Face Centred Cubic Multi-Component Equiatomic Solid Solutions in the Au-Cu-Ni-Pd-Pt System
Abstract
:1. Introduction
2. Outline
3. Materials and Methods
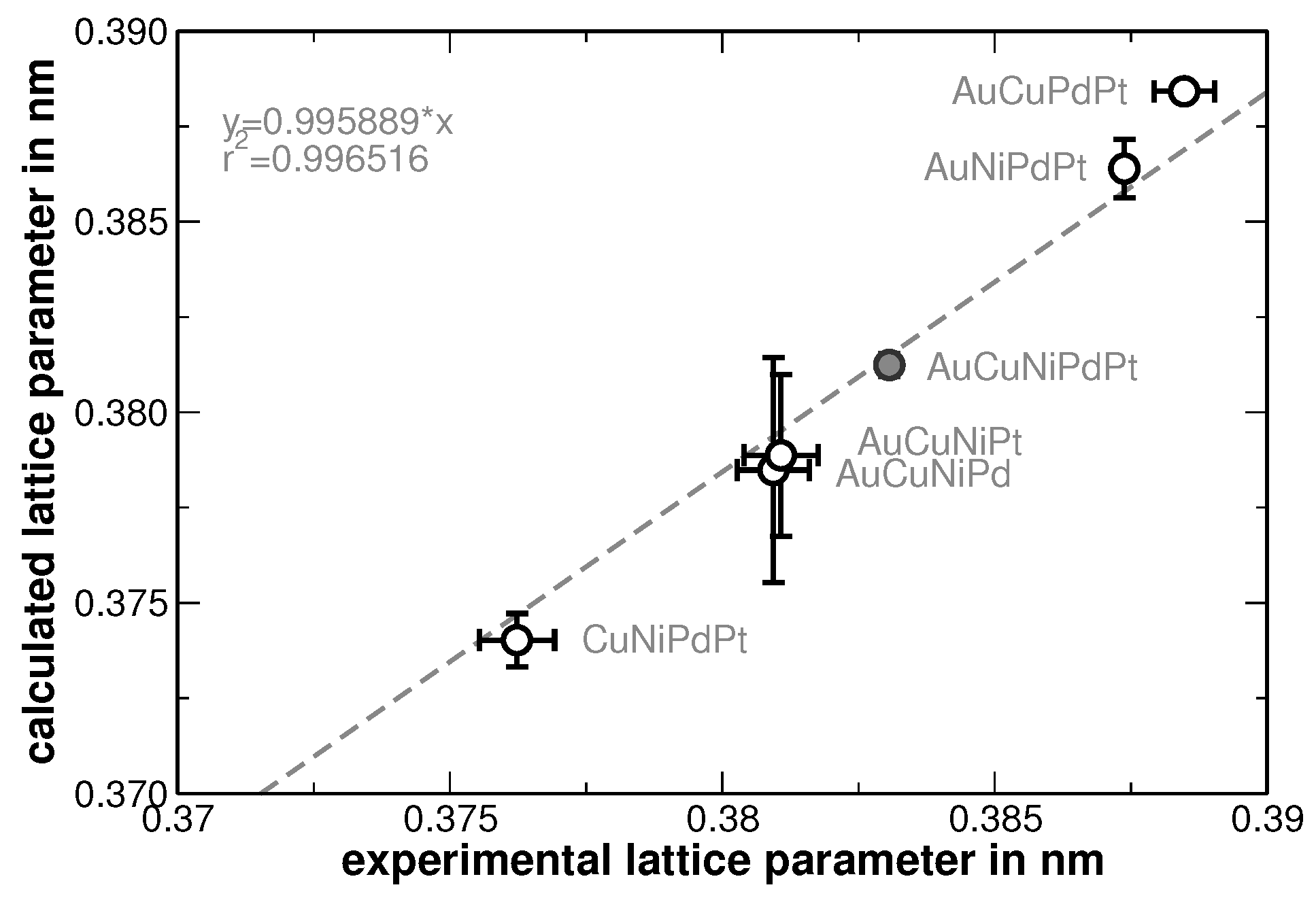
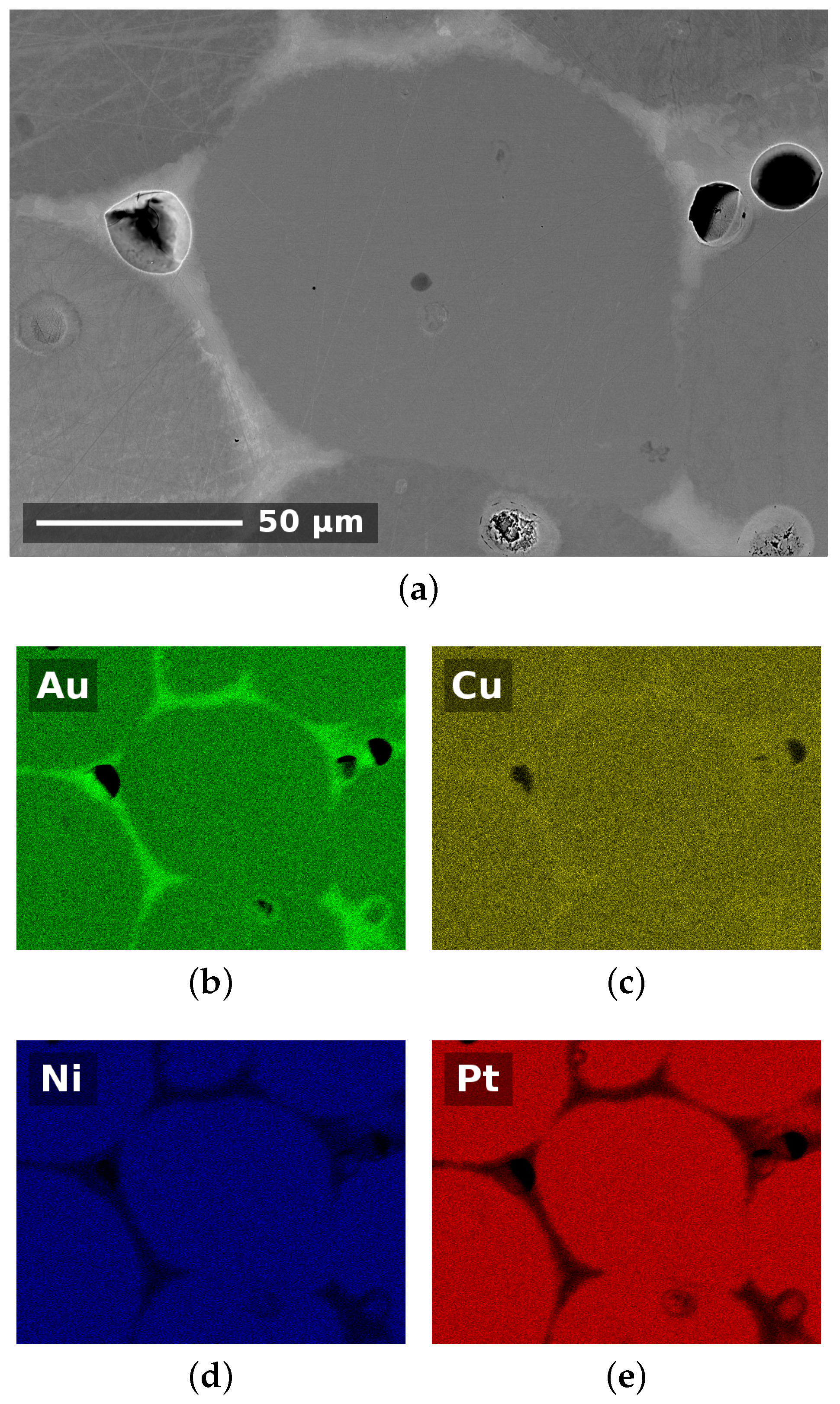
4. Results
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
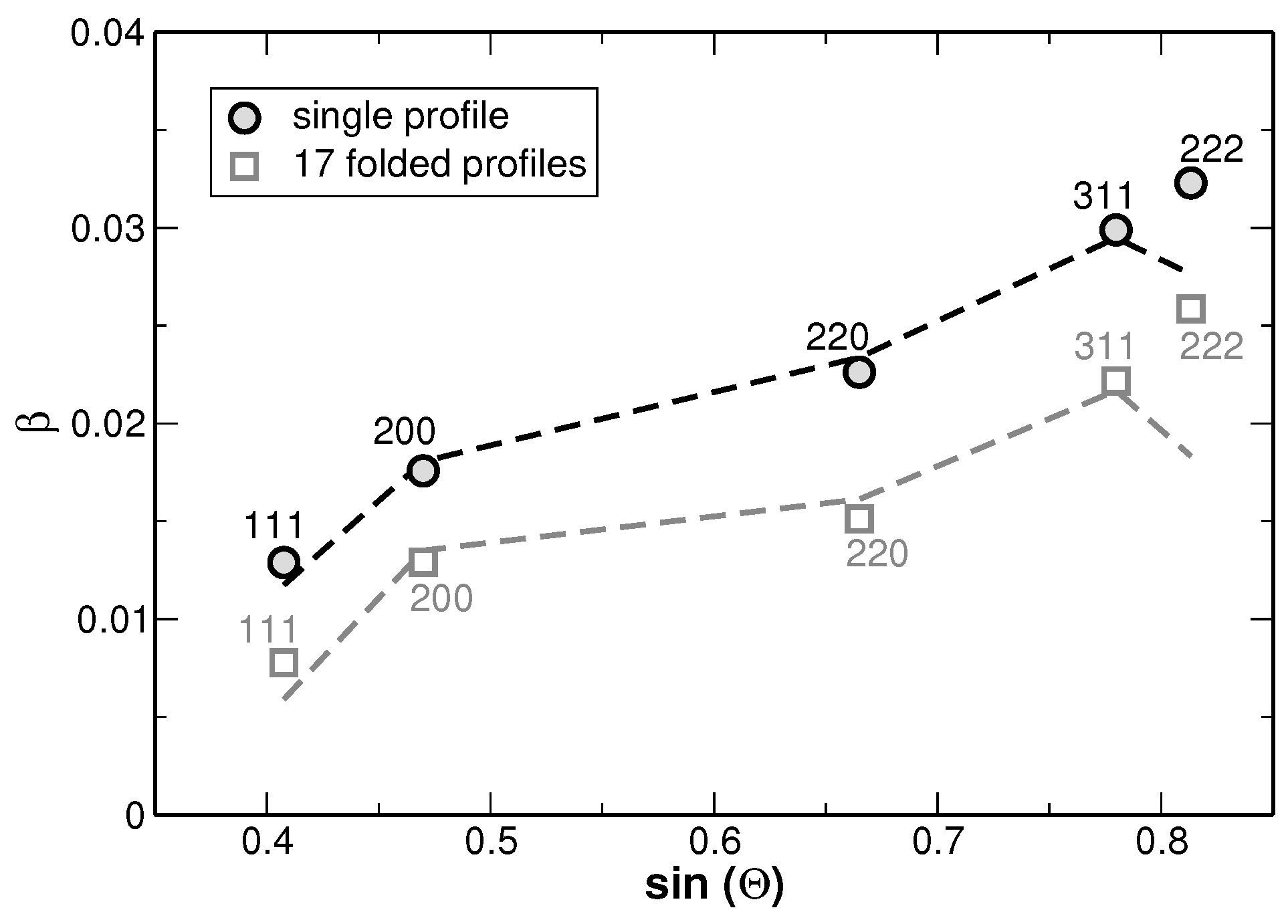
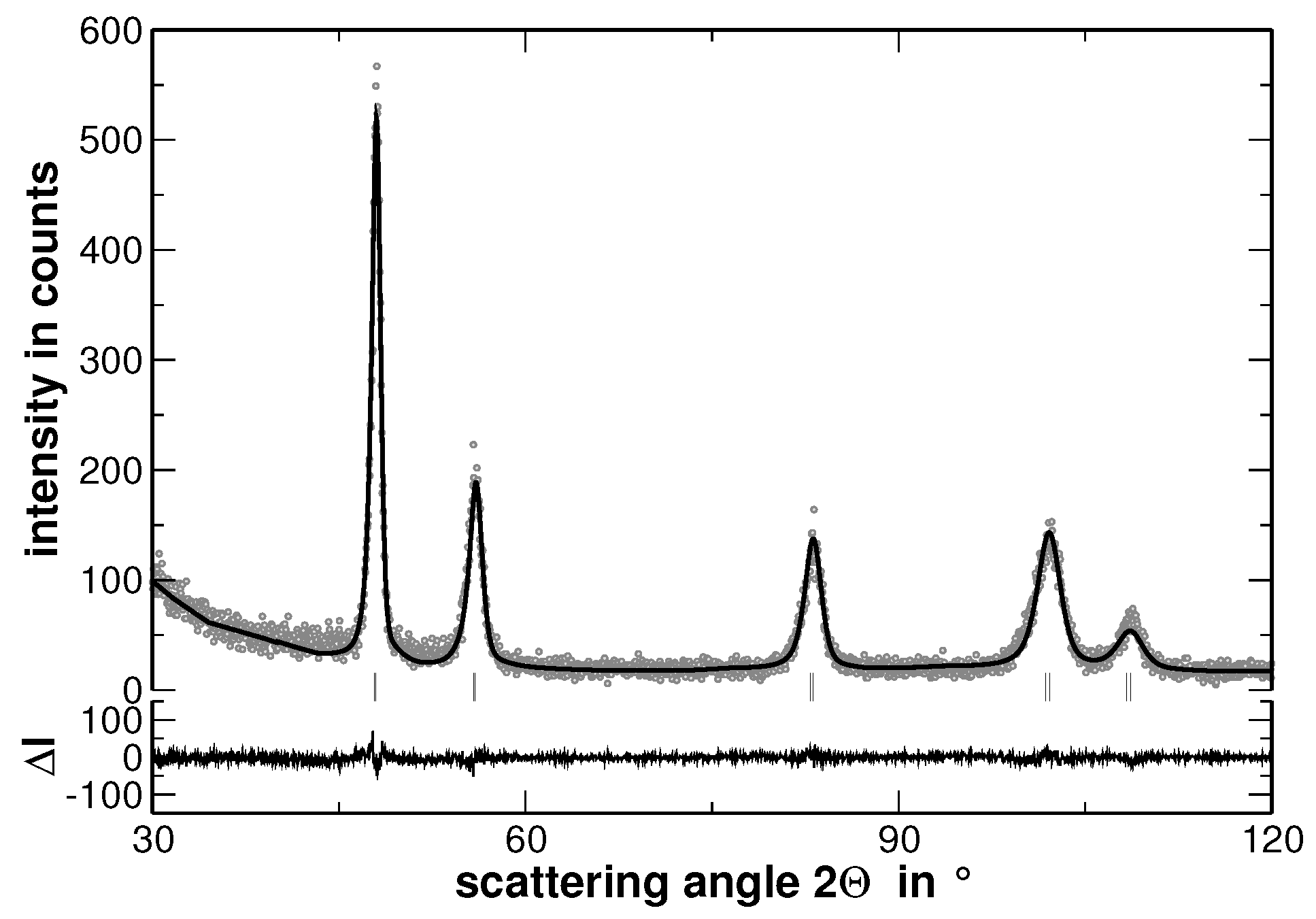
Appendix A. Rietveld Analyses

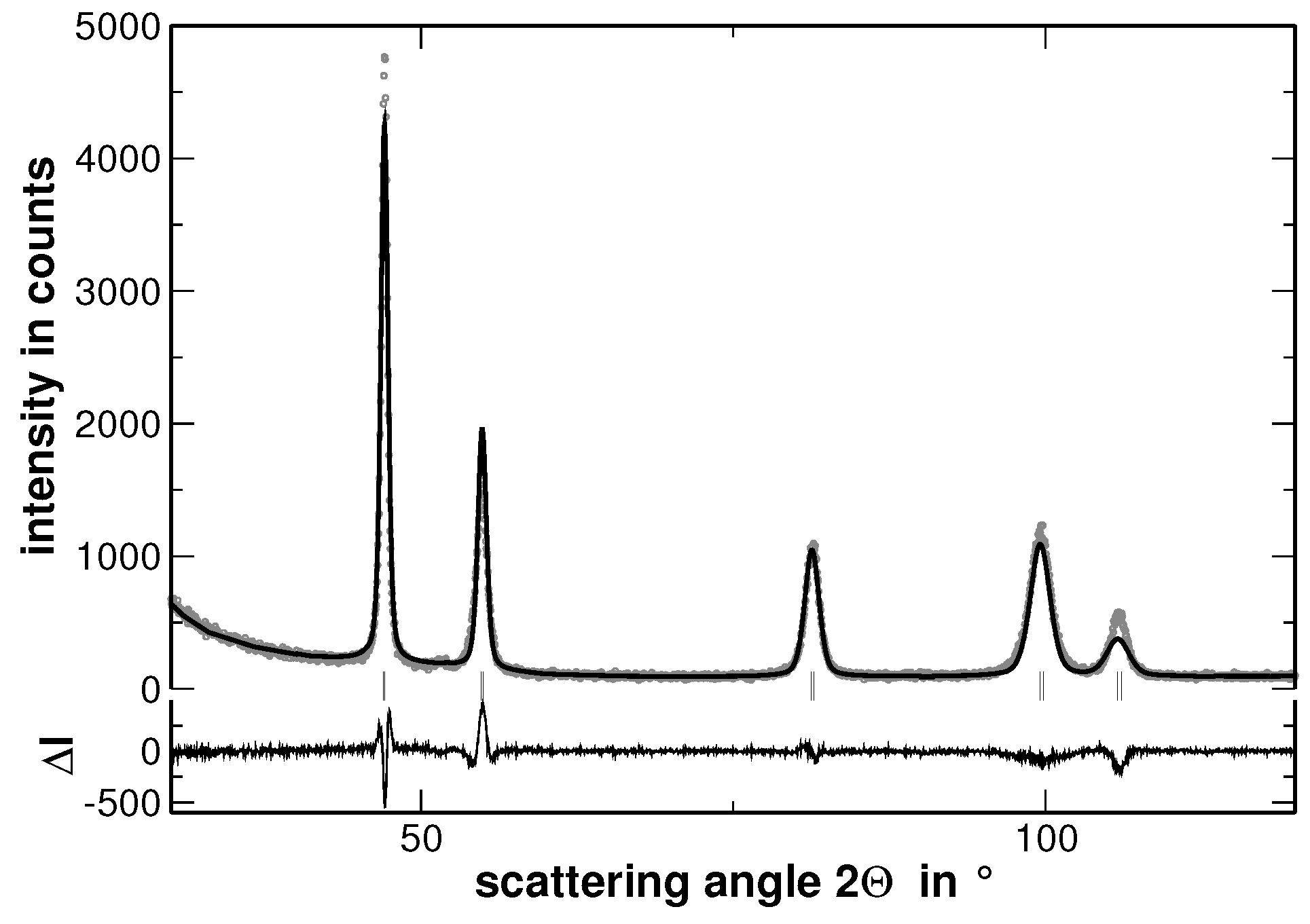

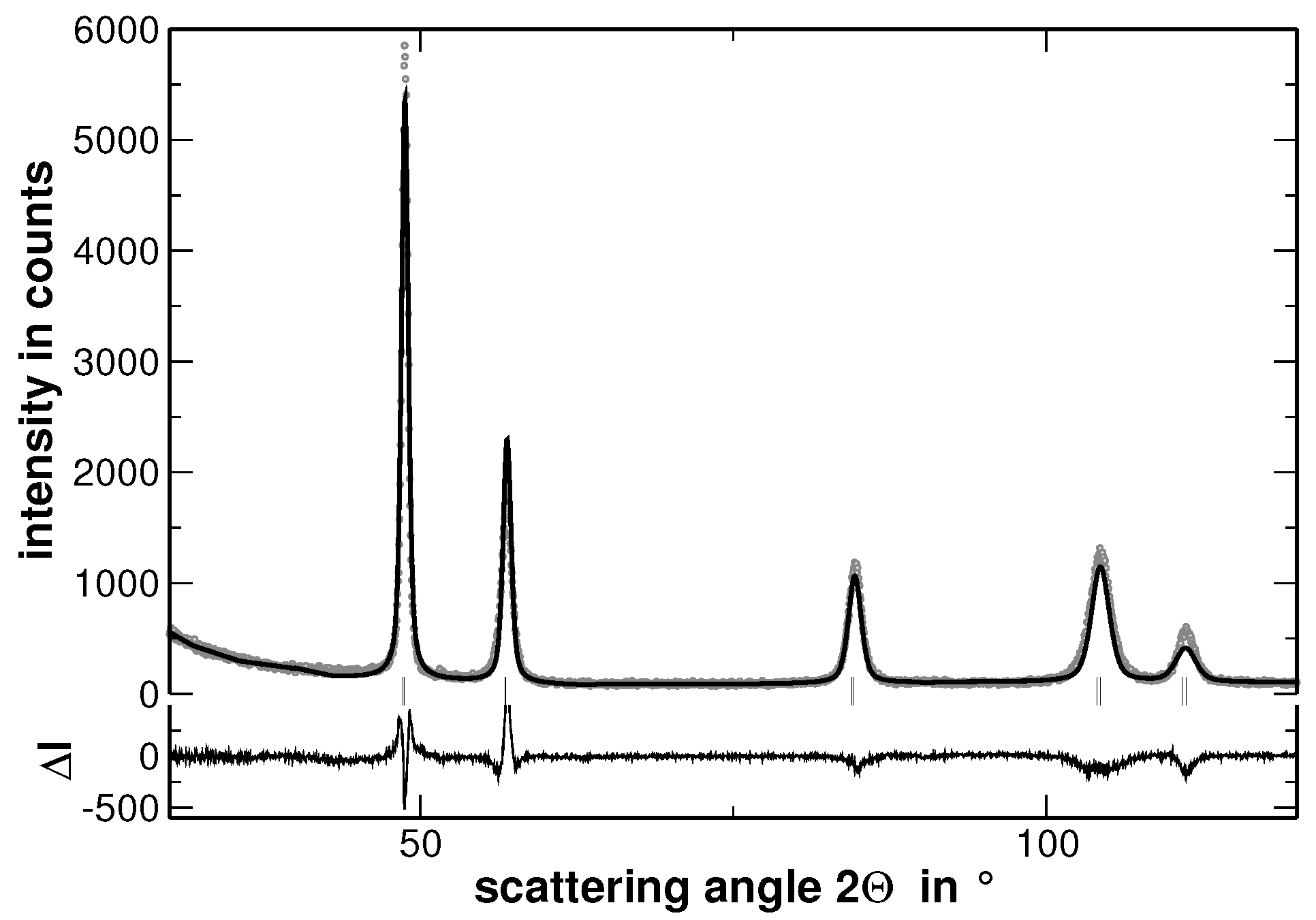
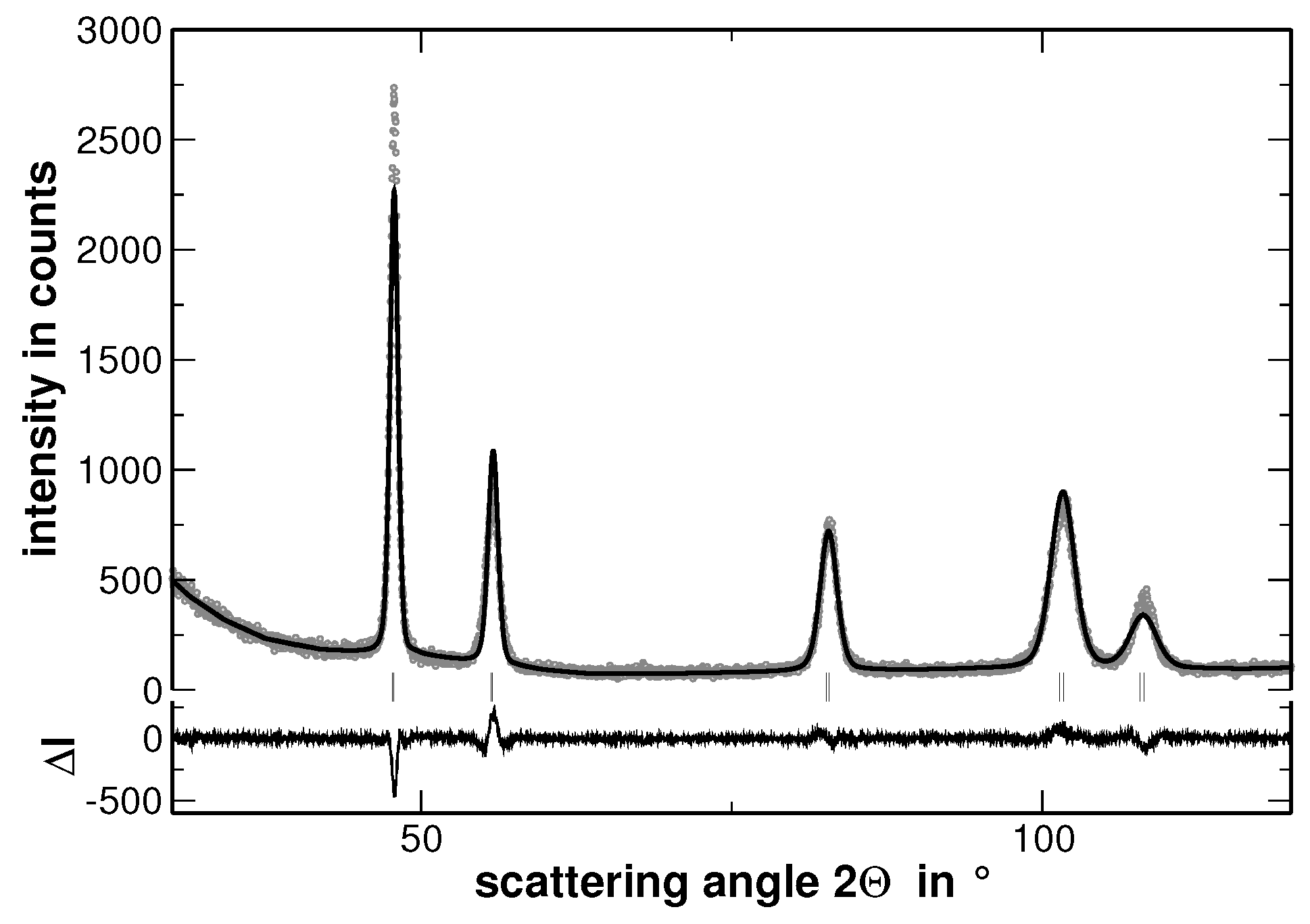
| Alloy | Lattice Parameter | |||||
|---|---|---|---|---|---|---|
| AuCuNiPd | 0.380(93) nm | 24.9 | 22.8 | 3.99 | 2.15 | 3.82 |
| AuCuNiPt | 0.381(07) nm | 32.6 | 32.6 | 8.09 | 5.64 | 1.26 |
| AuCuNiPt * | 0.381(00) nm | 19.8 | 20.9 | 7.78 | 5.15 | 4.07 |
| AuCuPdPt | 0.388(47) nm | 23.7 | 22.6 | 12.7 | 8.93 | 3.94 |
| AuNiPdPt | 0.3873(80) nm | 20.1 | 20.8 | 3.84 | 2.47 | 4.16 |
| CuNiPdPt | 0.376(22) nm | 23.6 | 22.9 | 6.99 | 4.32 | 4.69 |
| AuCuNiPdPt | 0.3830(66) nm | 22.0 | 20.9 | 7.74 | 4.90 | 2.62 |
Appendix B. DSC Results

Appendix C. Pair Mixing Enthalpies
| Cu | Ni | Pt | |
|---|---|---|---|
| Au | −5.2 | 7.6 | 5 |
| Cu | 2.3 | −11.4 | |
| Ni | −9.3 |
Appendix D. Atomic Scattering Factor
| Element | c | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | 12.8376 | 3.8785 | 7.292 | 0.2565 | 4.4438 | 12.1763 | 2.38 | 66.3421 | 1.0341 |
| Cu | 13.338 | 3.5828 | 7.1676 | 0.247 | 5.6158 | 11.3966 | 1.6735 | 64.8126 | 1.191 |
| Pd | 19.3319 | 0.698655 | 15.5017 | 7.98929 | 5.29537 | 25.2052 | 0.605844 | 76.8986 | 5.26593 |
| Pt | 27.0059 | 1.51293 | 17.7639 | 8.81174 | 15.7131 | 0.424593 | 5.7837 | 38.6103 | 11.6883 |
| Au | 16.8819 | 0.4611 | 18.5913 | 8.6216 | 25.5582 | 1.4826 | 5.86 | 36.3956 | 12.0658 |
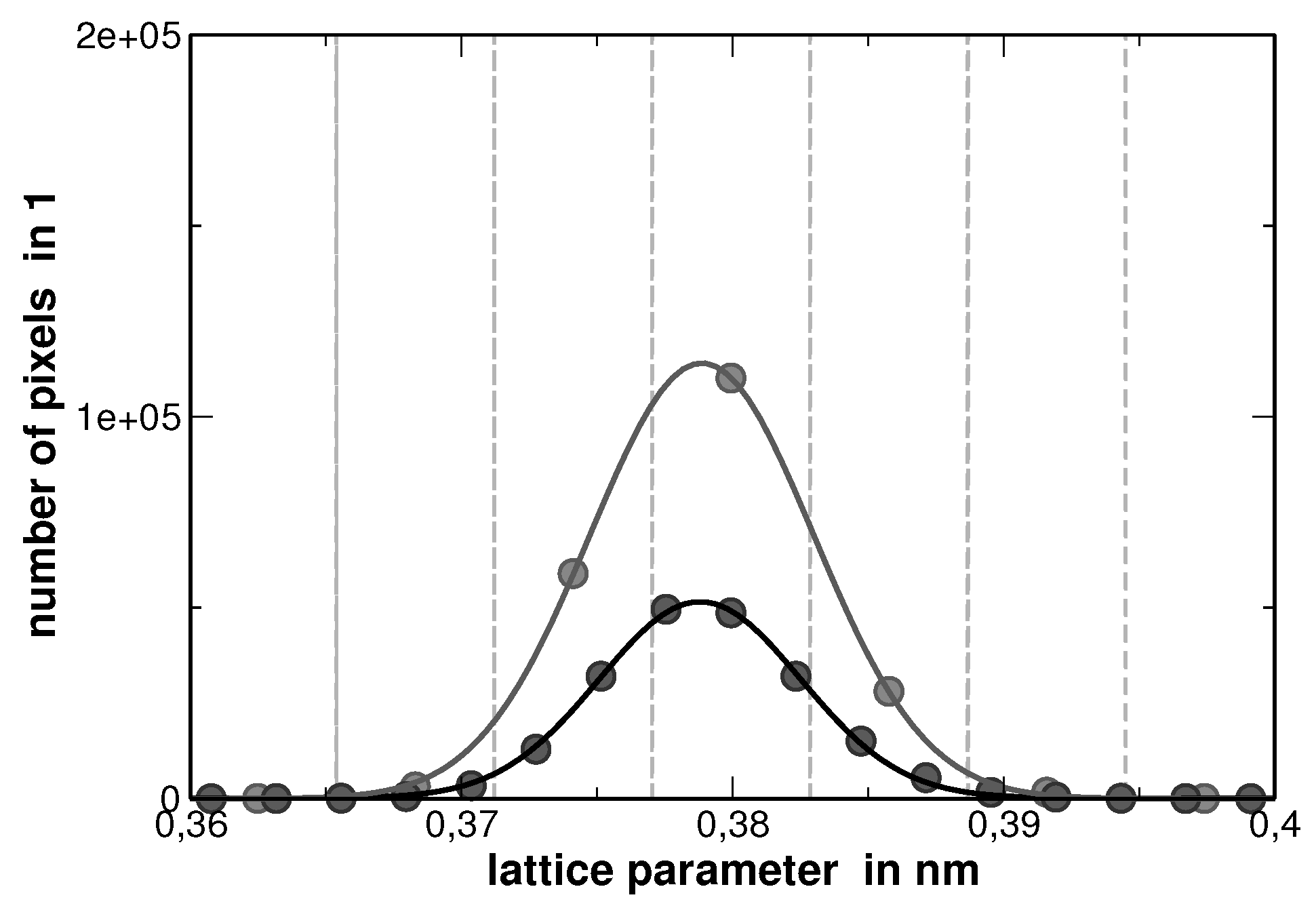
Appendix E. Modified Williamson-Hall Plot
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Ruiz-Yi, B.; Bunn, J.K.; Stasak, D.; Mehta, A.; Besser, M.; Kramer, M.J.; Takeuchi, I.; Hattrick-Simpers, J. The Different Roles of Entropy and Solubility in High Entropy Alloy Stability. ACS Comb. Sci. 2016, 18, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2016, 122, 448–551. [Google Scholar] [CrossRef]
- Kozak, R.; Sologubenko, A.; Steurer, W. Singe-phase high-entropy alloys—An overview. Z. Kristallogr. 2015, 230, 55–68. [Google Scholar]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A Novel Low-Density, High-Hardness, High-entropy Alloy with Close-packed Single-phase Nanocrystalline Structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Diao, H.; Gao, M.C.; Tang, Z.; Poplawsky, J.D.; Liaw, P.K. Understanding phase stability of Al-Co-Cr-Fe-Ni high entropy alloys. Mater. Des. 2016, 109, 425–433. [Google Scholar] [CrossRef]
- Song, H.; Tian, F.; Wang, D. Thermodynamic properties of refractory high entropy alloys. J. Alloys Compd. 2016, 682, 773–777. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Feuerbacher, M.; Heidelmann, M.; Thomas, C. Hexagonal High-entropy Alloys. Mater. Res. Lett. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Villars, P. SpringerMaterials Au-Ni Binary Phase Diagram 0–100 at.% Ni. Available online: http://materials.springer.com/isp/phase-diagram/docs/c_0906519 (accessed on 2 September 2016).
- Maiti, S.; Steurer, W. Structural-disorder and its effect on mechanical properties in single-phase TaNbHfZr high-entropy alloy. Acta Mater. 2016, 106, 87–97. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- The Periodic Table by WebElements. Available online: http://www.webelements.com (accessed on 27 November 2016).
- Wells, A.F. Structural Inorganic Chemistry, 5th ed.; Clarendon Press: Oxford, UK, 1984. [Google Scholar]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Rafaja, D.; Dopita, M.; Masimov, M.; Klemm, V.; Wendt, N.; Lengauer, W. Analysis of local composition gradients in the hard-phase grains of cermets using a combination of X-ray diffraction and electron microscopy. Int. J. Refract. Met. Hard Mater. 2008, 26, 263–275. [Google Scholar] [CrossRef]
- Rafaja, D. Deconvolution versus convolution—A comparison for materials with concentration gradient. Mater. Struct. 2000, 7, 43–50. [Google Scholar]
- Ungár, T.; Dragomir, I.; Revesz, A.; Borbély, A. The contrast factors of dislocations in cubic crystals: The dislocation model of strain anisotropy in practice. J. Appl. Cryst. 1999, 32, 992–1002. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Šimek, D.; Rafaja, D.; Motylenko, M.; Klemm, V.; Schreiber, G.; Brethfeld, A.; Lehmann, G. XRD analysis of local strain fields in pearlitic steels—Towards the fast examination of microstructure after rolling. Steel Res. Int. 2008, 79, 800–806. [Google Scholar] [CrossRef]
- Šimek, D.; Oswald, A.; Schmidtchen, R.; Motylenko, M.; Lehmann, G.; Rafaja, D. Prediction of Mechanical Properties of Carbon Steels After Hot and Cold Forming by Means of Fast Microstructure Analysis. Steel Res. Int. 2014, 85, 1369–1378. [Google Scholar] [CrossRef]
- Wüstefeld, C.; Rafaja, D.; Dopita, M.; Motylenko, M.; Baehtz, C.; Michotte, C.; Kathrein, M. Decomposition kinetics in Ti1−xAlxN coatings as studied by in-situ X-ray diffraction during annealing. Surf. Coat. Technol. 2011, 206, 1727–1734. [Google Scholar] [CrossRef]
- Rafaja, D.; Wüstefeld, C.; Baehtz, C.; Klemm, V.; Dopita, M.; Motylenko, M.; Michotte, C.; Kathrein, M. Effect of internal interfaces on hardness and thermal stability of nanocrystalline Ti0.5Al0.5N coatings. Metall. Mater. Trans. A 2011, 42, 559–569. [Google Scholar] [CrossRef]
- Rafaja, D.; Wüstefeld, C.; Dopita, M.; Motylenko, M.; Baehtz, C. Capability of X-ray diffraction for the study of microstructure of metastable thin films. IUCrJ 2014, 1, 446–456. [Google Scholar] [CrossRef] [PubMed]
- The Landolt Börnstein Database. Available online: http://materials.springer.com (accessed on 2 September 2016).
- Prince, E. (Ed.) International Tables for Crystallography: Mathematical, Physical and Chemical Tables; International Union of Crystallography: Chester, UK, 2006; Volume C. [Google Scholar]


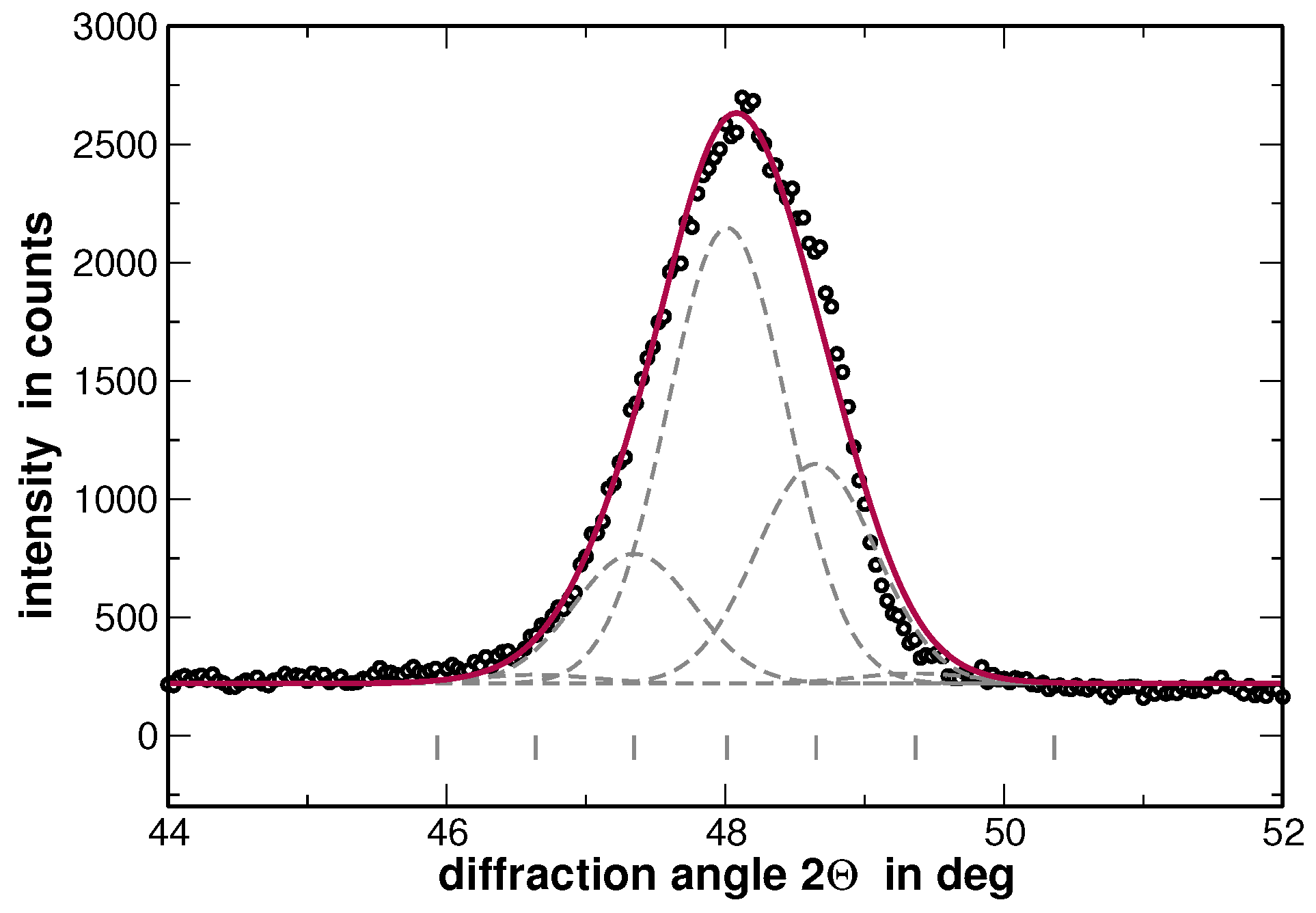


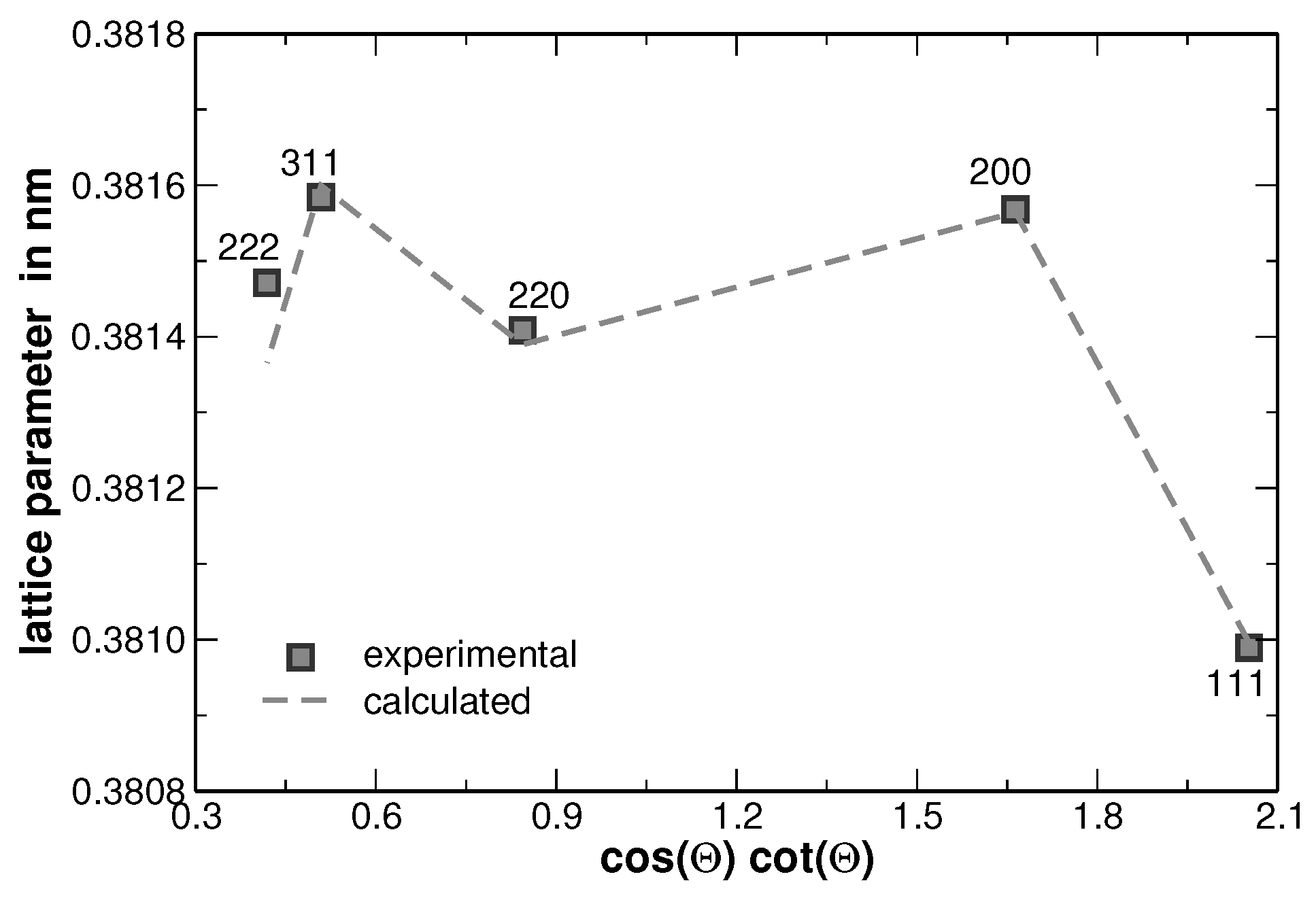
| Element | Lattice Parameter | Metallic Radius | Melting Temperature |
|---|---|---|---|
| Au | 407.82 | ||
| Cu | |||
| Ni | |||
| Pd | |||
| Pt |
| Element | Composition | |||
|---|---|---|---|---|
| ICP-OES | EDX | Grain Interior | Grain Boundary | |
| Au | ||||
| Cu | ||||
| Ni | ||||
| Pt | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freudenberger, J.; Rafaja, D.; Geissler, D.; Giebeler, L.; Ullrich, C.; Kauffmann, A.; Heilmaier, M.; Nielsch, K. Face Centred Cubic Multi-Component Equiatomic Solid Solutions in the Au-Cu-Ni-Pd-Pt System. Metals 2017, 7, 135. https://doi.org/10.3390/met7040135
Freudenberger J, Rafaja D, Geissler D, Giebeler L, Ullrich C, Kauffmann A, Heilmaier M, Nielsch K. Face Centred Cubic Multi-Component Equiatomic Solid Solutions in the Au-Cu-Ni-Pd-Pt System. Metals. 2017; 7(4):135. https://doi.org/10.3390/met7040135
Chicago/Turabian StyleFreudenberger, Jens, David Rafaja, David Geissler, Lars Giebeler, Christiane Ullrich, Alexander Kauffmann, Martin Heilmaier, and Kornelius Nielsch. 2017. "Face Centred Cubic Multi-Component Equiatomic Solid Solutions in the Au-Cu-Ni-Pd-Pt System" Metals 7, no. 4: 135. https://doi.org/10.3390/met7040135
APA StyleFreudenberger, J., Rafaja, D., Geissler, D., Giebeler, L., Ullrich, C., Kauffmann, A., Heilmaier, M., & Nielsch, K. (2017). Face Centred Cubic Multi-Component Equiatomic Solid Solutions in the Au-Cu-Ni-Pd-Pt System. Metals, 7(4), 135. https://doi.org/10.3390/met7040135