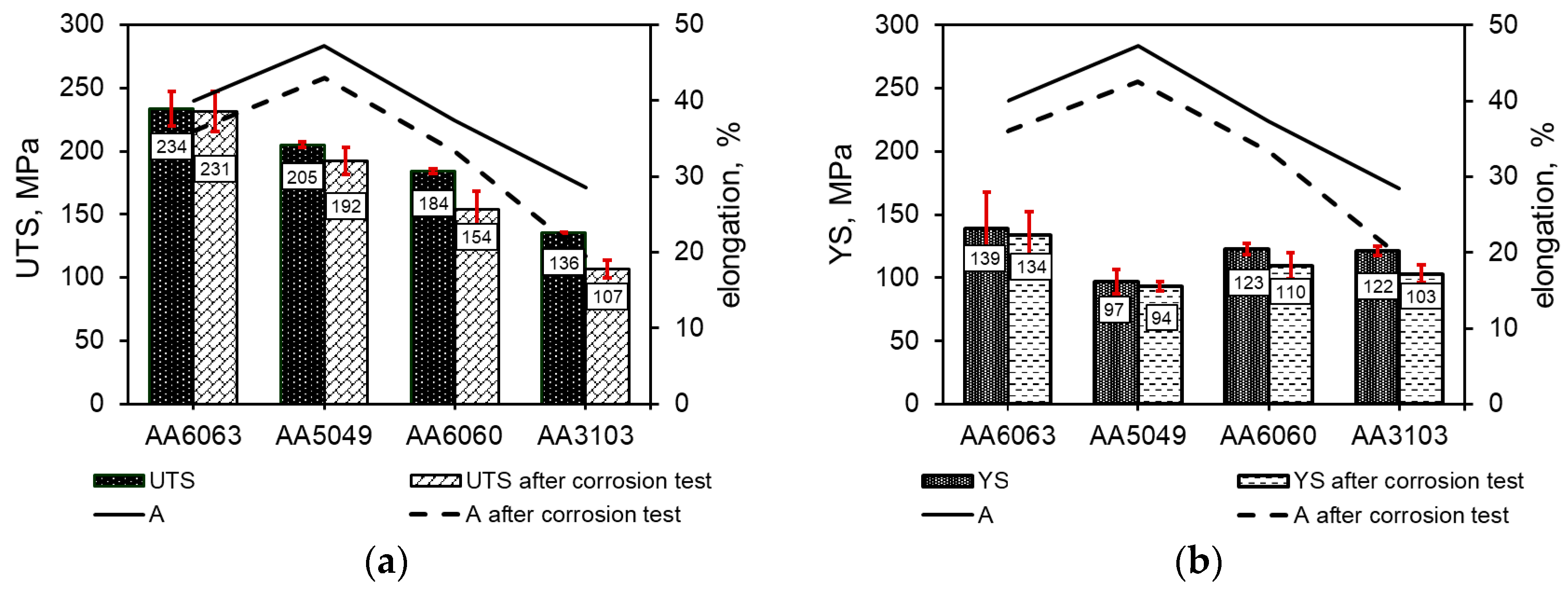
3.3. Corrosion Resistance Investigations
4. Conclusions
- Both AA3103 and AA5049 are stable within the entire range of the investigated temperatures. The average measured microhardness of AA3103 and AA5049 is 45 HV0.1 and 60 HV0.1, respectively. As regards AA6060 and AA6063, neither of them is stable in the investigated range of temperatures. The maximum HV0.1 occurs in the temperature range of 100–140 °C and is due to the precipitation process of the intermetallic phases.
- Pitting is the main corrosion process. It starts in or around the intermetallic particles present in the alloys and is the result of the difference of potential between the intermetallic phases and the matrix.
- The obtained results have demonstrated a decrease in the mechanical properties and elongation of about 5–20%, observed in all the examined alloys after the corrosion resistance test in a salt spray chamber. This is mainly due to the effect of corrosion that attacks the material and penetrates into its interior.
- Despite the adverse impact of corrosion deteriorating the strength properties of the tested alloys, the values obtained remain within the acceptable range. Hence the conclusion follows that all the tested alloys satisfy the imposed requirements and can be safely used for parts of the automotive air conditioning systems.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kahl, S.; Ekström, H.-E.; Mendoza, J. Tensile, Fatigue and Creep Properties of Aluminium Heat Exhanger Tube Alloys for Temperatures from 293 K to 573 K (20 °C to 300 °C). Metall. Mater. Trans. A 2014, 45, 663–681. [Google Scholar] [CrossRef]
- Hirsch, J. Aluminium in Innovative Light-Weight Car Design. Mater. Trans. 2011, 52, 818–824. [Google Scholar] [CrossRef]
- Fridlyander, N.; Sister, V.G.; Grushko, O.E.; Berstenev, V.V.; Sheveleva, L.M.; Ivanova, L.A. Aluminium alloys: Promising Materials in the Automotive Industry. Metal Sci. Heat Treat. 2002, 44, 365–370. [Google Scholar] [CrossRef]
- Kruse, H.; Suess, J. Research on the Behaviour of Refrigeration Compressors Using CO2 as the Refrigerant. Int. Compress. Eng. Conf. 1996, 111, 223–228. [Google Scholar]
- Paul, M.; Zhongjie, H. A review of carbon dioxide as a refrigerant in refrigeration technology. S. Afr. J. Sci. 2011, 111, 1–10. [Google Scholar]
- Skrzyniowski, A.; Skrzyniowska, D. Poszukiwania alternatywnego czynnika ziębniczego dla urządzeń klimatyzacyjnych w pojazdach samochodowych. J. KONES Powertrain Transp. 2015, 13, 165–175. [Google Scholar]
- Gaurav; Kumar, R. Sustainability of alternative material R-134a in mobile air-conditioning system: A review. Mater. Today Proc. 2017, 4, 112–118. [Google Scholar] [CrossRef]
- Rameez, U.D.; Borodo, K.; Morten, S.J.; Rajan, A. Accelerated growth of oxide film on aluminium alloys under steam: Part II: Effect of alloy chemistry and steam vapour pressure in corrosion and adhesion performance. Surf. Coat. Technol. 2015, 276, 106–115. [Google Scholar]
- Sukiman, N.L.; Zhou, X.; Birbilis, N.; Mol, J.M.; Garcia, S.J.; Zhou, X.; Thompson, G.E. Durability and corrosion of aluminium and its alloys: Overview, property space, techniques and developments. In Aluminium Alloys—New Trends in Fabrication and Applications; Ahmad, Z., Ed.; InTech: Rijeka, Croatia, 2013; pp. 47–97. ISBN 978-953-51-0861-0. [Google Scholar]
- Andersen, S.J.; Marioara, C.D.; Frøseth, A.; Vissers, R.; Zandbergen, H.W. Crystal structure of the orthorhombic U2-Al4Mg4Si4 precipitate in the Al-Mg-Si alloy system and its relation to the β’ and β” phases. Mater. Sci. Eng. A 2005, 390, 127–138. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Frøseth, A.G.; Høier, R.; Derlet, P.M.; Andersen, S.J.; Marioara, C.D. Bonding in MgSi and Al-Mg-Si compounds relevant to Al-Mg-Si alloys. Phys. Rev. B 2003, 67, 1–11. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakaguch, Y.; Miyata, Y.; Uetani, Y.; Sato, T.; Kamio, A.; Ikeno, S. Precipitation sequence of various kinds of metastable phases in Al-1.0 mass% Mg2Si-0.4 mass% Si alloy. J. Mater. Sci. 2000, 35, 179–189. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Zandbergen, H.W.; Andersen, S.J.; Jansen, J. Structure Determination of Mg5Si6 Particles in Al by Dynamic Electron Diffraction Studies. Science 1997, 277, 1221–1225. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Zandbergen, H.W.; Holmestad, R. The Influence of Alloy Composition on Precipitates of the Al-Mg-Si System. Metall. Mater. Trans. A 2005, 36, 691–702. [Google Scholar]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. The influence of temperature and storage time at RT on nucleation of the β” phase in a 6082 Al-Mg-Si alloy. Acta Mater. 2003, 51, 789–796. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Su, Y.; Xiao, Y.; Liu, J. Corrosion Behavior of 5A05 Aluminium Alloy in NaCl Solution. Acta Metall. Sin. 2013, 26, 581–587. [Google Scholar] [CrossRef]
- Alavi, A.; Corns, R.A. The Determination of pH, Potential and chloride concentration in corroding crevies on 304 stainless steel and 7475 aluminium alloy. Corros. Sci. 1987, 27, 443–451. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Localized alkaline corrosion of alloy AA5083 in neutral 3.5% NaCl solution. Corros. Sci. 2001, 43, 1657–1674. [Google Scholar] [CrossRef]
- Gudić, S.; Smoljko, I.; Kliškić, M. The effect of small addition of tin and indium on the corrosion behavior of aluminium in chloride solution. J. Alloys Compd. 2010, 505, 54–63. [Google Scholar] [CrossRef]
- Li, T.; Li, X.G.; Dong, C.F.; Cheng, Y.F. Characterization of Atmospheric Corrosion of 2A12 Aluminum Alloy in Tropical Marine Environment. J. Mater. Eng. Perform. 2010, 19, 591–598. [Google Scholar] [CrossRef]
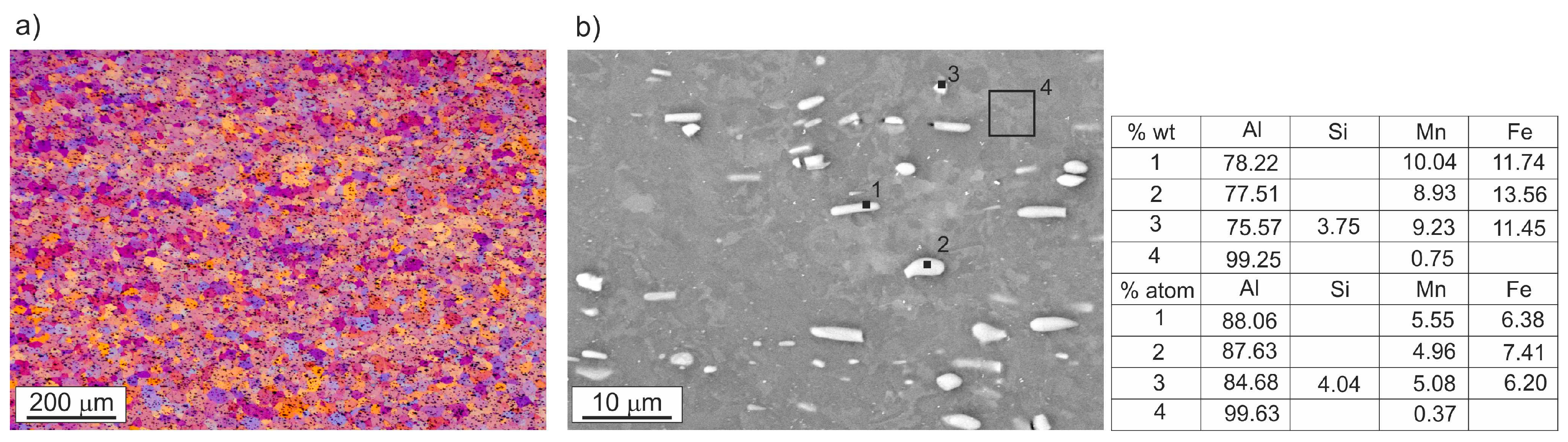
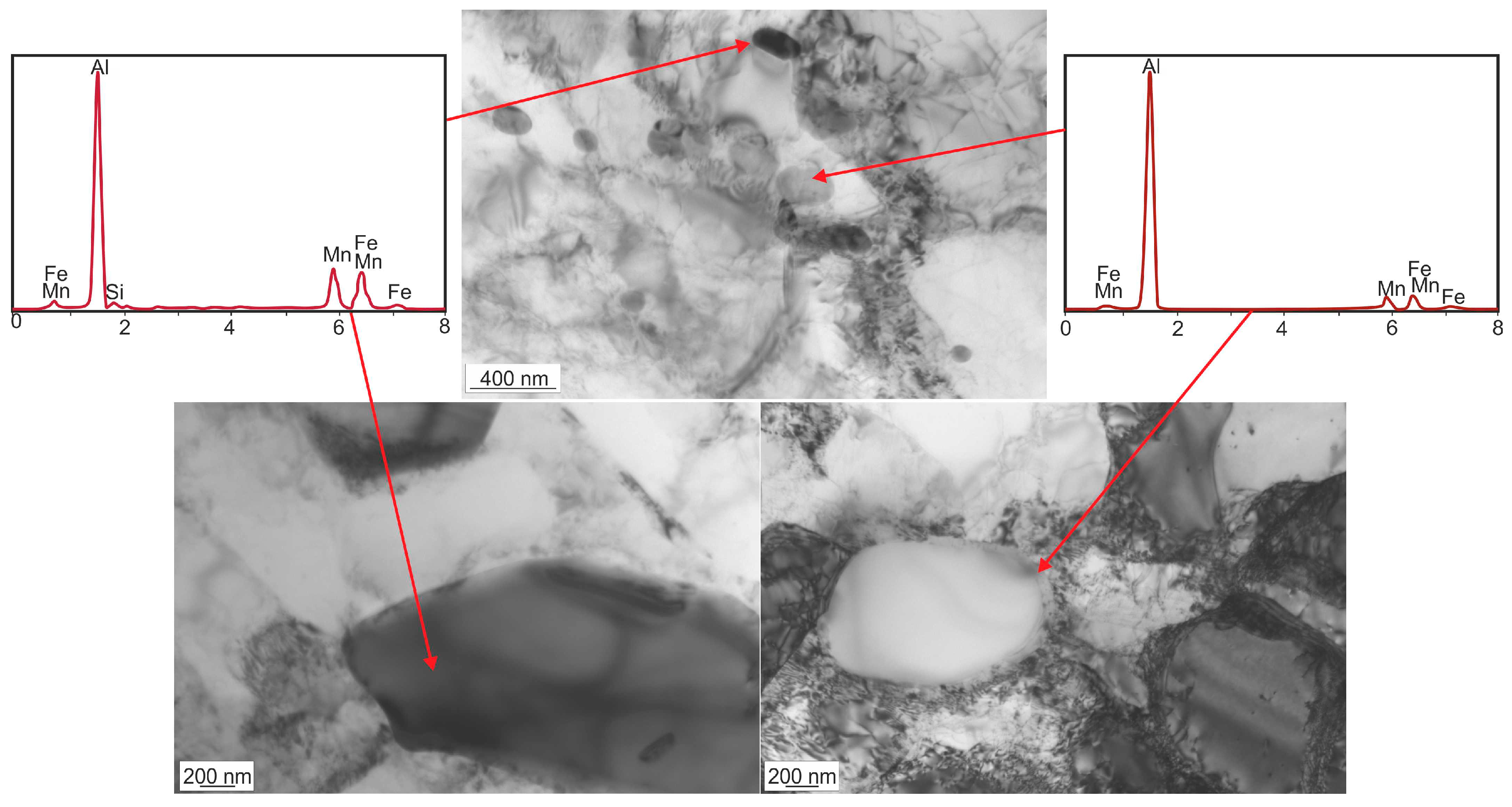
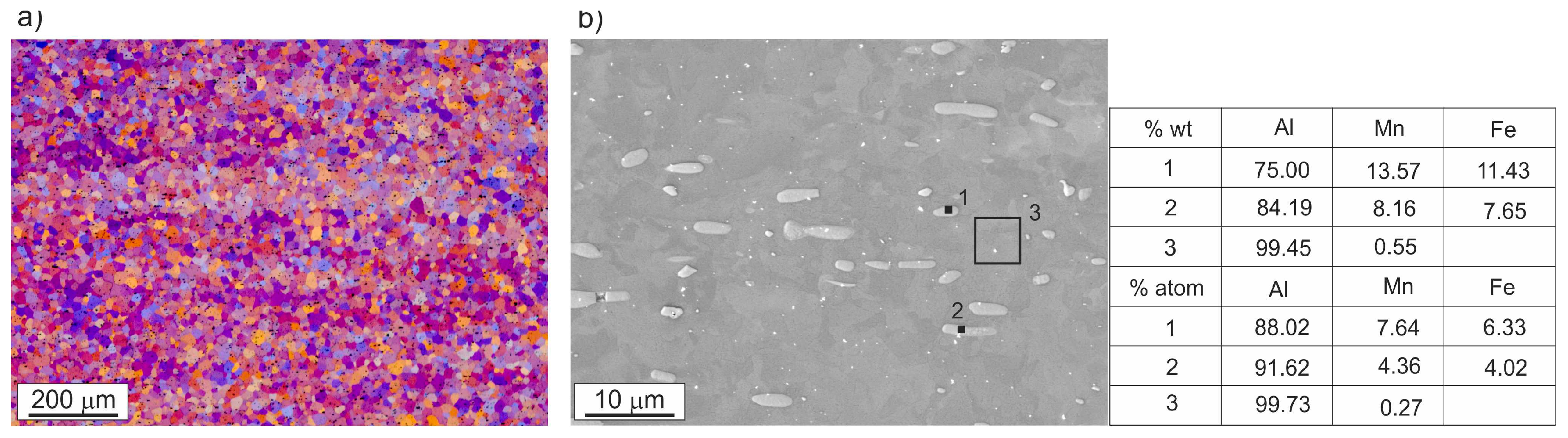
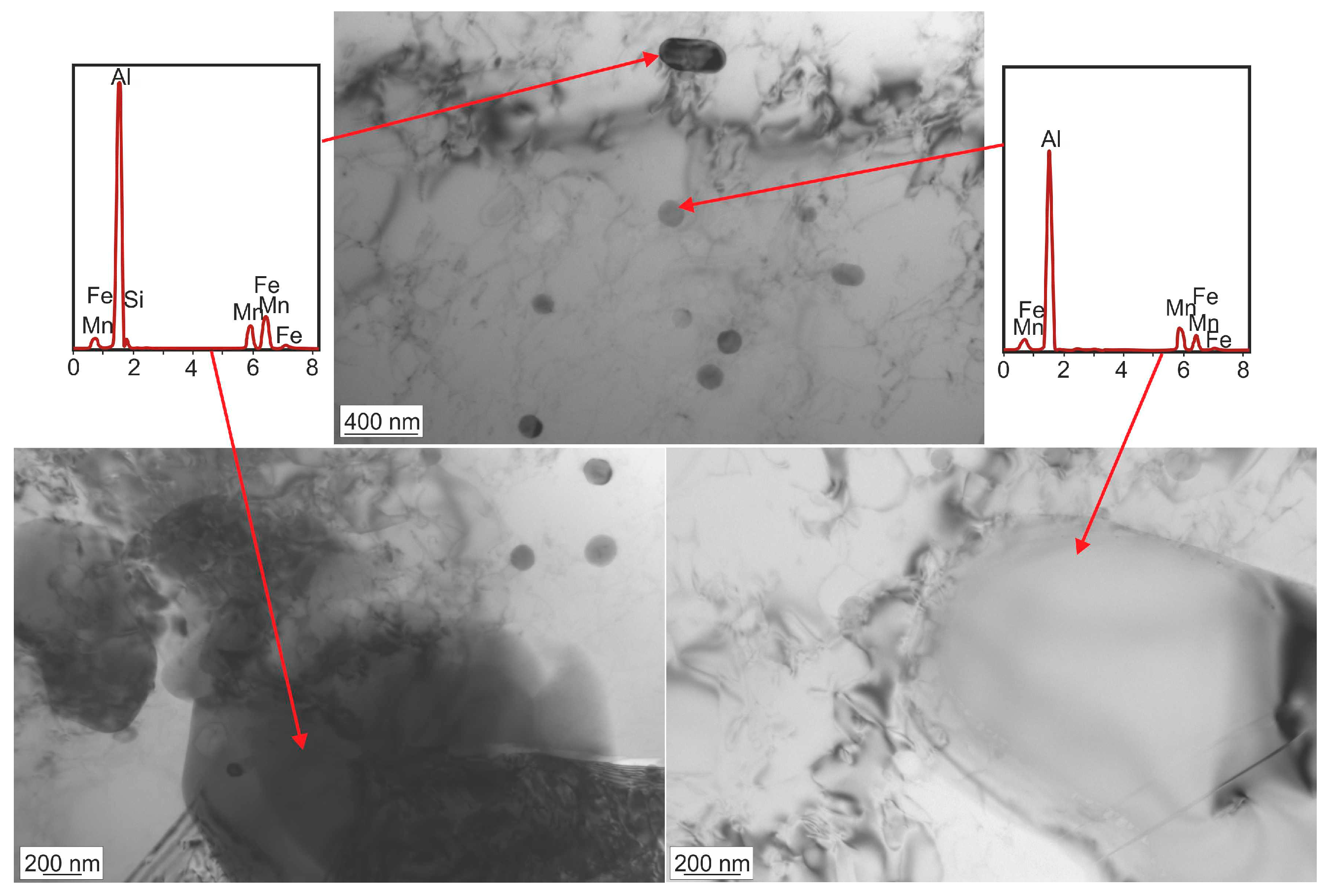

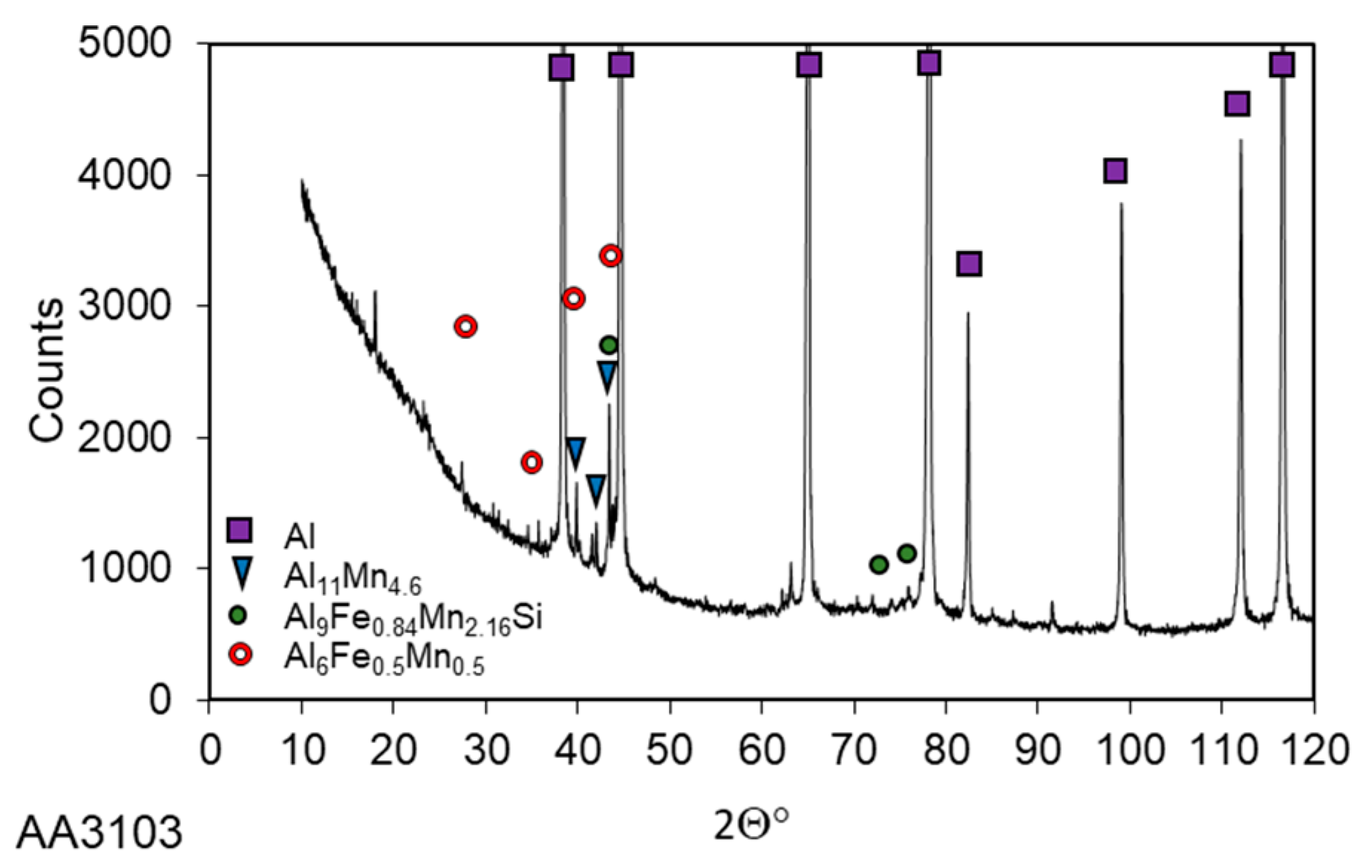
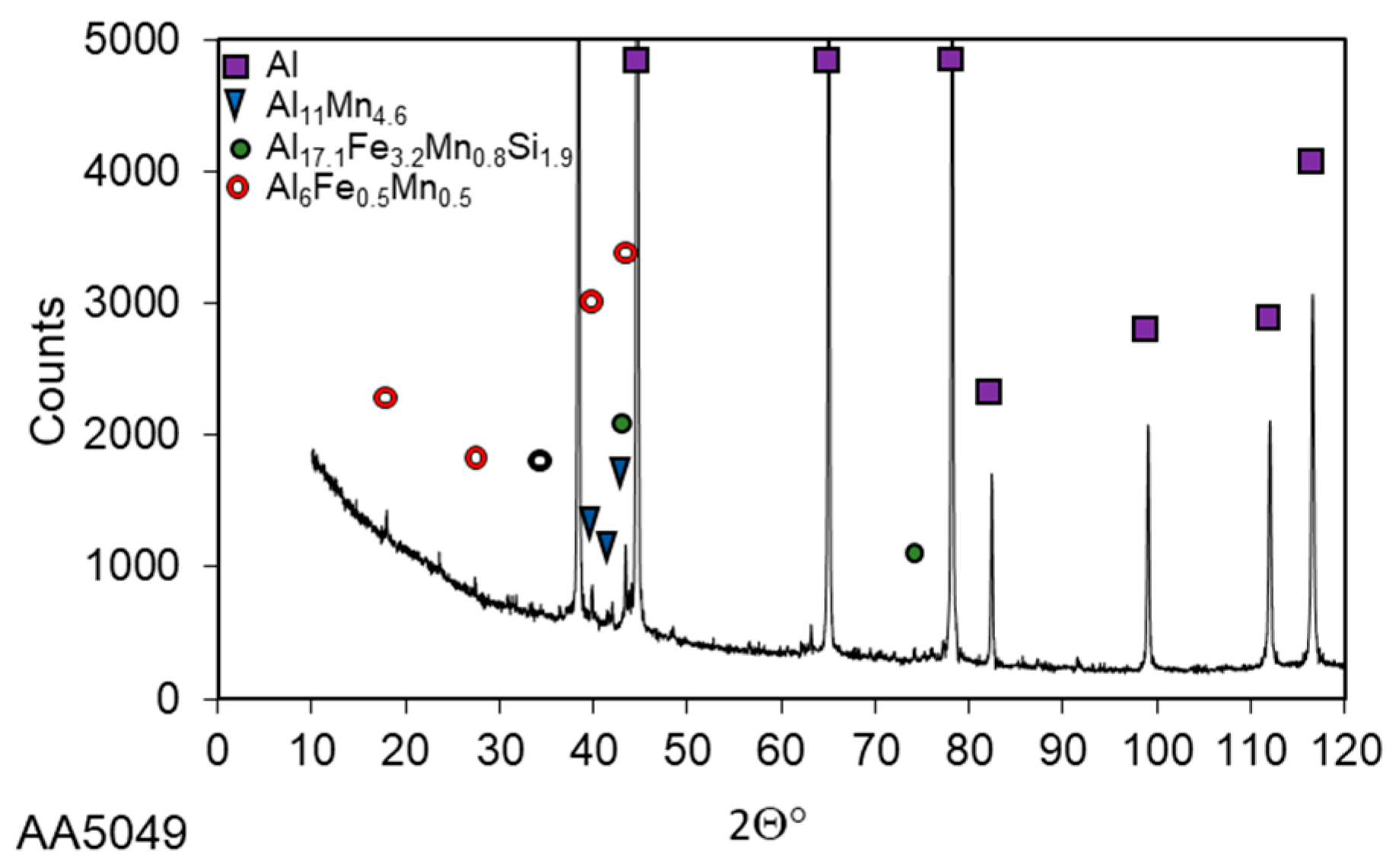
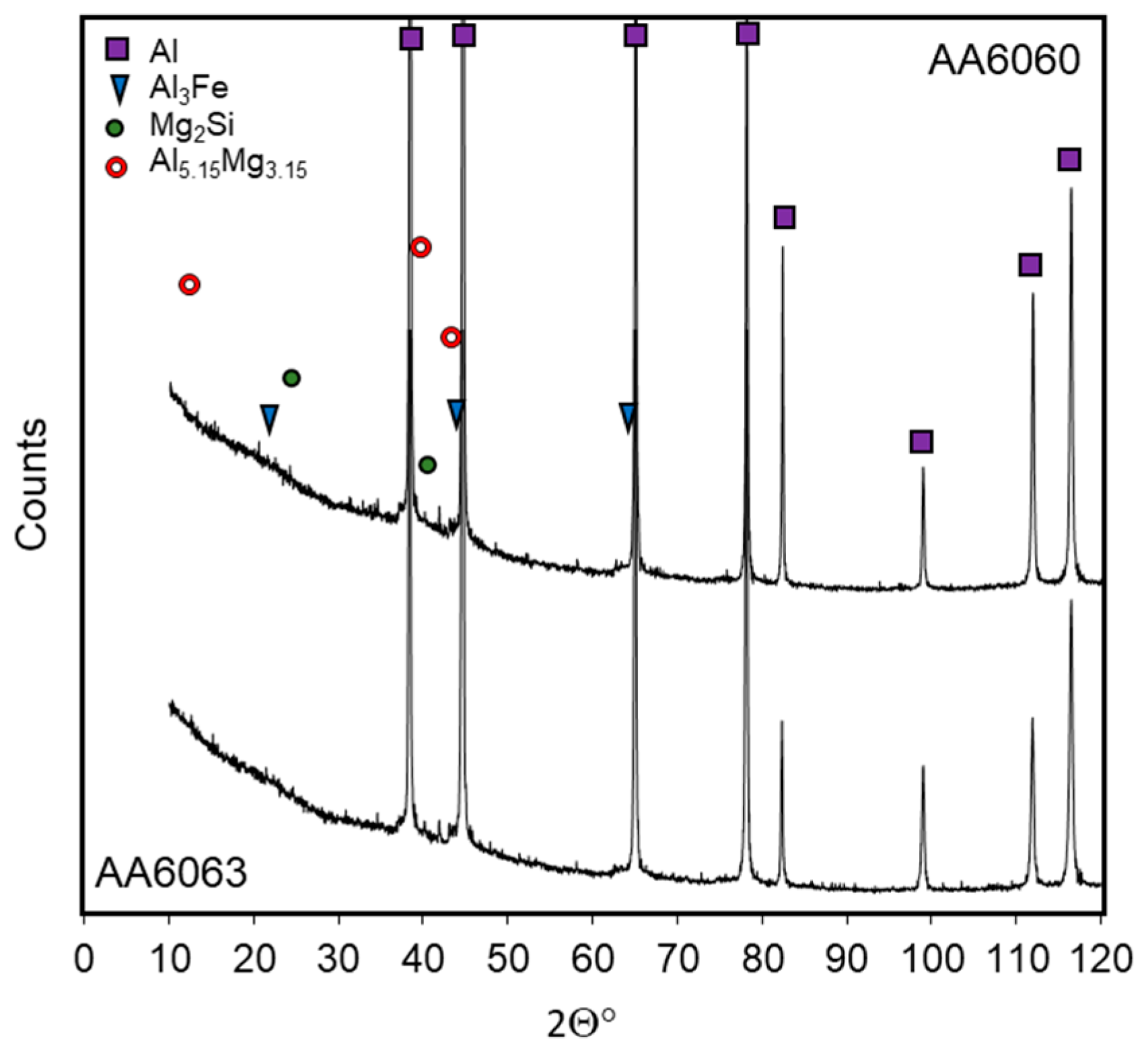
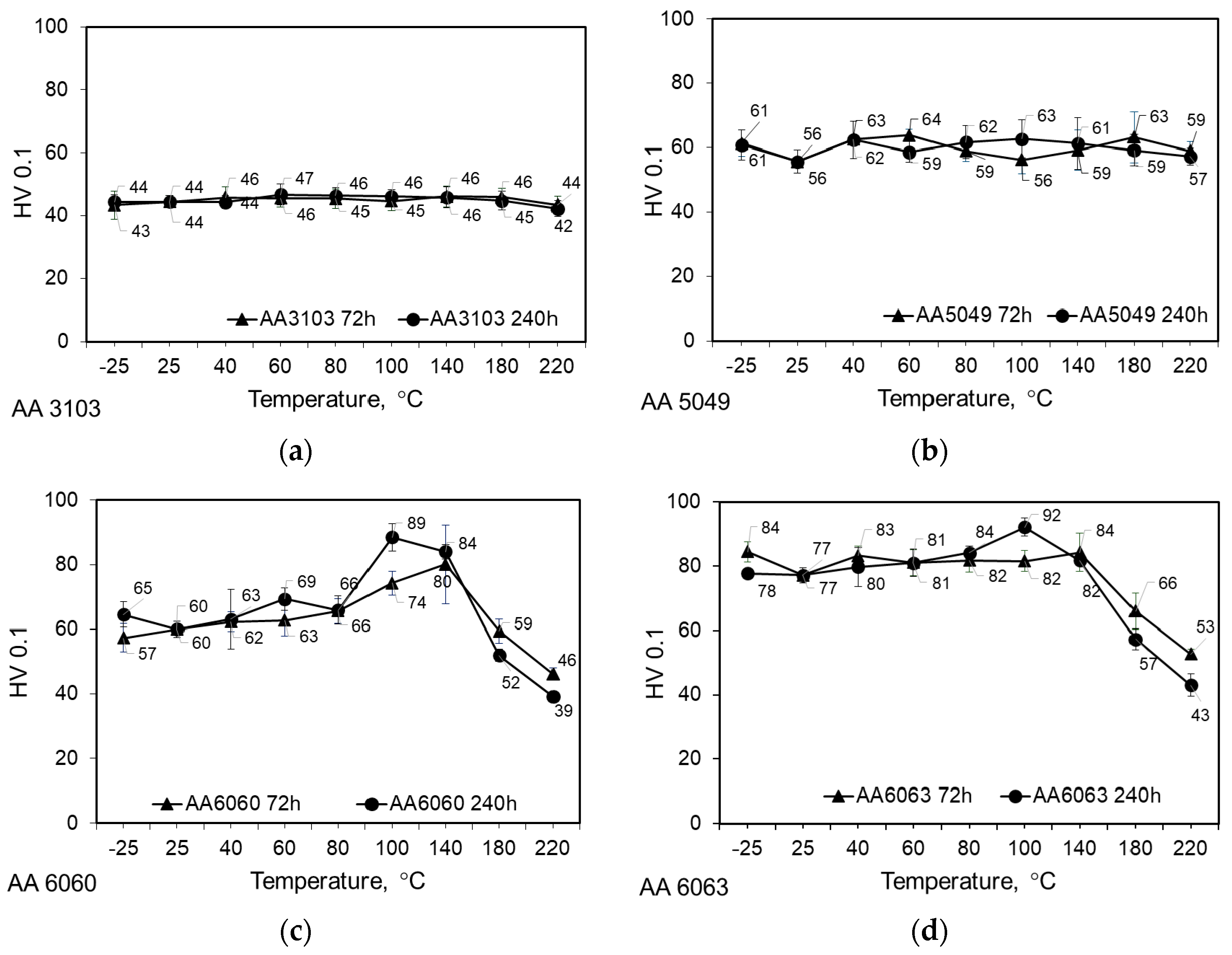



| Alloy | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti/Zr | Al |
|---|---|---|---|---|---|---|---|---|---|
| AA3103 | 0.50 | 0.70 | 1.10 | 0.90–1.50 | 0.30 | 0.10 | 0.20 | <0.10 | balance |
| AA5049 | 0.40 | 0.50 | 0.10 | 0.50–1.10 | 1.60–2.50 | <0.30 | 0.20 | <0.10 | balance |
| AA6060 | 0.30–0.60 | 0.10–0.30 | 0.10 | 0.1 | 0.35–0.60 | 0.05 | 0.15 | 0.15 | balance |
| AA6063 | 0.40–0.60 | 0.15–0.30 | 0.05 | 0.1 | 0.50–0.70 | 0.05 | 0.05 | - | balance |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leszczyńska-Madej, B.; Richert, M.; Wąsik, A.; Szafron, A. Analysis of the Microstructure and Selected Properties of the Aluminium Alloys Used in Automotive Air-Conditioning Systems. Metals 2018, 8, 10. https://doi.org/10.3390/met8010010
Leszczyńska-Madej B, Richert M, Wąsik A, Szafron A. Analysis of the Microstructure and Selected Properties of the Aluminium Alloys Used in Automotive Air-Conditioning Systems. Metals. 2018; 8(1):10. https://doi.org/10.3390/met8010010
Chicago/Turabian StyleLeszczyńska-Madej, Beata, Maria Richert, Anna Wąsik, and Adam Szafron. 2018. "Analysis of the Microstructure and Selected Properties of the Aluminium Alloys Used in Automotive Air-Conditioning Systems" Metals 8, no. 1: 10. https://doi.org/10.3390/met8010010





