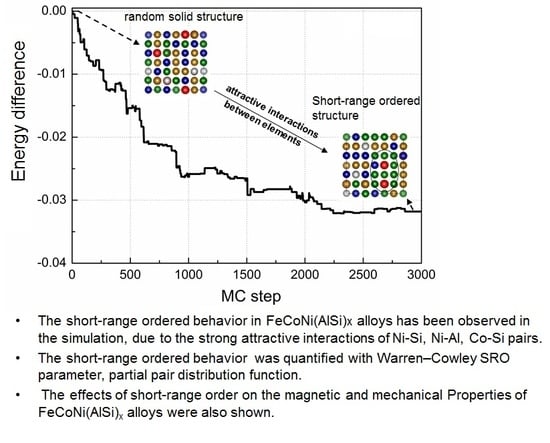
3.2. Short-Range Order (SRO) in FeCoNi(AlSi)x Alloys
The evolution of the relative potential energy and SRO parameters of FeCoNi (AlSi)
0.8 alloy are shown in
Figure 2. The relative potential energy is defined as the energy difference between the potential energy of the ith MC step and the initial potential energy. In the case that the 92-atom SQS structure is selected as the initial starting structure, all the constituent atoms are randomly distributed in the lattice positions,
and
(
Figure 2a,b).
Figure 2b shows that there are major deviations of SRO parameters between the finial structure and the SQS structure. The average numbers of Al-Al and Si-Si pairs are reduced by ~80%, the nearest neighbor number changes from 2.1 for the random case to about 0.5 for the SRO case, and the average number of Al-Si pairs is reduced by ~50%, indicating Al (or Si) atoms separate from each other. Meanwhile, it can be seen that there is rapid growth of the average number of Ni-Al pairs, followed by Co-Si, Co-Al and Ni-Si pairs. The rapid growth of Ni-Al pairs shows that the Ni-Al pair first formed during the solidification from the liquid phase. The Ni-Al pairs will form a B2-orderd parent crystal structure, which is an ordered structure based on BCC with Pearson symbol of cP2. Si element also have negative mixing enthalpies with Ni, Co and Fe elements, the binary mixing enthalpies of Ni-Si, Co-Si and Fe-Si pairs are −40, −38 and −35 kJ/mol, respectively. Si atoms prefer to separate with Al and Si atoms, and bond with Ni, Co and Fe atoms. Raja et al. studied the structural properties of Fe
3-xCo
xSi alloys (
) using X-ray powder diffraction [
35]. The results revealed that Si atoms occupy the body-centered positions, Fe and Co atoms occupy the body corners and form B2 and L2
1 ordered phases. The L2
1 phase is an ordered structure based on BCC with Pearson symbol of cF16. Just like Al, Si also tends to stabilize BCC structure. In the FeCoNi (AlSi)
0.4 alloy, although the fraction of Al element (x = 0.4) is smaller as compared to the case in Al
xCoCrFeNi alloy (BCC phase in x
), there will be a large amount of Ni-Al and Co-Si pairs first formed and other constituents will dissolve in the parent crystal structure due to the mixing entropy effect. Thus, the system forms a BCC structure.
In the case where the MaxEnt structure is selected as the initial starting structure (
Figure 2c,d), since each constituent atoms in the system strives for the maximum free space,
and
for FeCoNi(AlSi)
0.8 alloy. We obtained the lower potential energy at the 2200 MC step. After 800 MC steps searches, the MC method did not find the lower energy structure. The emergence of the SRO reduced the potential energy, which made the potential energy change from −325 to −359 meV per atom. In
Figure 2d, the SRO parameters of
and
decrease slightly.
changes from 0.92 to 0.86, and
changes from 0.92 to 0.84. We started from two different initial starting points to probe the change of the atomic nearest neighbor environment in FeCoNi (AlSi)
0.8 alloy, and obtained a similar trend to the SRO. It can be concluded that the potential energy curve is relatively converged after 3000 MC steps simulation. From the above comparison, it can be seen that in the process of searching for the SRO structure, using the MaxEnt model as the initial starting structure is more efficient. This is based on the following two points: on the one hand, the potential energy of the MaxEnt structure is lower than that of the SQS structure, which can be validated from the difference in formation energy listed in
Table 1. On the other hand, the SRO parameters
and
of the MaxEnt structure are closer to the SRO parameters of the finial SRO structure. Therefore, in the following probing of SRO, and the calculation of the magnetic and mechanical properties, the MaxEnt structure is selected as the initial structure.
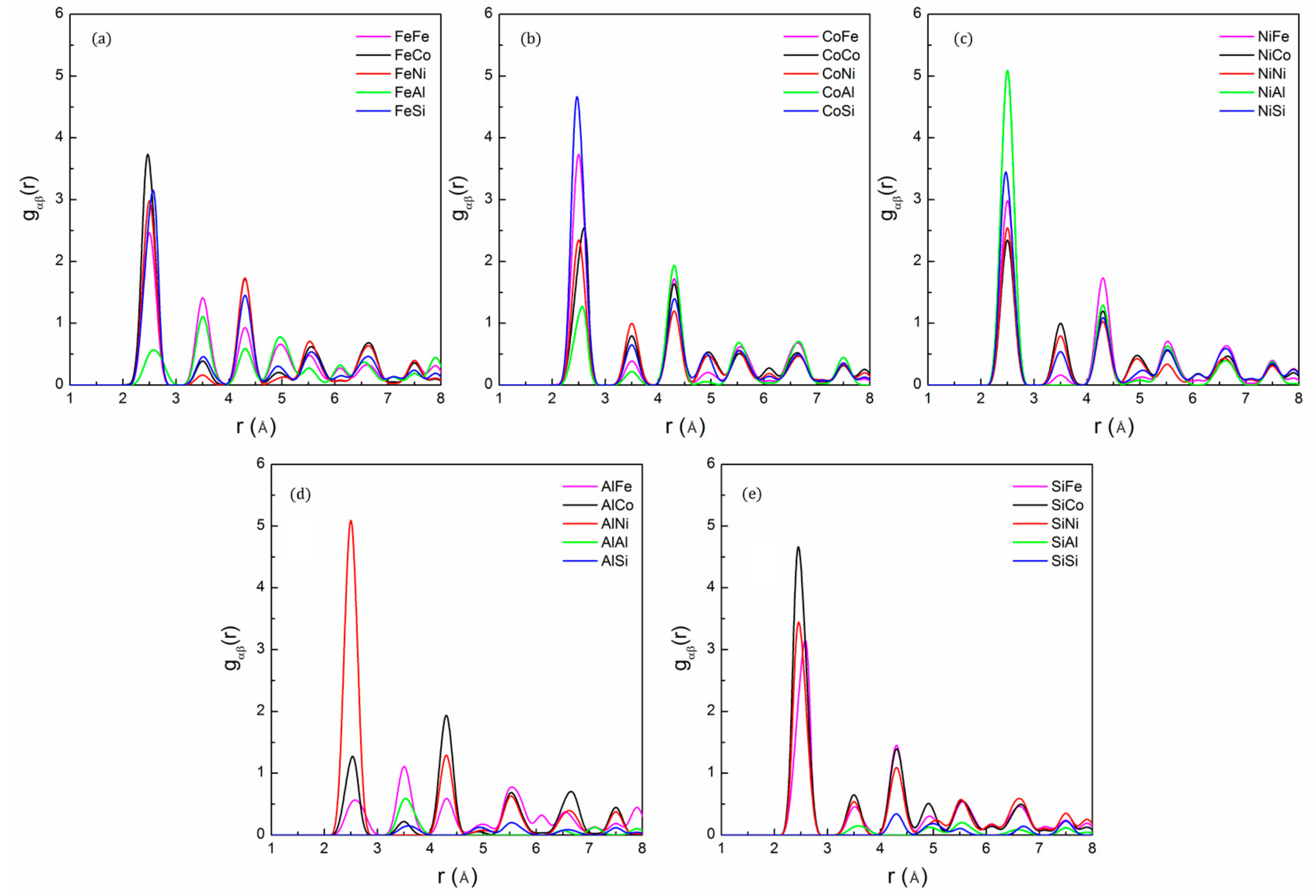
The partial pair distribution function can be used to describe the relative positional preferences of different constituent elements. The shape and position of the peaks provide detailed information about the atomic local environment [
36]. We used pair PDF to show the SRO structure of the FeCoNi(AlSi)
0.2 alloy, and the corresponding data are plotted in
Figure 3. We observe that there is no obvious lattice distortion in the alloy. The average bond lengths of Co-Si and Ni-Si are r ~2.45 Å, which is shorter than the metal–metal bonds because the Si atom has a smaller radius than the metal atoms (Fe, Co, Ni, and Al). The Ni-Al pair has a higher peak intensity, followed by the Co-Si and Fe-Co, Ni-Si pairs. The peaks of the Al-Al and Al-Si pairs have moved to the second nearest neighbor shell, and the peak of the Si-Si pair has moved to the third nearest neighbor shell, indicating that Al and Si prefer to bond with other elements rather than themselves. The results suggest the existence of the preferred Ni-Al, Co-Si, Fe-Co and Ni-Si pairs in the FeCoNi(AlSi)
0.2 alloy.
The partial PDF of the FeCoNi(AlSi)
0.8 alloy after the 3000 MC steps is shown in
Figure 4. It can be seen that the most preferred pair is Ni-Al, followed by the Co-Si, Co-Al, Fe-Si and Ni-Si pairs. The least favored pairs are the Si-Si, Al-Al pairs. Al and Si atoms prefer to bond with other elements to lower the potential energy. The results suggest the existence of a preferred short-range order of Ni-Al, Co-Si, Co-Al, Fe-Si and Ni-Si pairs in the alloy. In addition, there is obvious lattice distortion when the fraction of Al and Si atoms is larger. The lattice distortion makes the second intensity peak smear out and become less distinct, indicating that the atoms in the alloy deviate from the ideal lattice positions. The lattice distortion can significantly scatter free electrons, shortened electrons mean free paths, which will reduce the thermal and electrical conductivity of the alloy.
In
Table 2, the SRO parameters of the FeCoNi(AlSi)
x alloys as a function of Al and Si fraction x averaged after 3000 MC steps are shown. For FeCoNi alloy, we observed that the SRO parameter of the Fe-Ni pair is negative, and the Fe-Fe, Ni-Ni and Co-Co pairs are positive. Fe atoms prefer to bond with Ni atoms and form an SRO structure. Tamm et al. investigated the SRO behavior of NiCrCoFe alloys and found a negative Ni–Fe pair and positive Fe-Fe, Ni-Ni and Co-Co pairs [
16]. When Al and Si atoms are added to the FeCoNi-based alloy, the Fe-Ni pair become positive. Al and Si elements have relative stronger attractive interaction with other constituent elements. They will separate from each other and bond with Fe, Co and Ni elements to form an SRO structure. The strength of the attractive interaction can be seen from the binary mixing enthalpies [
37], that is, the more negative the binary mixing enthalpy, the stronger the attractive interaction between the binary pair. This is applicable to other HEAs as well. The SRO depends on the type of constituent elements present in the HEAs. If a constituent element has a stronger attractive interaction with the other constituent elements, the atoms of this element will separate from each other and bond with other constituent elements to form an SRO structure. Also, the concentration of the constituent elements can change the degree of SRO. We observed that the SRO parameter of Co-Al changes from 0.34 (x = 0.2) to −0.56 (x = 0.8) and Co-Co changes from 0.15 (x = 0.2) to 0.84 (x = 0.8). It indicates that the element concentrations also have a certain influence on the degree of SRO. We think that a possibility for the formation of SRO in the HEAs is the enrichment of the preferred element pairs during solidification. At high temperature, the atomistic structure of liquid alloy is generally thought to be totally random due to the high mixing entropy effect. As the temperature decreases, the enthalpy effect becomes more important, and the non-random configuration shows a tendency toward phase separation or chemical short-range order, and some ordered phases may form during the solidification. Santodonato et al. studied the structural evolution of Al
1.3CoCrCuFeNi alloy from the high temperature liquid phase to the room temperature phase [
38]. The results demonstrated that the alloy is a liquid above 1315 K with Al-Ni, Cr-Fe and Cu-Cu the preferred nearest-neighbor pairs. The results demonstrated that the alloy is a liquid above 1315 K with Al-Ni, Cr-Fe and Cu-Cu preferred nearest-neighbor pairs. During the cooling of the melt, the ordered phases will be preserved.
Due to the preferred Ni-Al, Co-Al and Fe-Si pairs in the BCC phase of FeCoNi (AlSi)
0.4 and FeCoNi (AlSi)
0.8 alloys, a B2-ordered phase structure may be formed. In the multi-component ordered BCC alloys, the crystal structure can be divided in two interpenetrating sublattices, designed by an
sublattice, and a
sublattice.
and
denote the molar fraction of the ith element on
and
sublattices. The B2-ordered parameter
was used to quantify the degree of ordering of the ith element in the sublattices [
38].
is described using:
The total order parameter
is calculated from
with the equation:
where
is the overall molar fraction.
indicates the random solid solution, and
shows the fully B2-ordered structure.
The mixing entropy for the case of a B2 structure can be calculated:
In
Table 3, we presented the ordering parameter
,
and the mixing entropy for the ideal, and taking into account the B2-ordered structure. For the FeCoNi (AlSi)
0.4 alloy,
,
and
, indicating that Al prefers to occupy the
sublattice and Ni and Si prefer the
sublattice to form the B2 phase. The total B2-ordered parameter was
0.294 when x = 0.8,
,
and
, indicating that Al prefers to occupy the
sublattice and Ni and Co prefer the
sublattice to form the B2 phase. With the increase of Al and Si content, the total B2-ordered parameter
reaches 0.427. The emergence of the B2 phase in the alloys reduces the configuration entropy from 1.521 to 1.475 R for the FeCoNi (AlSi)
0.4 alloy, forming 1.606 R to 1.509 R for the FeCoNi(AlSi)
0.8 alloy.
3.3. SRO on Magnetic Properties
As a reference, a series of calculations were carried out to determine the magnetic moments of Fe with BCC, Ni with FCC and Co with HEX structure, respectively. The calculated magnetic moments per Fe, Co and Ni atom are 2.18
, 1.67
, and 0.62
, which are in good agreement with the experimental values [
39]. The deviations between the calculated and the experimental lattice parameters (
a) are within 1%. Chandran et al. [
40] investigated Fe
1−xCo
x alloys in BCC structures and found that with the increase of Co contents, the magnetic moment per Fe atom increased from 2.22
(x = 0) to 2.76
(x = 0.5). Apiñaniz et al. [
41] studied the magnetic properties of ordered Fe
xAl
1-x alloys and found that the magnetic moment per Fe atom decreases from 2.22 to 0.64
with the increase in Al contents. The FeM, CoM and NiM binary ordered alloys (M = Fe, Co, Ni, Al and Si) with BCC primitive cells were constructed. Taking the FeM alloy as an example, the Fe atom was assigned to the body-centered position and the M atom to the body corners.
Table 4 listed the calculated magnetic moments per Fe, Co and Ni atoms with the M atom in the nearest neighbor shell. It can be seen that Co and Ni atoms in the nearest neighbor shell of Fe atoms greatly increase the magnetic moment of Fe atoms, while Al and Si atoms in the nearest neighbor shell of magnetic atoms will drastically decrease the magnetic moments of magnetic atoms. Therefore, the atomic nearest neighbor environment has considerable influence on the atomic magnetic moments in alloys.
There is obvious SRO behavior in the FeCoNi (AlSi)
x alloys. The SRO will significantly change the atomic nearest neighbor environment, which has an impact on the magnetic properties of the alloys. The saturation magnetizations and average atomic magnetic moments of the FeCoNi (AlSi)
x alloys with MaxEnt and SRO structures are summarized in
Table 5. For the FeCoNi alloy, the average atomic magnetic moments per Fe, Co and Ni atoms are 2.65, 1.64
and 0.62
with the initial structure. The Co and Ni atoms in the nearest neighbor of Fe atoms increase the magnetic moment of the Fe atoms. The effect of SRO on the magnetic moments of the FeCoNi alloy is relatively small. The calculated magnetic moments (
= 1.65 T) are consistent with the previous calculated values (
= 1.62 T) [
22]. For the FeCoNi(AlSi)
0.2 alloy, the SRO parameter of the Ni-Al pair reduces by −100% and the nearest neighbor number of the Ni to Al atom changes from 3.5 to 7 for the SRO structure. Similarly, the SRO parameters of the Co-Si, Ni-Si and Fe-Co pairs are also negative. According to the atomic coordinate analysis, Al atoms occupy the body corners, while Ni atoms occupy the face-centered positions and form an Ni-Al rich region. The negative SRO parameters of Ni-Al, Ni-Si and Fe-Co pairs present a similar trend with the experimental work [
14] that the dendritic area is rich in Fe and Co atoms, while the inter-dendritic area is rich in Al, Ni, and Si atoms in FeCoNi (AlSi)
0.2 alloy. In
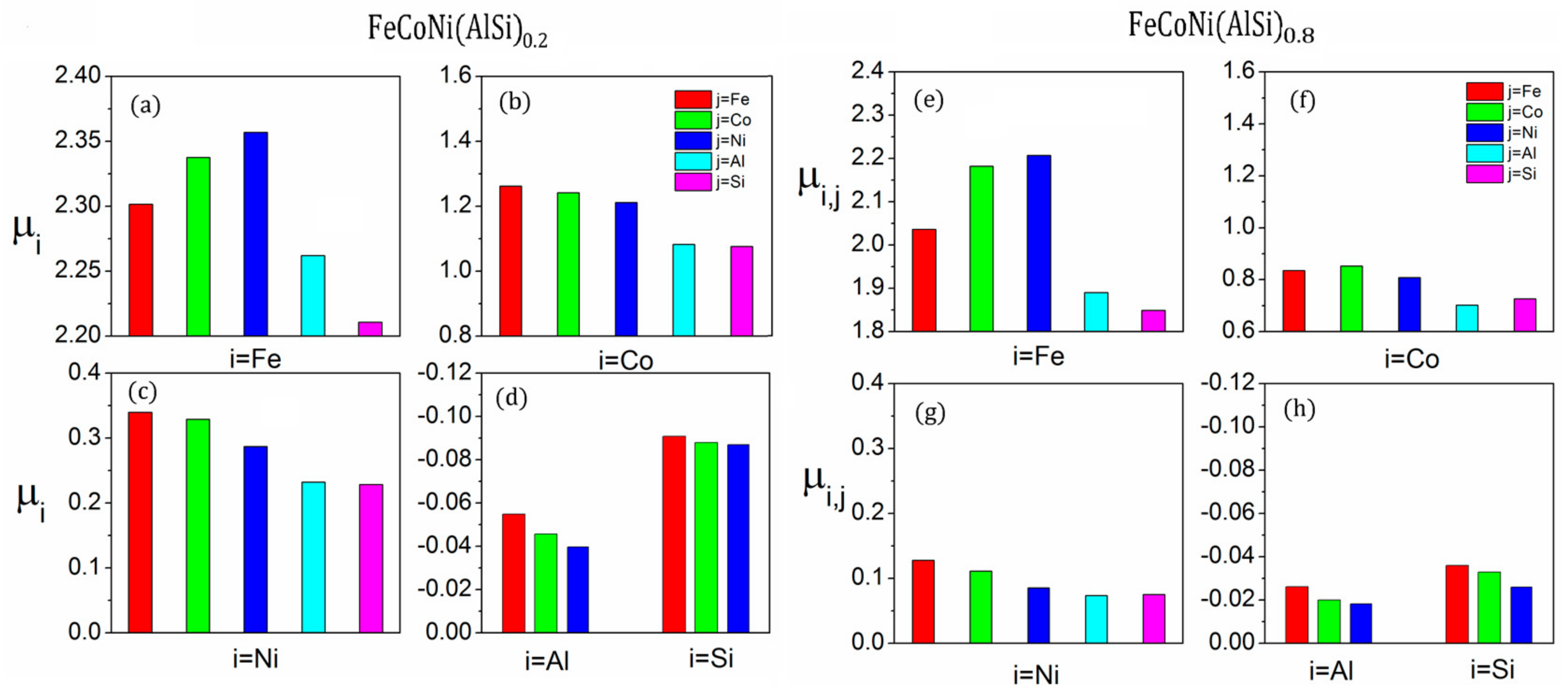
Figure 5, the average atomic magnetic moment
of the FeCoNi (AlSi)
0.2 and FeCoNi (AlSi)
0.8 alloys are presented.
is the average magnetic moment of the type
i element, which is calculated that there is type
j element in its nearest neighbor shell. The average magnetic moments
,
and
vary with different nearest neighbor elements. The results are different from the case of random solid solution. If each lattice is randomly occupied and the probability is proportional to the concentration of the element, the magnetic moment will be homogeneous. The SRO behavior decreases the atomic magnetic moments and the saturation magnetization of the system. The average magnetic moment of the Fe atom changes from 2.50 to 2.32
, the Co atom from 1.43 to 1.19
and the Ni atom from 0.51 to 0.32
. Compared to the previously-calculated saturation magnetization value
= 1.31 T with the random structure model [
22], the saturation magnetization with SRO is
= 1.17 T, which is closer to the experimental value
= 1.15 T [
22].
The FeCoNi(AlSi)
0.8 phase contains more Al and Si components. The SRO parameters of Ni-Al, Co-Si, Co-Al, Fe-Si and Ni-Si pairs are more negative. It is obvious that the Fe, Co and Ni atoms prefer to gather around the Si atom, while the Ni and Co atoms prefer to gather around the Al atom. The SRO greatly alters the local environment of the magnetic atoms, which will further reduce the average magnetic moments of the magnetic atoms. The average magnetic moment of the Fe atoms changes from 2.10 to 2.03
, Co atoms from 0.94 to 0.76
. Due to the enrichment of Al and Si atoms in the nearest neighboring shell of Ni atoms, the magnetic moment of Ni atoms vanishes. The saturation magnetization changes from 0.67 T with the random structure to 0.6 T with the SRO structure. The saturation magnetization with SRO is closer to the experimental value = 0.46 T [
22].
3.4. SRO on Elastic Properties
Mechanical properties are a vital aspect in material selection. It is important to understand the relationship of component, structure and mechanical properties for material applications. FeCoNi(AlSi)
x alloys form FCC or BCC phase structures with different Al and Si fractions. There are three independent elastic constants
,
, and
for the present cubic lattice.
and
can be determined from the bulk modulus (
) and tetragonal shear modulus (
) with
, and
. The bulk modulus can be obtained by fitting energy-volume data with the three-order Birch–Murnaghan equation of state [
42]. Tetragonal shear modulus can be extracted from
by applying orthorhombic strain (
) to the cubic lattice. The elastic constant
can be obtained from fitting the energy-strain equation:
by applying the monoclinic strain (
) to the base lattice [
43]. The shear strain, strain matrix and energy-strain equations are shown in
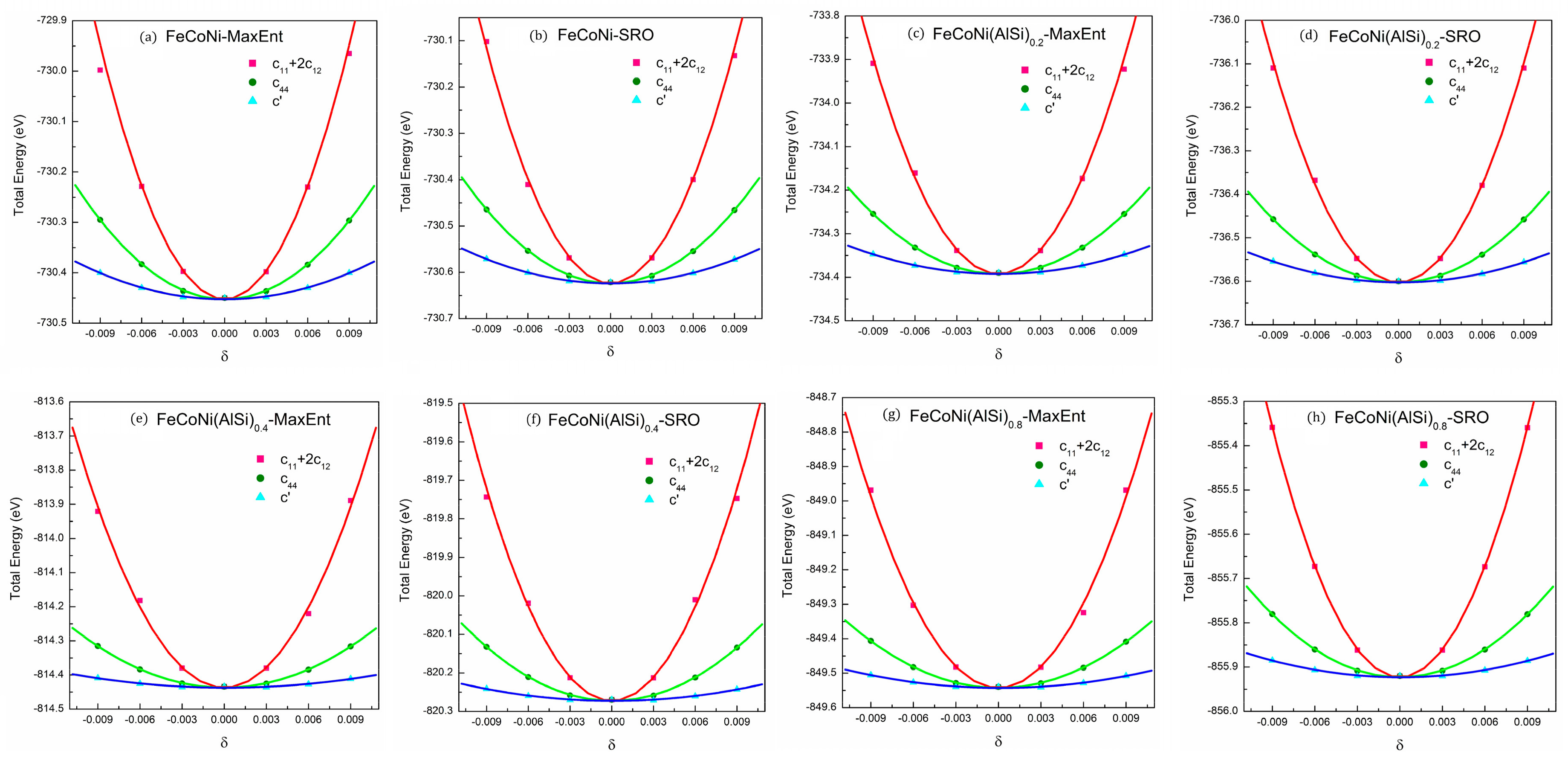
Table 6. In order to keep the elastic behavior of crystals, the applied strains should be relatively small, so the strains adopted are
= −0.009, −0.006, −0.003, 0, 0.003, 0.006, 0.009. The MaxEnt structure is a cubic lattice structure. We modified the basis vector matrix according to the strain matrix to apply different deformations to the supercell structures. The calculations of the total energy were conducted with
k-points to increase the accuracy. The energy curves of different deformations for the FeCoNi(AlSi)
x alloys are shown in
Figure 6.
The shear modulus
is calculated by the Hill average
, and the Voigt and Reuss bounds shear modulus can be obtained by using Equations (6) and (7):
Young’s modulus (
) and Poisson’s ratio (
) are calculated from the bulk modulus (
) and the shear modulus (
):
The calculated lattice parameters, three elastic moduli
,
,
and derived elastic moduli of the FeCoNi(AlSi)
x alloys with the MaxEnt and SRO structures are presented in
Table 7. From
Table 7, all the calculated elastic constants (
,
and
) fulfill the mechanical stability criteria:
,
and
, which demonstrates that the FeCoNi (AlSi)
x alloys are mechanically stable. The experimental information on the elastic modulus is very limited. The calculated lattice parameter for the FeCoNi alloy is
Å, and the experimental value is 3.599 Å [
22]. The calculated lattice parameter is closer to the value
Å calculated with EMTO [
44]. The calculated bulk modulus is
, shear modulus
, and Young’s modulus
. The corresponding results calculated with EMTO are
,
and
The bulk modulus is a measure of the resistance to compressibility of a material. From the calculations, it can be seen that the fractions of Al and Si have significant influence on bulk modulus of FeCoNi (AlSi)x alloys. It is observed that the elastic constants, and and the three elastic moduli (and) decrease with increase of Si and Al fractions. The bulk modulus changes from 184.7 (x = 0) to 157.1 (x = 0.8). Note that Al and Si have a smaller bulk modulus compared to Fe, Co and Ni components. Also the addition of Al and Si make the alloy structure change from the FCC to BCC phase (the packing factor changes from 0.74 to 0.68), which will result in a decrease of the bulk modulus. Taking into account the effect of SRO, SRO behavior prefers to make the affinity atoms locate together, resulting in an increase of the average bonding strength and a decrease of alloy volume. The FeCoNi(AlSi)x alloys with SRO have a higher bulk modulus as compared to that of random solid structures. We also observed that SRO has similar effects on shear modulus and Young’s modulus.
The Pugh’s ratio (
) [
45] and Poisson’s ratio (
) [
46] can be used to qualify the ductile properties of a material. It was reported that an alloy material is ductile when
> 1.75 and
, otherwise it is brittle. For FeCoNi(AlSi)
x alloys, the Cauchy pressure (
), Pugh’s ratio and Poisson’s ratio increase with the increase of Al and Si fractions. The trends of Cauchy pressure, Pugh’s ratio
and Poisson’s ratio
indicate the addition of Al and Si to FeCoNi alloys improves the ductility of alloys. However, it is observed that the SRO behavior reduces the Pugh’s ratio, Poisson’s ratio and Cauchy pressure, showing that SRO results in a reduction in the ductility of the material. The Zener ratio
and the ratio
are used to describe the isotropy property of FeCoNi (AlSi)
x alloys.
, and
. For an isotropic cubic material,
and
. The values of
indicate the relative degree of the elastic anisotropy. For FeCoNi(AlSi)
x alloys, the Zener ratio
changes from 2.94 to 4.29 and
changes from 0.13 to 0.23 with the increase of Al and Si fractions. The Al and Si enhance the anisotropy of the FeCoNi (AlSi)
x alloys. When taking into account the effect of SRO, the
changes from 2.99 to 4.36 and the
changes from 0.14 to 0.24. The SRO behavior further enhances the anisotropy of the FeCoNi (AlSi)
x alloys. From the above results, it can be seen that although SRO has a positive effect on B, G and E, it has a negative effect on the ductility and isotropic properties of the materials.