Microstructure and Wear Resistance of Laser-Clad (Co, Ni)61.2B26.2Si7.8Ta4.8 Coatings
Abstract
:1. Introduction
2. Design of the Cladding Material
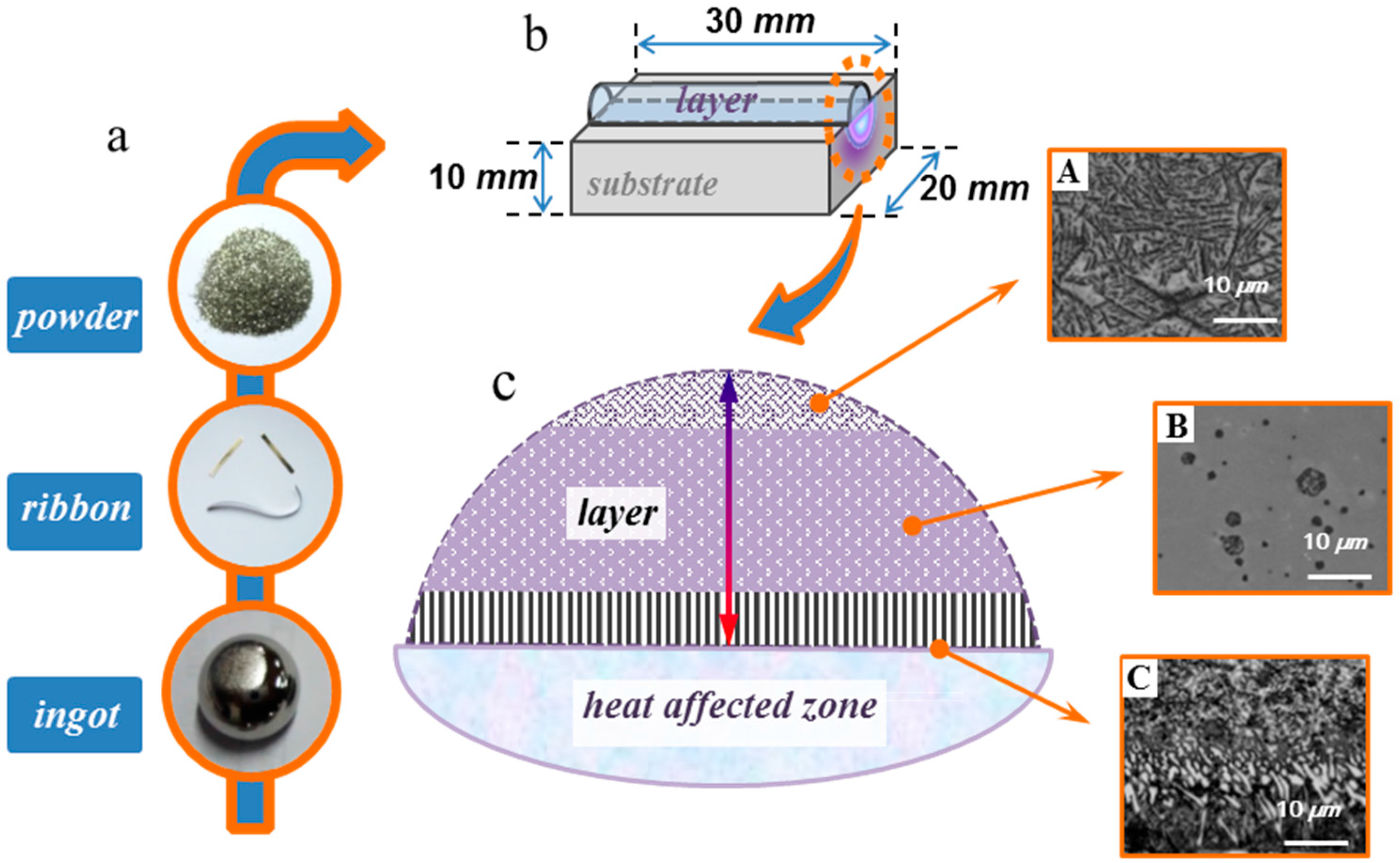
3. Experimental Materials and Procedure
4. Results and Discussion
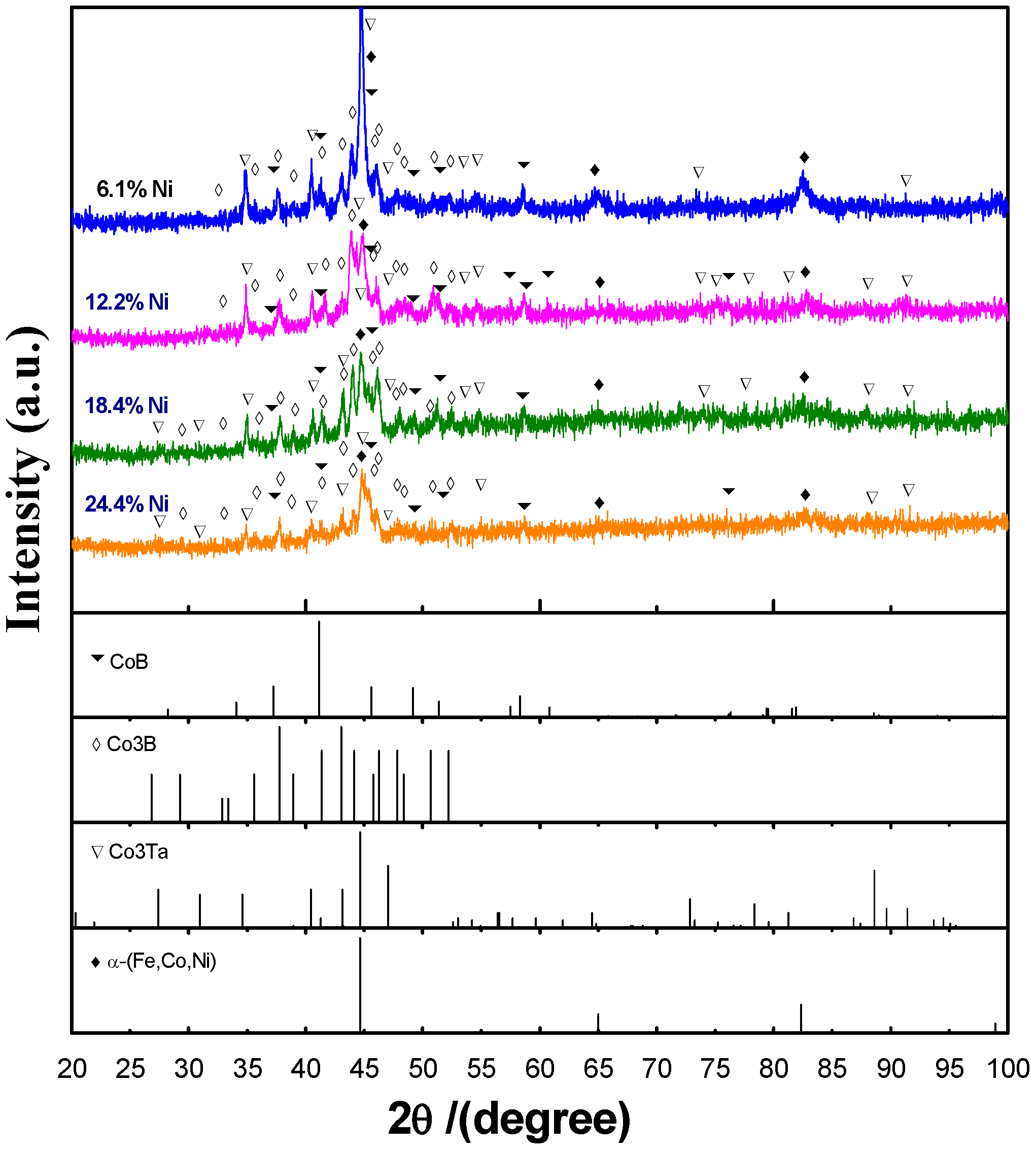
4.1. Microstructure Characterization
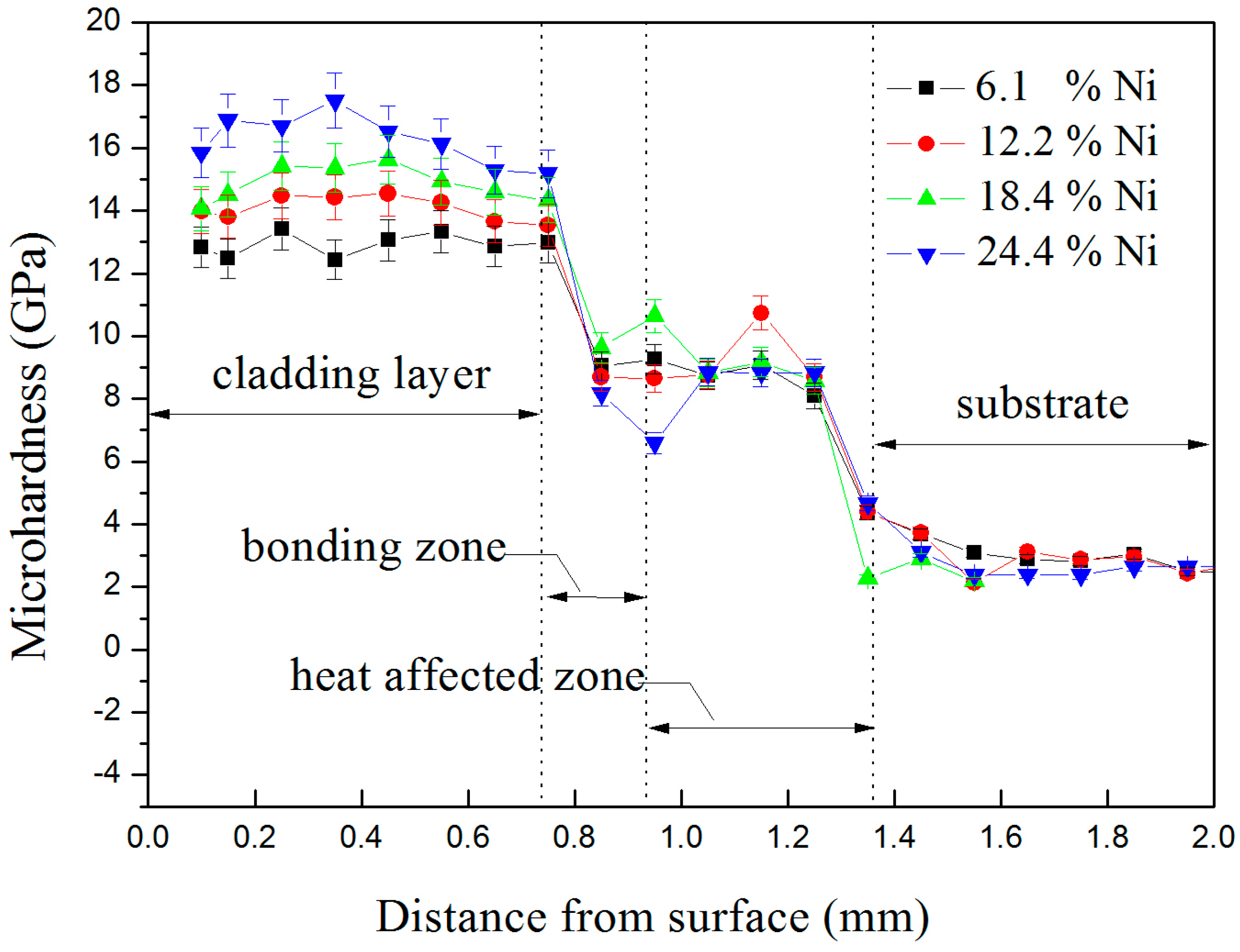
4.2. Mechanical Properties
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaskell, P.H. Models for the Structure of Amorphous Metals. In Glassy Metal II; Beck, H., Güntherodt, H.J., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1983; Volume 53, pp. 5–49. [Google Scholar]
- Greer, A.L. Metallic glasses. Science 1995, 267, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Takeuchi, A. Recent development and applications of bulk glassy alloys. Int. J. Appl. Glass Sci. 2010, 1, 273–295. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, H.; Liaw, P.K. Metallic glass matrix composites. Mater. Sci. Eng. R Rep. 2016, 100, 1–69. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Viswanathan, A. Laser Processing on Metals and Metal Alloys; VDM Verlag: Saarbrucken, Germany, 2010. [Google Scholar]
- Hong, X.; Tan, Y.; Wang, X.; Xu, T.; Gao, L. Microstructure and wear resistant performance of TiN/Zr-base amorphous-nanocrystalline composite coatings on titanium alloy by electrospark deposition. Surf. Coat. Technol. 2016, 305, 67–75. [Google Scholar] [CrossRef]
- Huang, K.; Xie, C.; Yue, T.M. Microstructure of Cu-based amorphous composite coatings on AZ91D magnesium alloy by laser cladding. J. Mater. Sci. Technol. 2009, 25, 492–498. [Google Scholar]
- Jin, Y.J.; Li, R.F.; Zheng, Q.C.; Li, H.; Wu, M.F. Structure and properties of laser-cladded Ni-based amorphous composite coatings. Mater. Sci. Technol. 2016, 32. [Google Scholar] [CrossRef]
- Chang, Z.K.; Gong, J.; Sun, C. Co-based Amorphous/nanocrystalline composite coatings deposited by arc ion plating. J. Mater. Sci. Technol. 2013, 29, 806–812. [Google Scholar] [CrossRef]
- Zhu, Q.J.; Wang, X.H.; Qu, S.Y.; Zou, Z.D. Microstructure and wear properties of laser clad Fe based amorphous composite coatings. Surf. Eng. 2009, 25, 201–205. [Google Scholar] [CrossRef]
- Hitit, A.; Şahin, H.; Öztürk, P.; Aşgın, A.M. A new Ni-based metallic glass with high thermal stability and hardness. Metals 2015, 5, 162–171. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Chang, C.T. Fe-and Co-based bulk glassy alloys with ultrahigh strength of over 4000 MPa. Intermetallics 2006, 14, 936–944. [Google Scholar] [CrossRef]
- Zhu, C.L.; Wang, Q.; Wang, Y.M.; Qiang, J.B.; Dong, C. Co-B-Si-Ta bulk metallic glasses designed using cluster line and alloying. J. Alloys Compd. 2010, 504, 34–37. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.S.; Han, L.Y.; Dong, C. Influence of laser power on microstructure and properties of laser clad Co-based amorphous composite coatings. Surf. Interfaces 2017, 6, 18–23. [Google Scholar] [CrossRef]
- Zhu, C.L. Compositions Design and Properties of (Co,Ni,Fe)-B-Based Bulk Metallic Glasses Designed from Eutectic Cluster Formulae. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 16 January 2011. [Google Scholar]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 4th ed.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 1998; pp. 86–88. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, C.; Qian, S.; Yu, Q.; Dong, C. Microstructure and Wear Resistance of Laser-Clad (Co, Ni)61.2B26.2Si7.8Ta4.8 Coatings. Metals 2017, 7, 419. https://doi.org/10.3390/met7100419
Zhang L, Wang C, Qian S, Yu Q, Dong C. Microstructure and Wear Resistance of Laser-Clad (Co, Ni)61.2B26.2Si7.8Ta4.8 Coatings. Metals. 2017; 7(10):419. https://doi.org/10.3390/met7100419
Chicago/Turabian StyleZhang, Luan, Cunshan Wang, Shengnan Qian, Qun Yu, and Chuang Dong. 2017. "Microstructure and Wear Resistance of Laser-Clad (Co, Ni)61.2B26.2Si7.8Ta4.8 Coatings" Metals 7, no. 10: 419. https://doi.org/10.3390/met7100419
APA StyleZhang, L., Wang, C., Qian, S., Yu, Q., & Dong, C. (2017). Microstructure and Wear Resistance of Laser-Clad (Co, Ni)61.2B26.2Si7.8Ta4.8 Coatings. Metals, 7(10), 419. https://doi.org/10.3390/met7100419





