The Effect of Cold Rolling on the Hydrogen Susceptibility of 5083 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
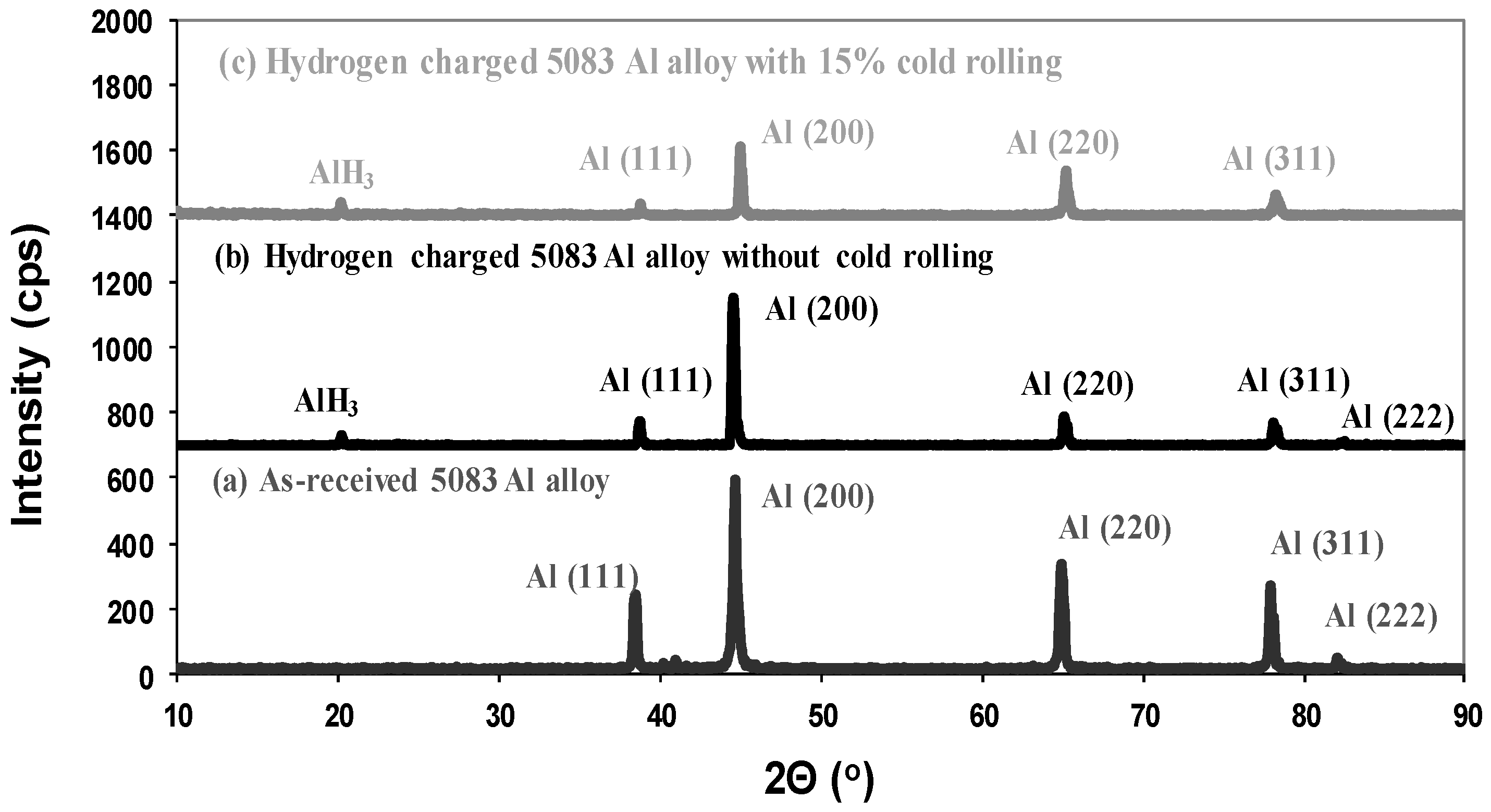
- AlH3 hydrides were detected on the surface layers of 5083 aluminum alloy with and without cold rolling, meaning that hydrogen concentration was above the solubility limit of hydrogen in the aluminum matrix.
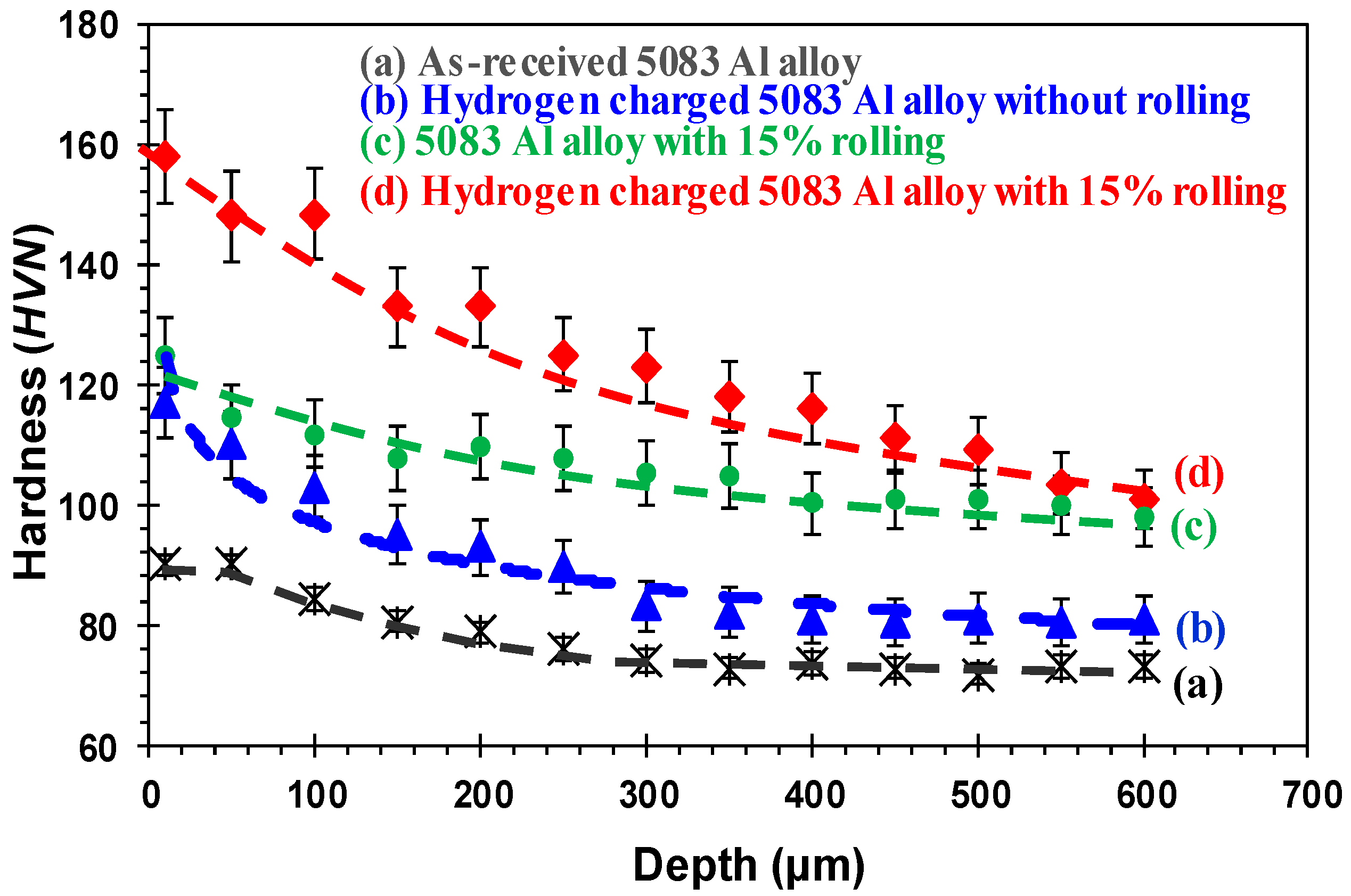
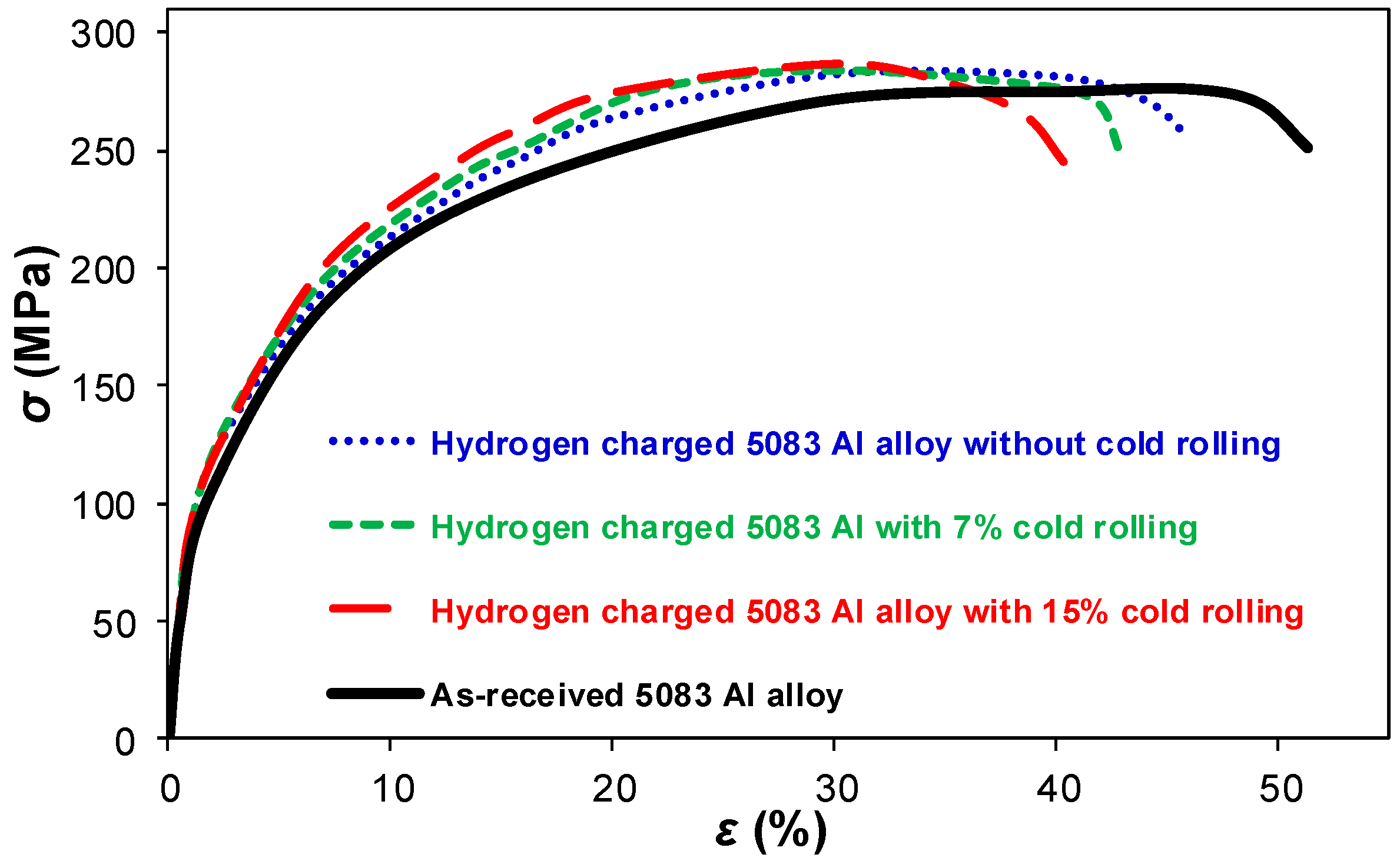
- The tensile tests revealed that the ductility of hydrogen charged 5083 aluminum alloy significantly decreased with increasing rolling deformation, for the examined charging and testing conditions. This is attributed to the higher number of micro-defects in the surface layers of the rolled alloys, which act as “trapping sites” and “diffusion paths” for hydrogen atoms.
- The ultimate tensile strength of the hydrogen charged alloy was observed to be approximately independent of the rolling process.
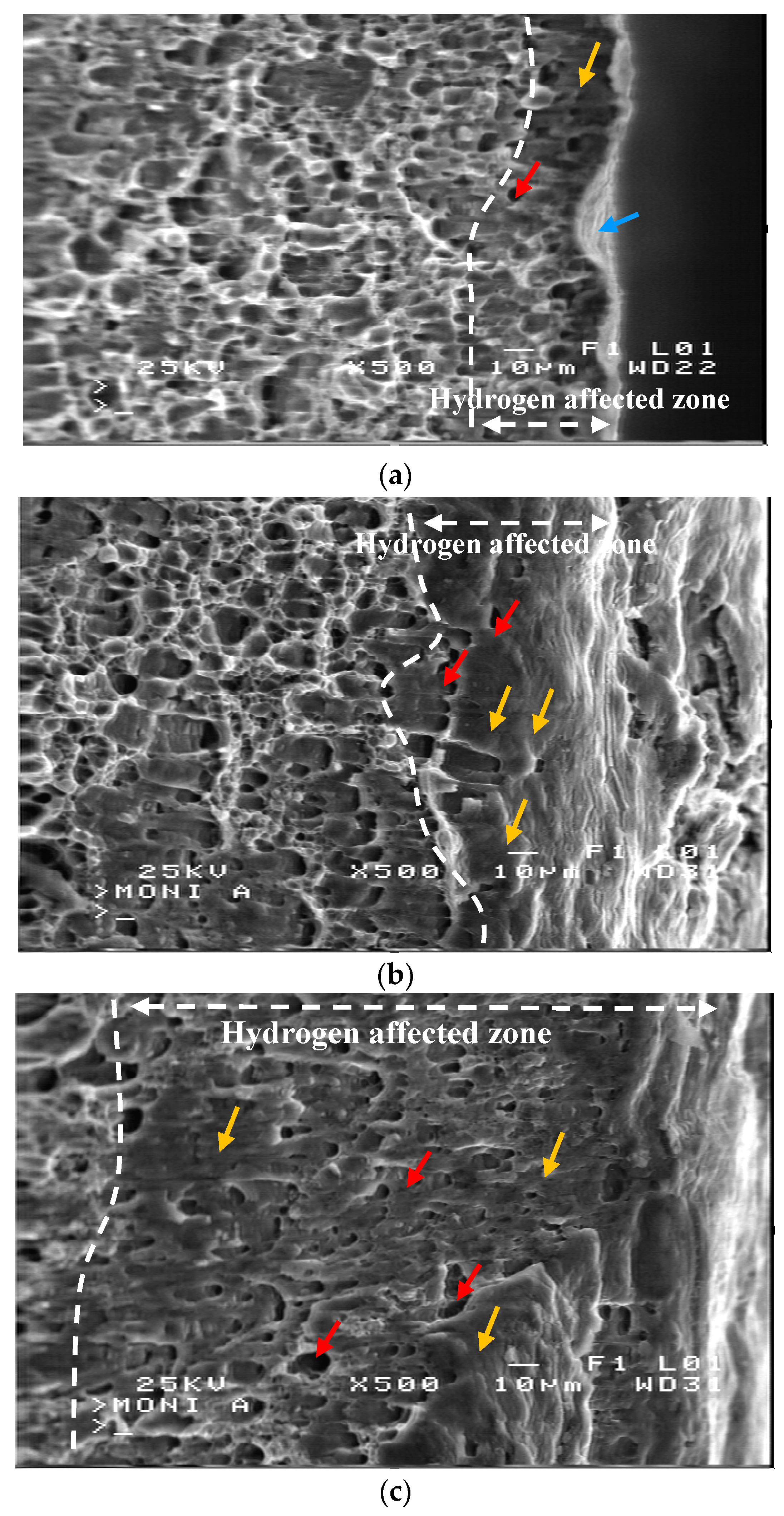
- All hydrogen charged samples exhibited a mixed fracture pattern with brittle transcrystalline cleavage along with ductile intergranular fracture at their surface layers, whereas only ductile intergranular fracture was observed at the bulk of the alloy.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Cicconardi, S.P.; Jannelli, E.; Spazzafumo, G. Hydrogen energy storage: Hydrogen and oxygen storage subsystems. Int. J. Hydrogen Energy 1997, 22, 897–902. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P.; Lagaris, D.A. Cathodic Hydrogen Charging of Aluminium Alloys. In Aluminum Alloys: Preparation, Properties and Applications; Persson, E.L., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011. [Google Scholar]
- Jones, R.H. The influence of hydrogen on the stress-corrosion cracking of low-strength Al-Mg alloys. JOM 2003, 55, 42–46. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P. The effect of hydrogen charging on the mechanical behaviour of 5083 wrought aluminum alloy. Corros. Sci. 2007, 49, 4443–4451. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen embrittlement understood. Metall. Mater. Trans. B 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Troiano, A. The role of hydrogen and other interstitials in the mechanical behavior of metals. Trans. Am. Soc. Met. 1960, 52, 54–80. [Google Scholar] [CrossRef]
- Watson, J.W.; Shen, Y.Z.; Meshii, M. Effect of cathodic charging on the mechanical properties of aluminum. Metall. Trans. A 1988, 19, 2299–2304. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking. Metall. Trans. A 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Robertson, I.M. The effect of hydrogen on dislocation dynamics. Eng. Fract. Mech. 2001, 68, 671–692. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P.; Chaliampalias, D. Cathodic hydrogen charging of zinc. Corros. Sci. 2014, 79, 16–20. [Google Scholar] [CrossRef]
- Hardwick, D.A.; Thompson, A.W.; Bernstein, I.M. The effect of copper content and heat treatment on the hydrogen embrittlement of 7050-type alloys. Corros. Sci. 1988, 28, 1127–1137. [Google Scholar] [CrossRef]
- Wilde, B.E.; Manohar, M.; Albright, C.E. The influence of laser surface melting on the resistance of AISI 4135 low alloy steel to hydrogen-induced brittle fracture. Mater. Sci. Eng. A 1995, 198, 43–49. [Google Scholar] [CrossRef]
- Amira, S.; Huot, J. Effect of cold rolling on hydrogen sorption properties of die-cast and as-cast magnesium alloys. J. Alloys Compd. 2012, 520, 287–294. [Google Scholar] [CrossRef]
- Marquez, J.A.; Matsushima, I.; Uhlig, H.H. Effect of cold rolling on resistance of Ni-Fe alloys to hydrogen cracking. Corrosion 1970, 26, 315–322. [Google Scholar] [CrossRef]
- Metals Handbook, Properies and Selection of Non-Ferrous Alloys, 9th ed.; ASM: Metals Park, OH, USA, 1985.
- Qiua, C.; Opalkab, S.M.; Olsonc, G.B.; Anton, D.L. Thermodynamic modeling of the sodium alanates and the Na-Al-H system. Int. J. Mater. Res. 2006, 97, 1484–1494. [Google Scholar] [CrossRef]
- Kenmochi, K.; Yarita, I.; Abe, H.; Fukuhara, A.; Komatu, T.; Kaito, H. Effect of micro-defects on the surface brightness of cold-rolled stainless-steel strip. J. Mater. Proc. Technol. 1998, 69, 106–111. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Akhtar, D. Effect of cold work on hydrogen embrittlement susceptibility of Ni60Nb40 glass. Mater. Res. Bull. 1986, 21, 1473–1479. [Google Scholar] [CrossRef]
- Chou, S.L.; Tsai, W.T. Effect of grain size on the hydrogen-assisted cracking in duplex stainless steels. Mater. Sci. Eng. A 1999, 270, 219–224. [Google Scholar] [CrossRef]
- Fukai, Y. Site preference of interstitial hydrogen in metals. J. Less-Common Met. 1984, 101, 1–16. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Lo, Y.-C.; Neeraj, T.; Srinivasan, R.; Ding, X.; Sun, J.; Qi, L.; Gumbsch, P.; Li, J. The interaction of dislocations and hydrogen-vacancy complexes and its importance for deformation-induced proto nano-voids formation in α-Fe. Int. J. Plast. 2015, 74, 175–191. [Google Scholar] [CrossRef]
- Fukai, Y.; Ōkuma, N. Evidence of copious vacancy formation in Ni and Pd under high hydrogen pressure. Jpn. J. Appl. Phys. 1993, 32, 1256–1259. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; El-Amoush, A.S.; Agathocleous, P.E. Hydrogen-induced cracking and blistering in α-brass. Corros. Sci. 1998, 40, 1837–1844. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Papapanayiotou, P. The influence of cathodic hydrogen charging on the mechanical behaviour of Al-4Zn-1Mg alloy. J. Mater. Sci. 1995, 30, 3449–3456. [Google Scholar] [CrossRef]
- Lufrano, J.; Sofronis, P.; Birnbaum, H.K. Elastoplastically accommodated hydride formation and embrittlement. J. Mech. Phys. Solids 1998, 46, 1497–1520. [Google Scholar] [CrossRef]
- Eliezer, D.; Tal-Gutelmacher, E.; Boellinghaus, T. Hydrogen embrittlement in hydride and non-hydride forming systems-microstructural/phase changes and cracking mechanisms. In Proceedings of the 11th International Conference on Fracture (ICF11), Turin, Italy, 20–25 March 2005. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiou, E.P.; Celis, J.-P.; Panagopoulos, C.N. The Effect of Cold Rolling on the Hydrogen Susceptibility of 5083 Aluminum Alloy. Metals 2017, 7, 451. https://doi.org/10.3390/met7110451
Georgiou EP, Celis J-P, Panagopoulos CN. The Effect of Cold Rolling on the Hydrogen Susceptibility of 5083 Aluminum Alloy. Metals. 2017; 7(11):451. https://doi.org/10.3390/met7110451
Chicago/Turabian StyleGeorgiou, E.P., J.-P. Celis, and C.N. Panagopoulos. 2017. "The Effect of Cold Rolling on the Hydrogen Susceptibility of 5083 Aluminum Alloy" Metals 7, no. 11: 451. https://doi.org/10.3390/met7110451




