Microwave and Ultrasound Augmented Leaching of Complicated Zinc Oxide Ores in Ammonia and Ammonium Citrate Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Leaching Experiments
2.4. Reaction Mechanism
3. Results and Discussion
3.1. Effect of Microwave Roasting on % Leaching of Zinc
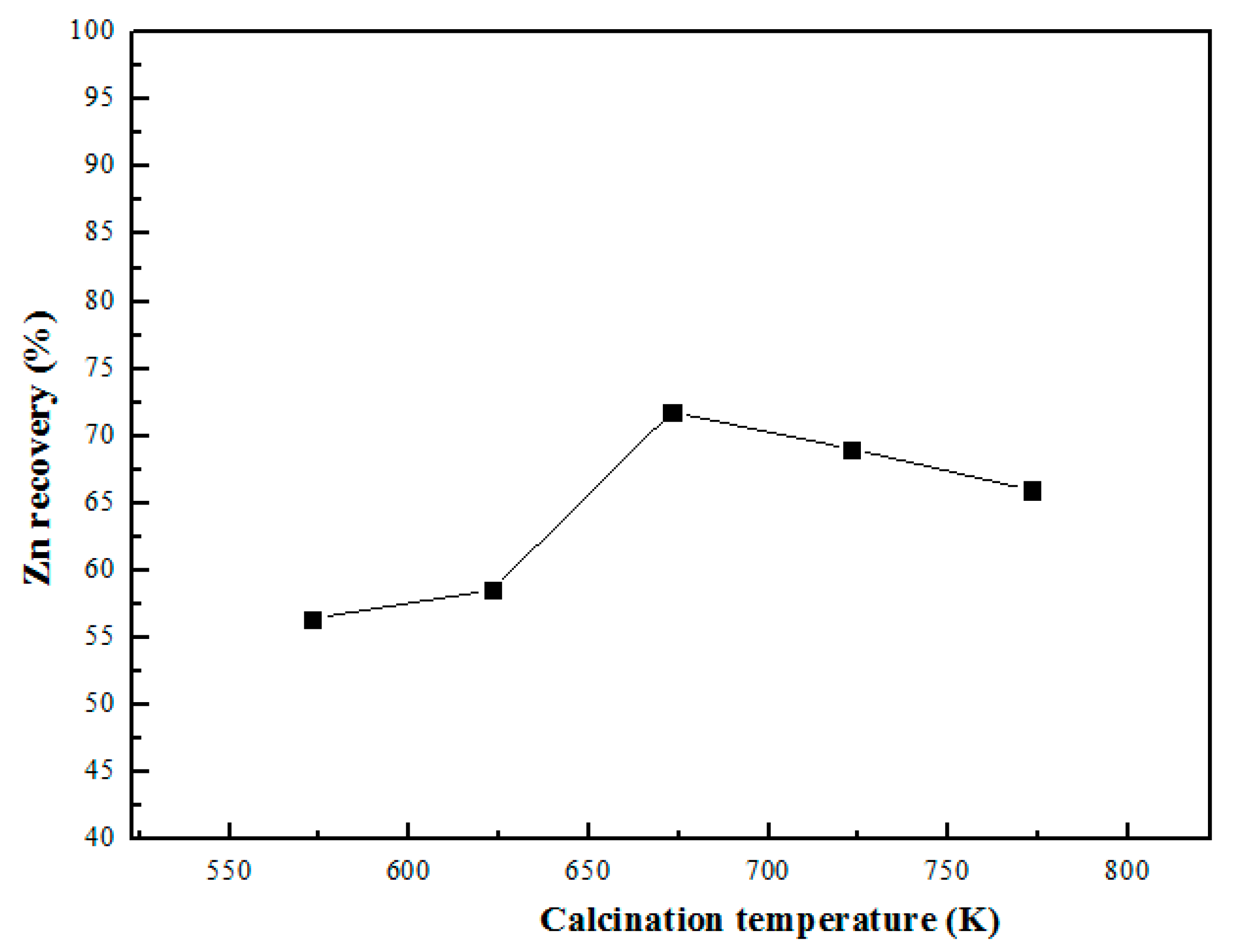
3.2. Effect of Microwave Roasting Temperature
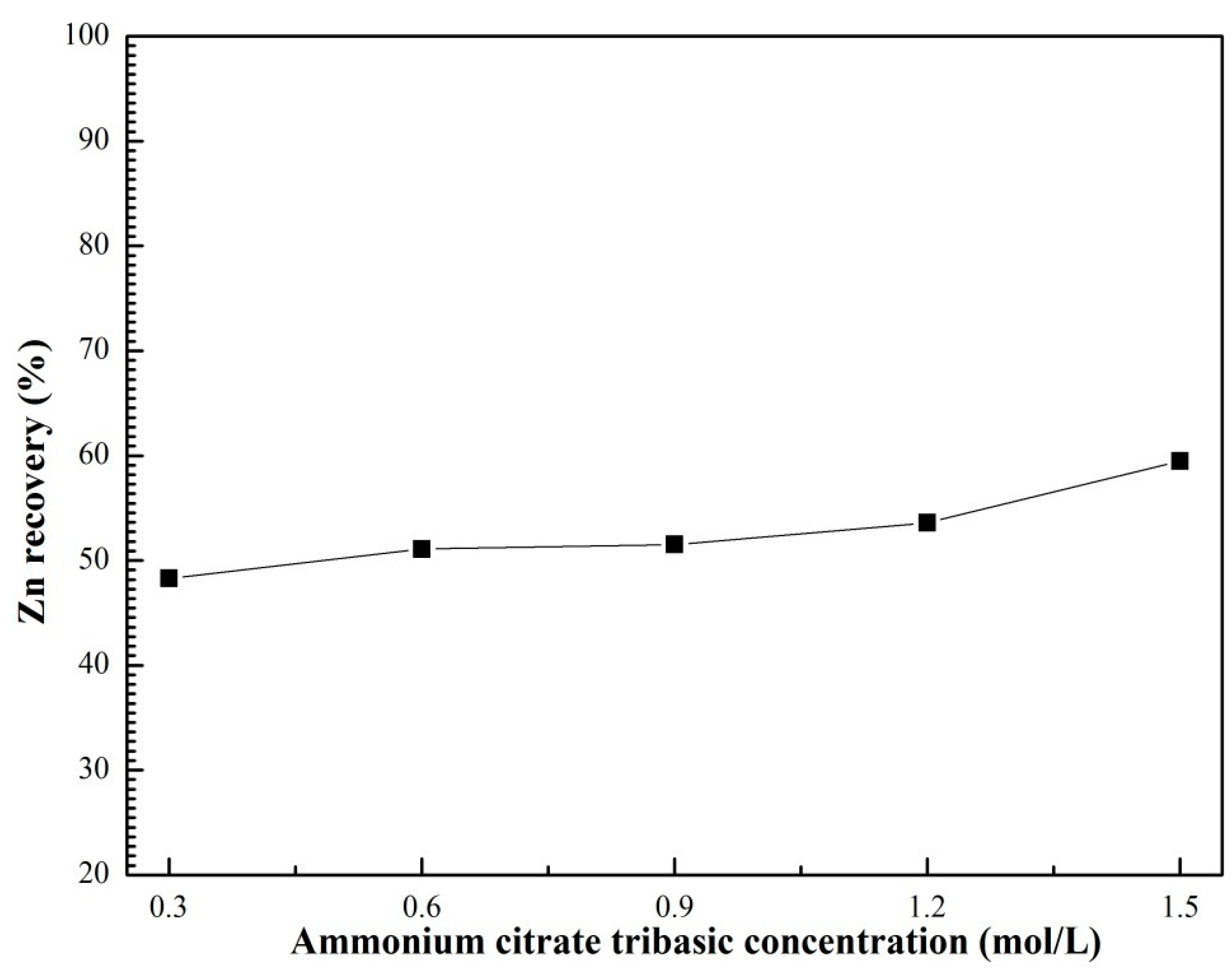
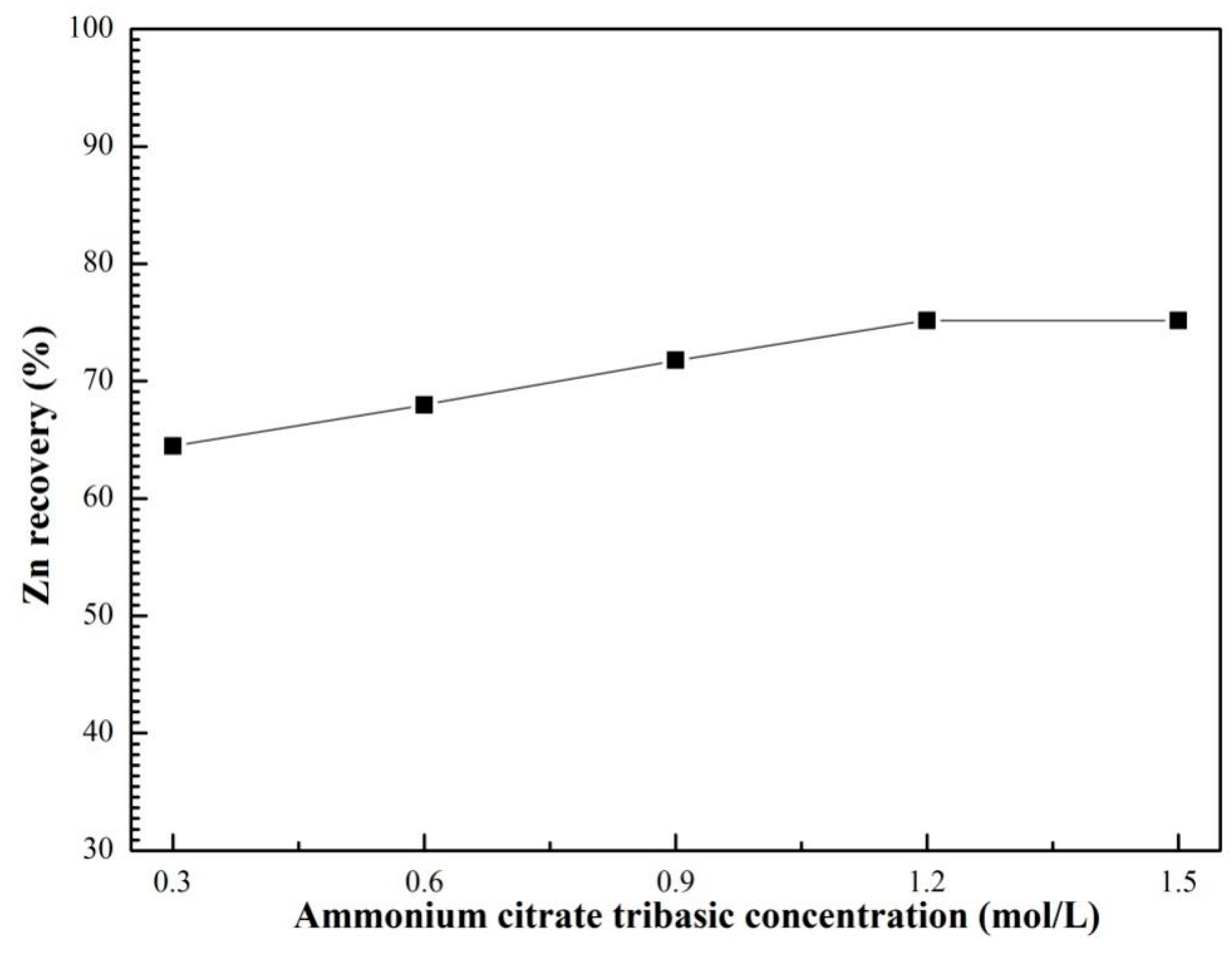
3.3. Effects of Ultrasound Power
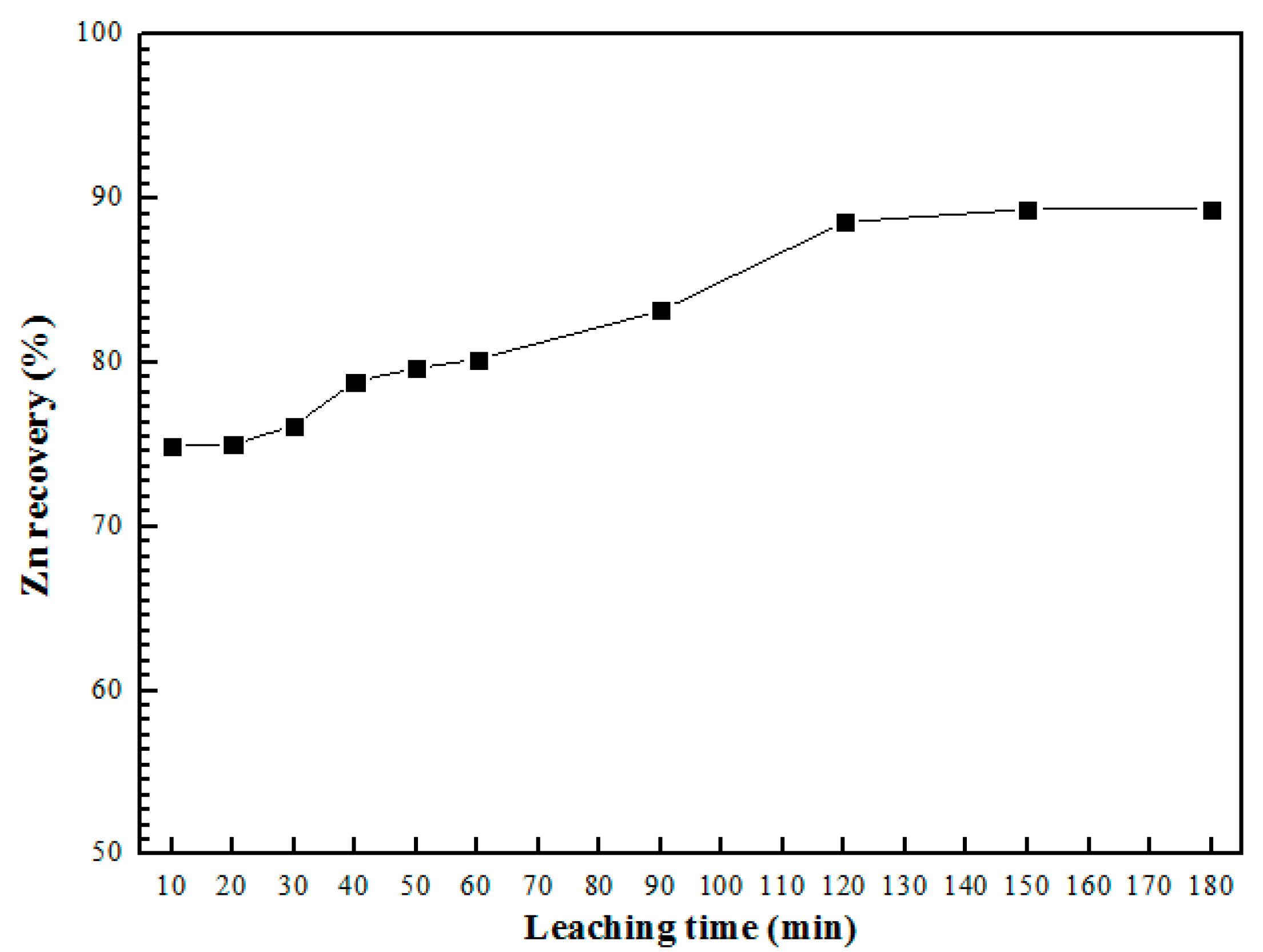
3.4. Effect of Leaching Time with Ultrasound
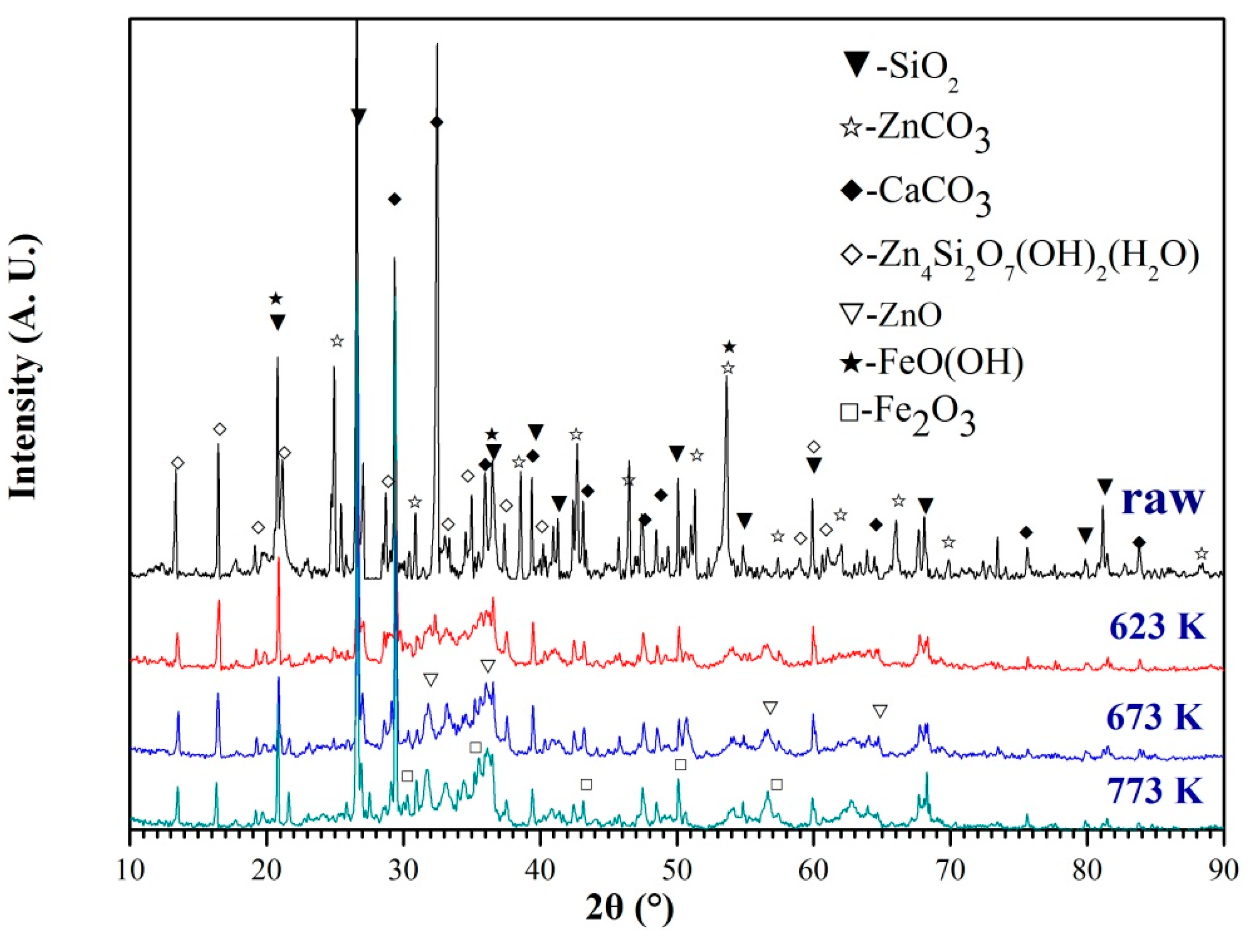
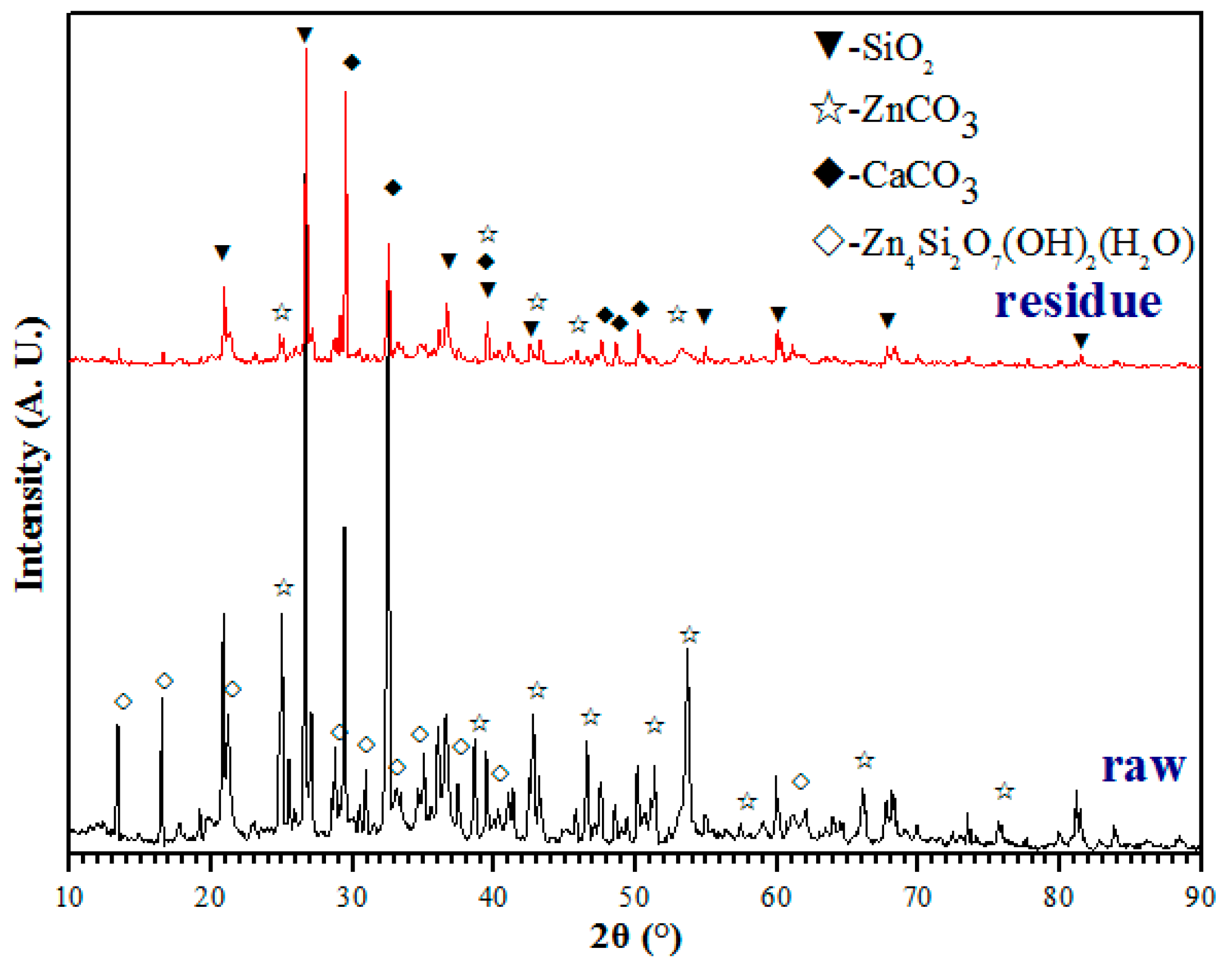
3.5. Characterization Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, A.; Zhao, Z.W.; Jia, X.; Long, S.; Huo, G.S.; Chen, X.Y. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore. Hydrometallurgy 2009, 97, 228–232. [Google Scholar] [CrossRef]
- Ferdous, R.; Khan, F.; Veitch, B.; Amyotte, P.R. Methodology for computer-aided fault tree analysis. Process Saf. Environ. Prot. 2007, 85, 70–80. [Google Scholar] [CrossRef]
- Yang, T.; Rao, S.; Zhang, D.; Wen, J.; Liu, W.; Chen, L. Leaching of low grade zinc oxide ores in nitrilotriacetic acid solutions. Hydrometallurgy 2016, 161, 107–111. [Google Scholar] [CrossRef]
- Moradi, S.; Monhemius, A.J. Mixed sulfide-oxide lead and zinc ores: Problems and solutions. Miner. Eng. 2011, 24, 1062–1076. [Google Scholar] [CrossRef]
- Anjum, F.; Shahid, M.; Akcil, A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 2012, 117–118, 1–12. [Google Scholar] [CrossRef]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part III. Application to oxide, hydroxide and sulphide minerals. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Ding, Z.; Yin, Z.; Hu, H.; Chen, Q. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution. Hydrometallurgy 2010, 104, 201–206. [Google Scholar] [CrossRef]
- Lovás, M.; Murová, I.; Mockovciaková, A.; Rowson, N.; Jakabský, Š. Intensification of magnetic separation and leaching of Cu-ores by microwave radiation. Sep. Purif. Technol. 2003, 31, 291–299. [Google Scholar] [CrossRef]
- Pinto, I.S.S.; Soares, H.M.V.M. Microwave-assisted selective leaching of nickel from spent hydrodesulphurization catalyst: A comparative study between sulfuric and organic acids. Hydrometallurgy 2013, 140, 20–27. [Google Scholar] [CrossRef]
- Wang, X.; Srinivasakannan, C.; Duan, X.H.; Peng, J.H.; Yang, D.J.; Ju, S.H. Leaching kinetics of zinc residues augmented with ultrasound. Sep. Purif. Technol. 2013, 115, 66–72. [Google Scholar]
- Choi, J.K.; Jayaprakash, A.; Chahine, G.L. Scaling of cavitation erosion progression with cavitation intensity and cavitation source. Wear 2012, 278, 53–61. [Google Scholar] [CrossRef]
- Lei, Y.; Chang, H.; Guo, X.; Li, T.; Xiao, L. Ultrasonic cavitation erosion of 316L steel weld joint in liquid Pb–Bi eutectic alloy at 550 °C. Ultrason. Sonochem. 2017, 39, 77–86. [Google Scholar] [CrossRef]
- Lebon, G.S.B.; Tzanakis, I.; Djambazov, G.; Pericleous, K.; Eskin, D.G. Numerical modelling of ultrasonic waves in a bubbly Newtonian liquid using a high-order acoustic cavitation model. Ultrason. Sonochem. 2017, 37, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Fischmann, A.J.; Dixon, D.G. Awaruite (Ni3Fe) as a nickel resource-leaching with ammoniacal–ammonium solution containing citrate and thiosulfate. Hydrometallurgy 2009, 99, 214–224. [Google Scholar] [CrossRef]
- Zhang, Y. The High Temperature Aqueous Solution Thermodynamics Data Calculation Handbook; Metallurgical Industry Press: Beijing, China, 1983. [Google Scholar]
- Zhang, Y.; Deng, J.; Chen, J.; Yu, R.; Xing, X. Leaching of zinc from calcined smithsonite using sodium hydroxide. Hydrometallurgy 2013, 131–132, 89–92. [Google Scholar] [CrossRef]
- Li, J.G.; Zhao, R.S.; Liu, H.C. Recovery of metal values from spent lithium-ion batteries. Acta Scientiarum Nat. Univ. Sunyatseni 2007, 46, 256–257. [Google Scholar]


| Zn | Pb | Fe | Si | Ca | S |
|---|---|---|---|---|---|
| 15.3 | 3.68 | 13.24 | 13.67 | 4.86 | 0.89 |
| Zn | Pb | Fe | Si | Ca | S |
|---|---|---|---|---|---|
| 2.73 | 5.23 | 18.22 | 20.1 | 7.31 | 0.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, H.; Peng, J.; Srinivasakannan, C.; Li, S.; Yin, S. Microwave and Ultrasound Augmented Leaching of Complicated Zinc Oxide Ores in Ammonia and Ammonium Citrate Solutions. Metals 2017, 7, 216. https://doi.org/10.3390/met7060216
Zhang L, Li H, Peng J, Srinivasakannan C, Li S, Yin S. Microwave and Ultrasound Augmented Leaching of Complicated Zinc Oxide Ores in Ammonia and Ammonium Citrate Solutions. Metals. 2017; 7(6):216. https://doi.org/10.3390/met7060216
Chicago/Turabian StyleZhang, Libo, Haoyu Li, Jinhui Peng, Chandrasekar Srinivasakannan, Shiwei Li, and Shaohua Yin. 2017. "Microwave and Ultrasound Augmented Leaching of Complicated Zinc Oxide Ores in Ammonia and Ammonium Citrate Solutions" Metals 7, no. 6: 216. https://doi.org/10.3390/met7060216




