Optimization of Copper Removal from Aqueous Solutions Using Emulsion Liquid Membranes with Benzoylacetone as a Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
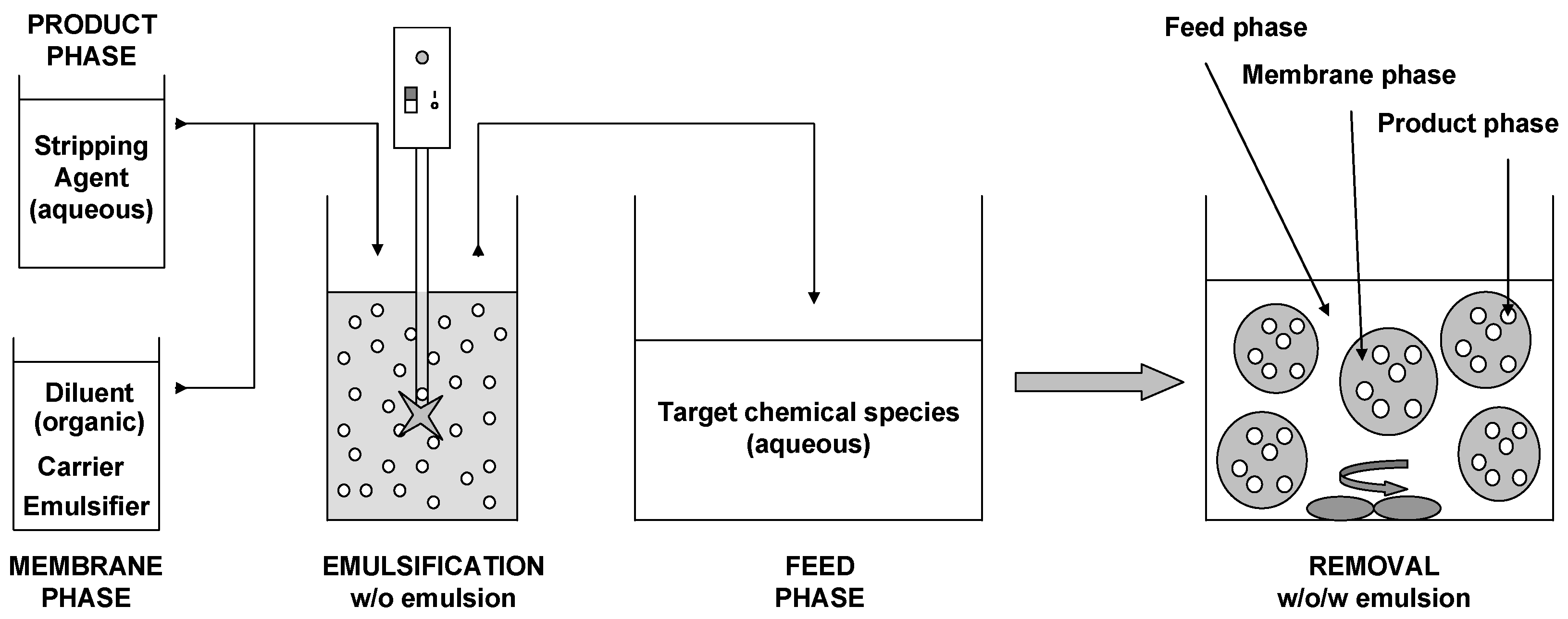
2.2. Procedure
2.3. Methods
3. Results
3.1. Emulsion Stability
3.2. Copper(II) Removal
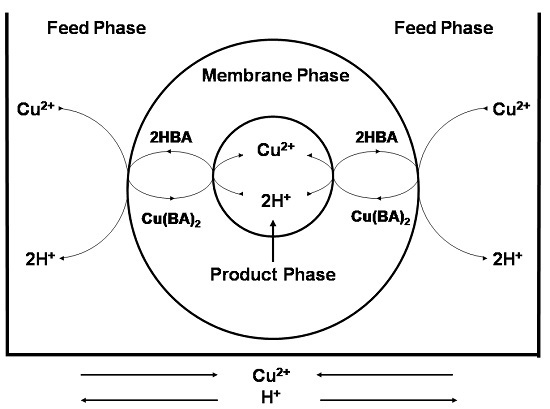
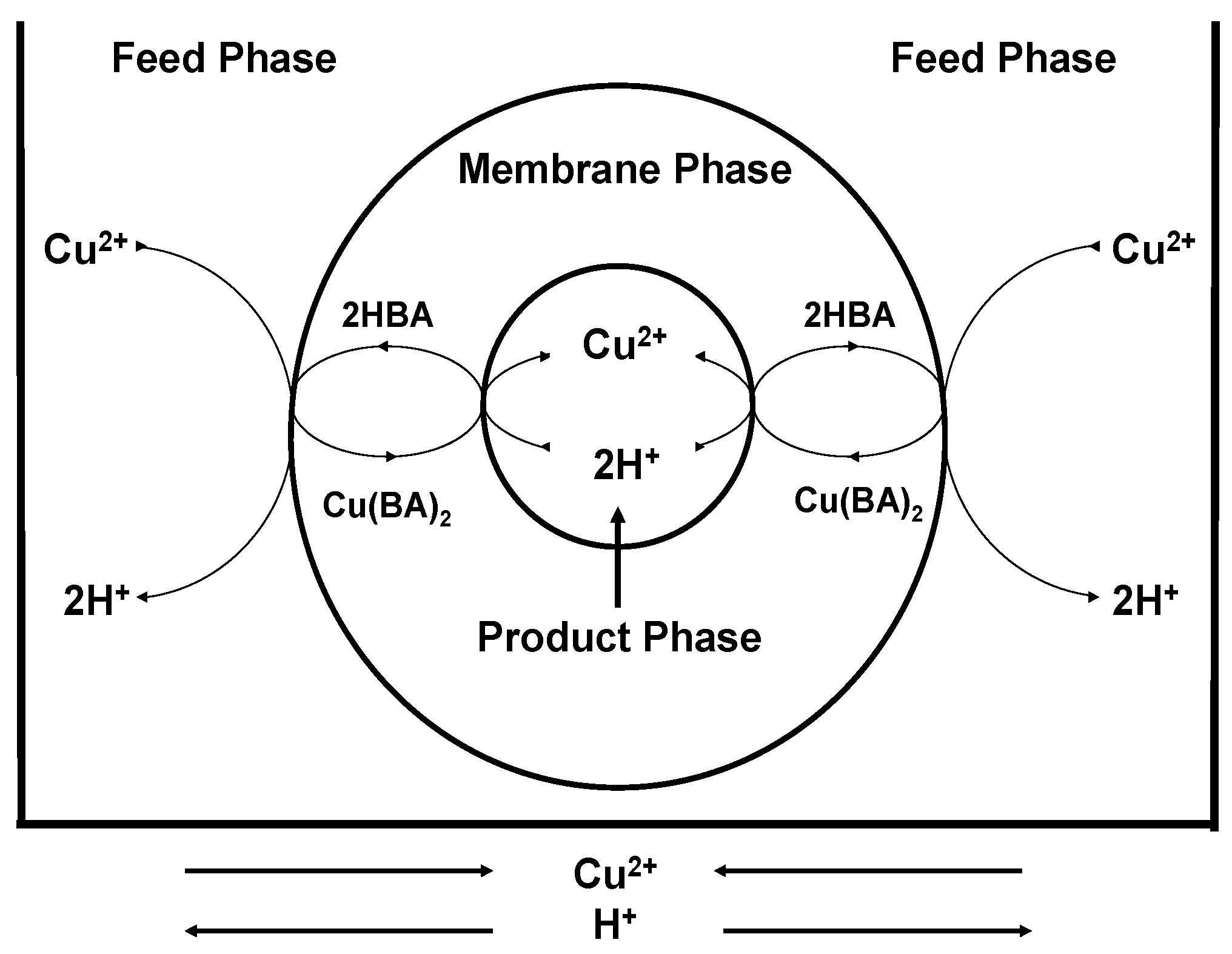
3.2.1. Transport Mechanism
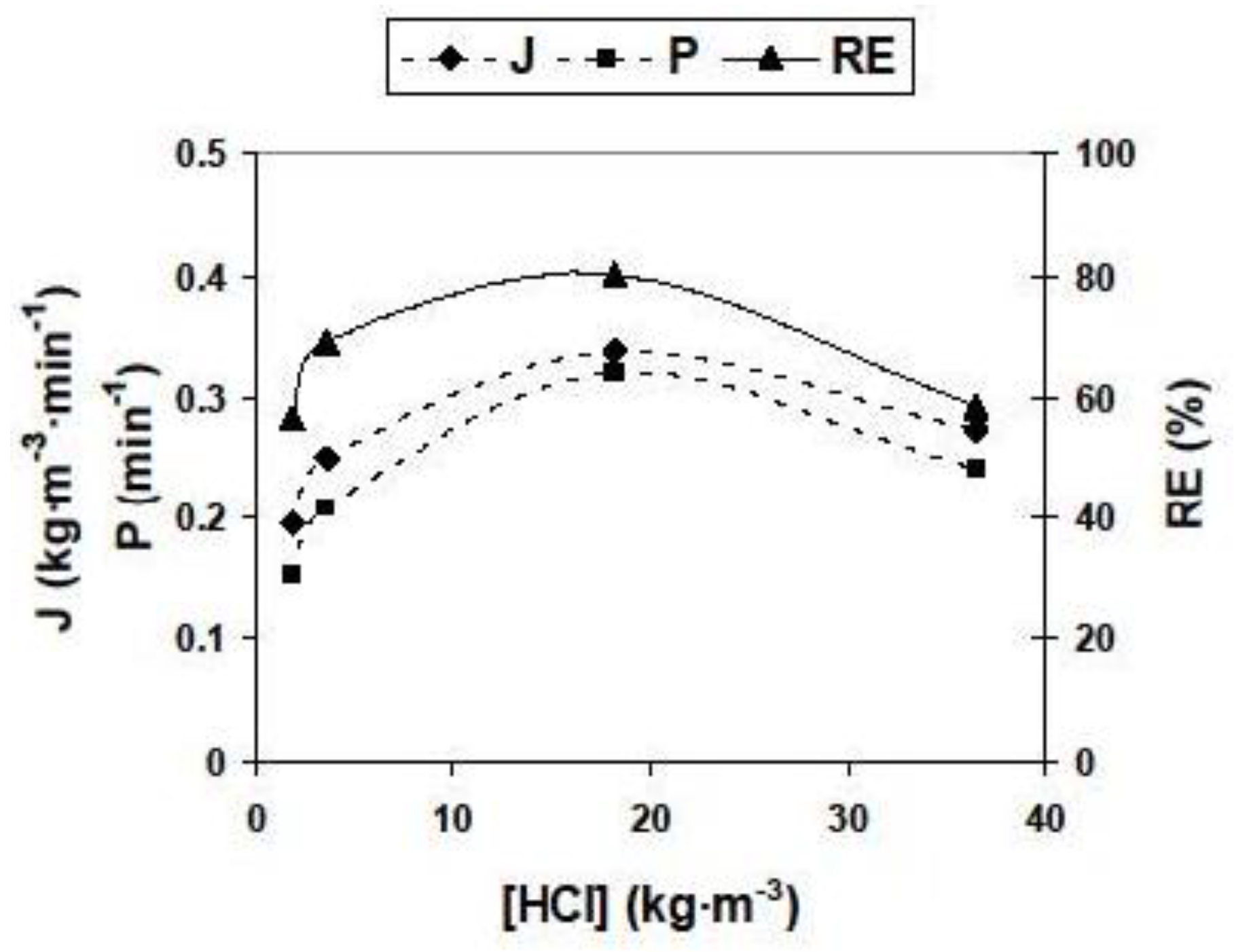
3.2.2. Influence of Different Experimental Parameters on Flux, Permeability and Removal Efficiency
3.2.3. Optimal Removal Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gleick, P.H. Global fresh water resources: Soft-path solutions for the 21st century. Science 2003, 302, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Goyer, R.A.; Guaalkes, M.P. Toxic effects of metals. In Casarett & Doull’s Toxicology: The Basic Science of Poisons, 7th ed.; Klaassen, C.D., Ed.; McGraw-Hill: New York, NY, USA, 2007; pp. 931–980. [Google Scholar]
- Bourgeois, W.; Burgess, J.E.; Stuets, R.M. On-line monitoring of wastewater quality: A review. J. Chem. Technol. Biotechnol. 1991, 76, 337–348. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Awual, M.R.; Yaita, T.; El-Safty, S.A.; Shiwaku, H.; Suzuki, S.; Okamoto, Y. Copper(II) ions capturing from water using ligand modified a new type mesoporous adsorbent. Chem. Eng. J. 2013, 221, 322–330. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Copper ions removal from water using functionalized carbon nanotubes-mullite composite as adsorbent. Mater. Res. Bull. 2015, 68, 54–59. [Google Scholar] [CrossRef]
- Strausak, D.; Mercer, J.F.B.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2001, 55, 175–185. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Aquatic Life Ambient Freshwater Quality Criteria-Copper 2007 Revision; EPA-822-R-07-001; Office of Water/Office of Science and Technology: Washington, DC, USA, 2007.
- Sampaioa, R.M.M.; Timmers, R.A.; Xua, Y.; Keesmanb, K.J.; Lens, P.N.L. Selective precipitation of Cu from Zn in a pS controlled continuously stirred tank reactor. J. Hazard. Mater. 2009, 165, 256–265. [Google Scholar] [CrossRef] [PubMed]
- El-Ashtoukhy, E.S.Z.; Andel-Aziz, M.H. Removal of copper from aqueous solutions by cementation in a bubble column reactor fitted with horizontal screens. Int. J. Miner. Process. 2013, 121, 65–69. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, H.; Yang, C. Chitosan membrane adsorber for low concentration copper ion removal. Carbohydr. Polym. 2016, 146, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Lee, S.; Park, J.A.; Park, C.; Lee, S.G.; Kim, S.B.; An, B.; Yun, S.T.; Lee, S.H.; Choi, J.W. Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 2017, 166, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Amirnia, S.; Ray, M.B.; Margaritis, A. Copper ion removal by Acer saccharum leaves in a regenerable continuous-flow column. Chem. Eng. J. 2016, 287, 755–764. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.J.; Ryu, C.; Park, Y.K. Removal of copper(II) in aqueous solution using pyrolytic biochars derived from red macroalga Porphyra tenera. J. Ind. Eng. Chem. 2016, 36, 314–319. [Google Scholar] [CrossRef]
- Taskin, O.S.; Kiskan, B.; Aksu, A.; Balkis, N.; Yagci, Y. Copper(II) removal from the aqueous solution using microporous benzidine-based adsorbent material. J. Environ. Chem. Eng. 2016, 4, 899–907. [Google Scholar] [CrossRef]
- Ntimbani, R.N.; Simate, G.S.; Ndlovu, S. Removal of copper ions from dilute synthetic solution using staple ion exchange fibres: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2016, 4, 3143–3150. [Google Scholar] [CrossRef]
- Wang, F.H.; Ji, Y.X.; Wang, J.J. Synthesis of heavy metal chelating agent withfour chelating groups of N1,N2,N4,N5-tetrakis(2-mercaptoethyl)benzene-1,2,4,5-tetracarboxamide (TMBTCA) and its application for Cu-containing wastewater. J. Hazard. Mater. 2012, 241–242, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Akbal, F.; Camc, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 2011, 269, 214–222. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Cai, Z.; Chen, R.; Sun, Q.; Sun, M. Removal of copper and nickel from municipal sludge using an improved electrokinetic process. Chem. Eng. J. 2017, 307, 1008–1016. [Google Scholar] [CrossRef]
- Rahimi, M.; Schoener, Z.; Zhu, X.; Zhang, F.; Gorski, C.A.; Logan, B.E. Removal of copper from water using a thermally regenerative electrodeposition battery. J. Hazard. Mater. 2017, 322, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Ennigrou, D.J.; Ali, M.B.S.; Dhahbi, M. Copper and Zinc removal from aqueous solutions by polyacrylic acid assisted-ultrafiltration. Desalination 2014, 343, 82–87. [Google Scholar] [CrossRef]
- Ghaemi, N.; Daraei, P. Enhancement in copper ion removal by PPy@Al2O3 polymeric nanocomposite membrane. J. Ind. Eng. Chem. 2016, 40, 26–33. [Google Scholar] [CrossRef]
- Lu, D.; Chang, Y.; Wang, W.; Xie, F.; Asselin, E.; Dreisinger, D. Copper and Cyanide Extraction with Emulsion Liquid Membrane with LIX 7950 as the Mobile Carrier: Part 1, Emulsion Stability. Metals 2015, 5, 2034–2047. [Google Scholar] [CrossRef]
- León, L.; León, G.; Senent, J.; Guzmán, M.A. Kinetic study of Cu(II) simultaneous extraction/stripping from aqueous solutions by bulk liquid membranes using coupled transport mechanisms. Metals 2016, 6, 212. [Google Scholar] [CrossRef]
- Duan, H.; Wang, Z.; Yuan, X.; Wang, S.; Guo, S.; Yang, X. A novel sandwich supported liquid membrane for simultaneous separation of copper, nickel and cobalt in ammoniacal solution. Sep. Purif. Technol. 2017, 173, 323–329. [Google Scholar] [CrossRef]
- Kislik, V.S. Introduction, General Description, Definitions and Classification. Overview. In Liquid Membranes. Principles and Applications in Chemical SEPARATION and Wastewater Treatment; Kislik, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–15. [Google Scholar]
- Lende, A.B.; Kulkarni, P.S. Selective recovery of tungsten from printed circuit board recycling unit wastewater by using emulsion liquid membrane process. J. Water Process Eng. 2015, 8, 75–81. [Google Scholar] [CrossRef]
- Noah, N.F.M.; Othman, N.; Jusoh, N. Highly selective transport of palladium from electroplating wastewater using emulsion liquid membrane process. J. Taiwan Inst. Chem. Eng. 2016, 64, 134–141. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Khaleque, M.A.; Sheik, M.C. Treatment of copper containing wastewater by a newly developed ligand based facial conjugate materials. Chem. Eng. J. 2016, 288, 368–376. [Google Scholar] [CrossRef]
- Awual, M.R. New type mesoporous conjugate material for selective optical copper(II) ion monitoring & removal from polluted water. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar]
- León, G. Facilitated transport. In Encyclopedia of Membranes, 1st ed.; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 763–764. [Google Scholar]
- Gyves, J.; Rodríguez, E. Metal ion separations by supported liquid membranes. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar] [CrossRef]
- Dâas, A.; Hamdaoui, O. Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. J. Hazard. Mater. 2010, 178, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Bouranene, S.; Samar, M.E.H.; Abbaci, A. Extraction of cobalt and lead from waste water using a liquid surfactant membrane emulsion. Acta Chem. Slov. 2003, 50, 663–675. [Google Scholar]
- Kedari, C.S.; Pandit, S.S.; Parikh, K.J.; Tripathi, S.C. Removal of 241Am from aqueous nitrate solutions by liquid surfactant membrane containing 2-ethylhexyl phosphonic acid mono 2-ethylhexyl ester as ion carrier. Chemosphere 2010, 80, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Abou-Nemech, I.; van Poteghem, A.P. Some aspects of emulsion inestability on using sorbitan monoleate (SPAN 80) as a surfactant in liquid emulsion membranes. Chem. Ing. Tech. 1990, 62, 420–421. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bhattacharya, C.; Datta, S. Emulsion liquid membranes: Definitions and classification, theories, module design, applications, new directions and perspectives. In Liquid Membranes. Principles and Applications in Chemical Separation and Wastewater Treatment; Kislik, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 141–199. [Google Scholar]
- Kulkarni, P.S.; Mahajani, V.V. Application of liquid emulsion membrane (LEM) process for enrichment of Molybdenum from aqueous solutions. J. Membr. Sci. 2002, 201, 123–135. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Kusumastuti, A.; Derek, C.J.C.; Ooi, B.S. Emulsion liquid membranes for heavy metal removal: An overview on emulsion stabilization and destabilization. Chem. Eng. J. 2011, 171, 870–882. [Google Scholar] [CrossRef]
- García, M.G.; Acosta, A.O.; Marchese, J. Emulsion liquid membrane pertraction of Cr(III) from aqueous solutions using PC-88A as carrier. Desalination 2013, 318, 88–96. [Google Scholar] [CrossRef]

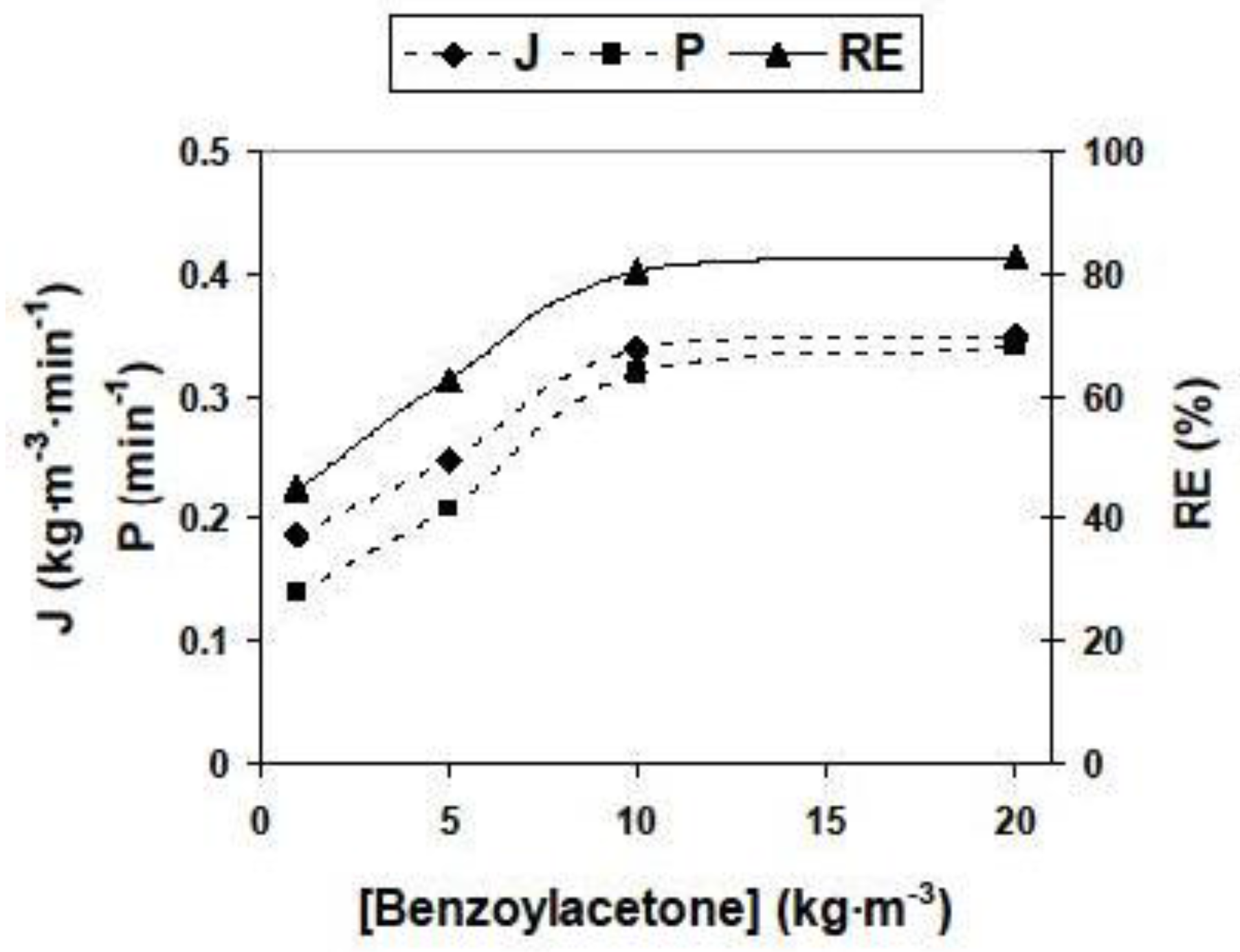
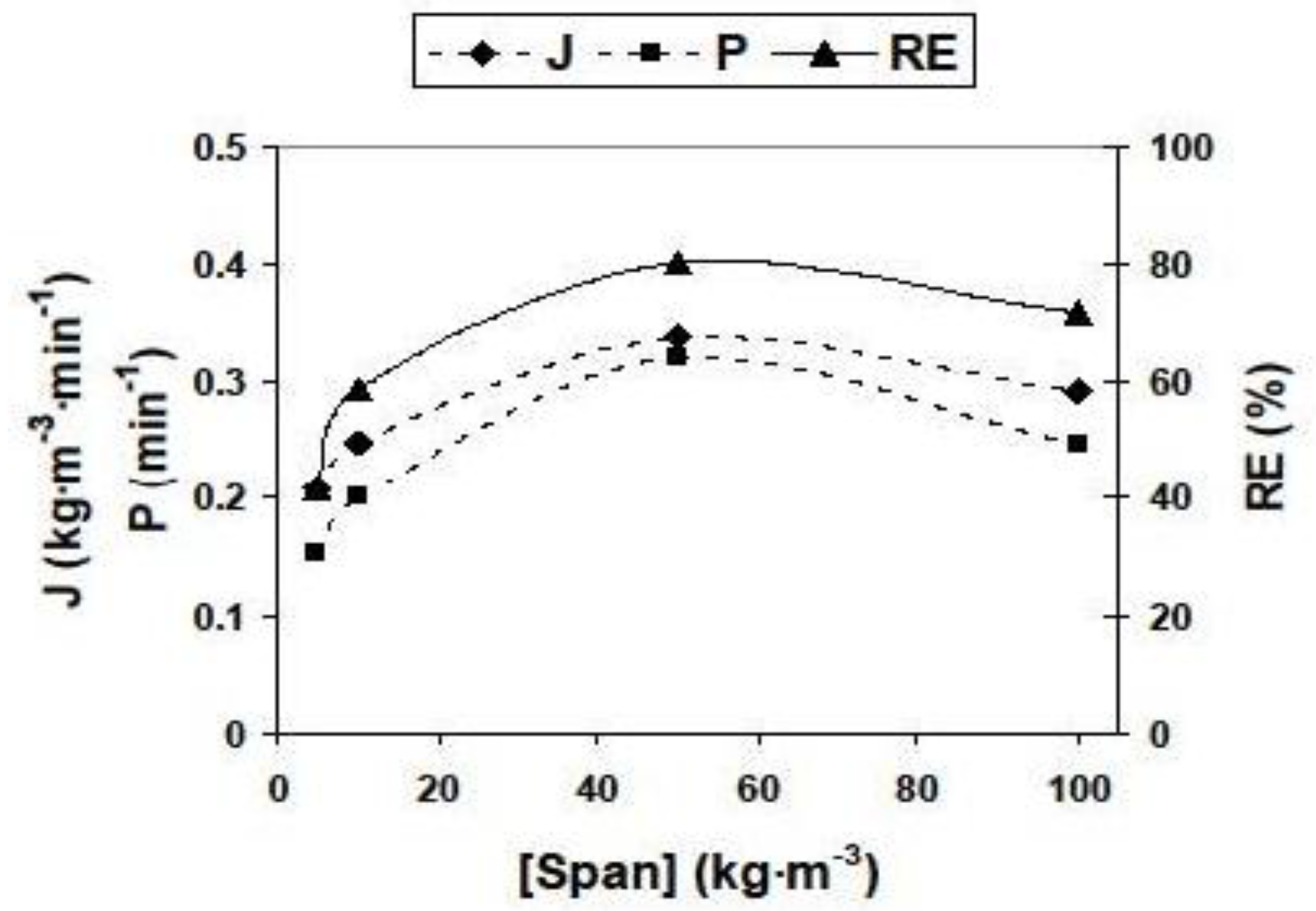
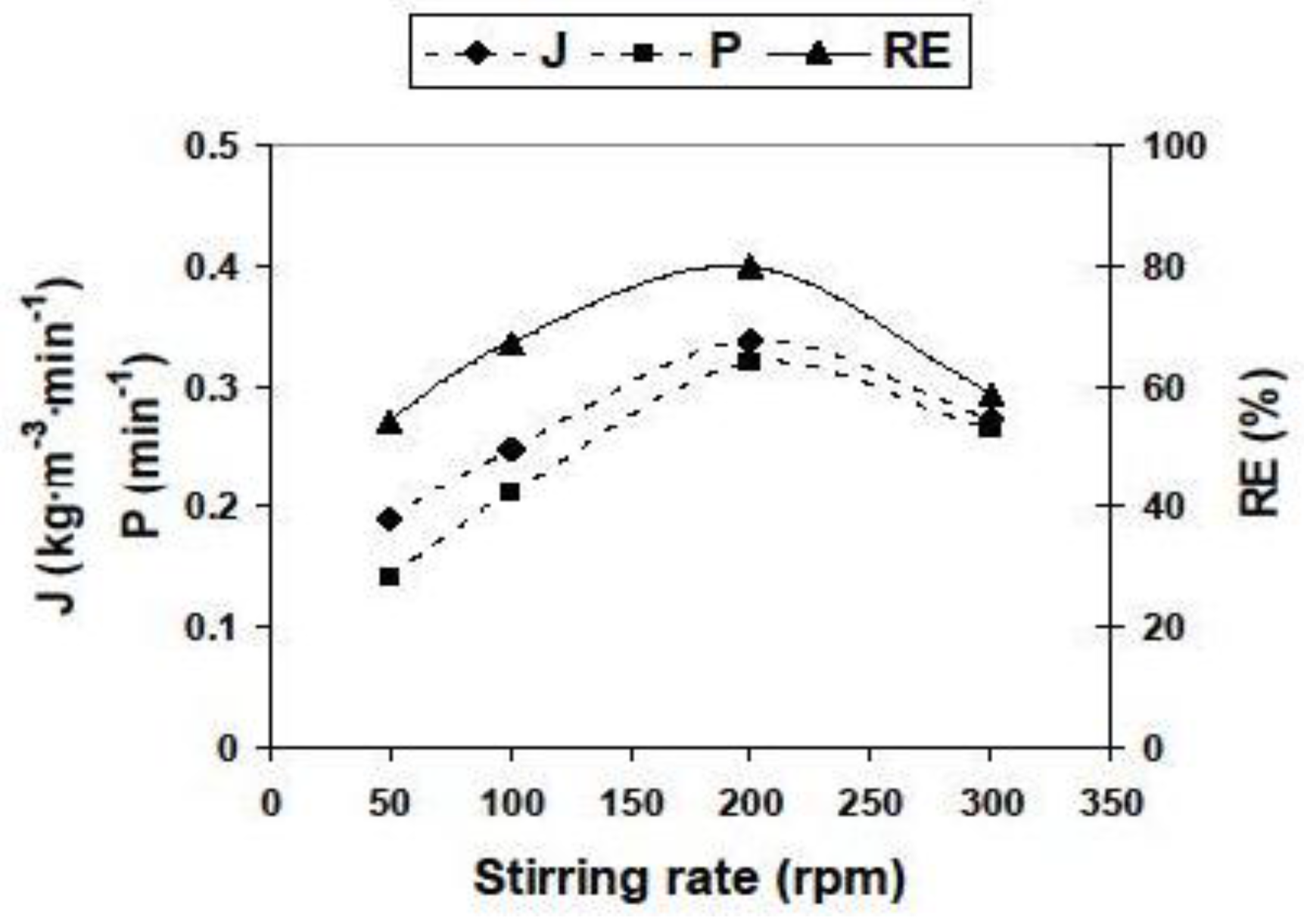


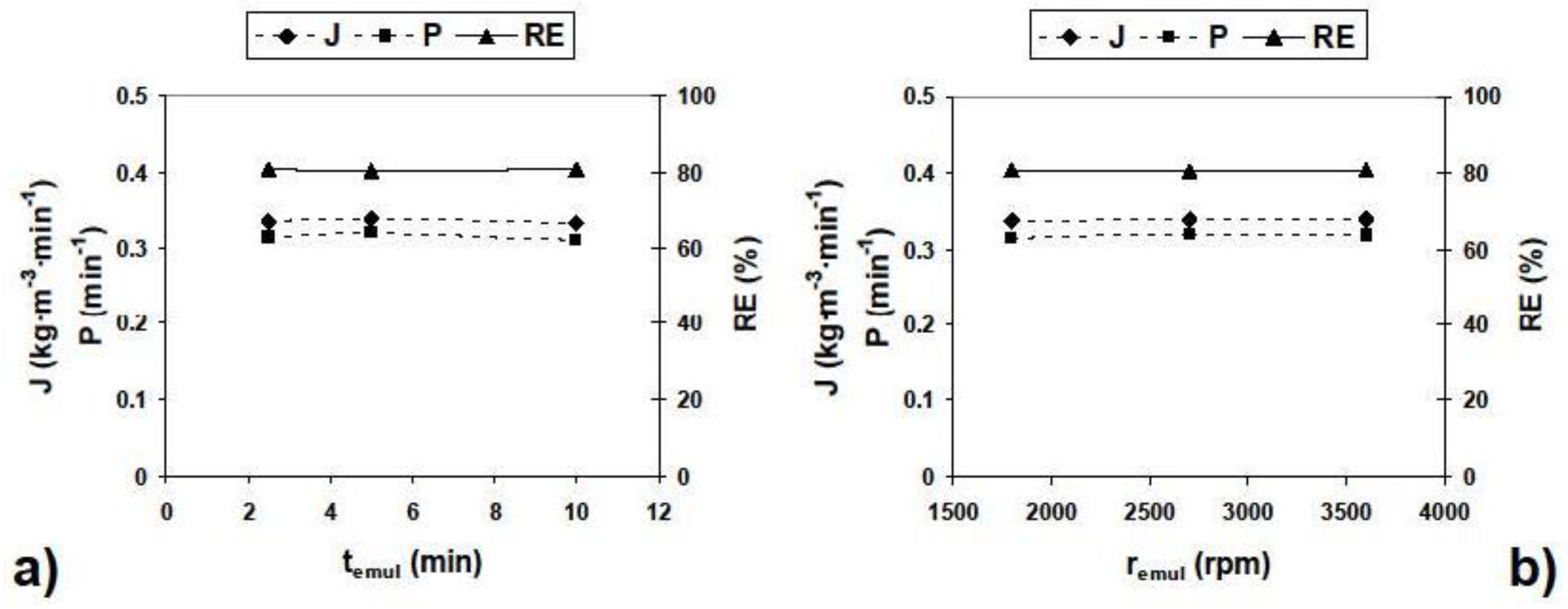
| Parameter | Values | Parameter | Values |
|---|---|---|---|
| Feed pH | 4.0; 4.5; 5.0; 5.5 * | [HCl] (kg/m3) | 1.825; 3.650; 18.250 *; 36.500 |
| [HBA] (kg/m3) | 1; 5; 10 *; 20 | [Span 80] (kg/m3) | 5; 10; 50 *; 100 |
| rstir (rpm) | 50; 100; 200 *; 300 | Vf/Vemul | 1; 2 *; 4; 8 |
| Vp/Vm | 0.75; 1.00 *; 1.25; 1.50 | temul (min) | 2.5; 5.0 *; 10.0 |
| remul (rpm) | 1800; 2700 *; 3600 |
| Parameter | Value | B (%) | Parameter | Value | B (%) |
|---|---|---|---|---|---|
| Feed pH | 4.0 | 0.8 | [HCl] (kg/m3) | 1.825 | 0.5 |
| 4.5 | 0.9 | 3.650 | 0.8 | ||
| 5.0 | 0.9 | 18.250 | 0.9 | ||
| 5.5 | 0.8 | 36.500 | 4.2 | ||
| [HBA] (kg/m3) | 1 | 0.8 | [Span 80] (kg/m3) | 5 | 4.1 |
| 5 | 0.8 | 10 | 2.8 | ||
| 10 | 0.9 | 50 | 0.9 | ||
| 20 | 1.3 | 100 | 0.3 | ||
| rstir (rpm) | 50 | 0.6 | Vf/Vemul | 1 | 2.5 |
| 100 | 0.8 | 2 | 0.9 | ||
| 200 | 0.9 | 4 | 0.6 | ||
| 300 | 3.7 | 8 | 0.4 | ||
| Vp/Vm | 0.75 | 0.4 | temul (min) | 2.5 | 0,6 |
| 1.00 | 0.9 | 5.0 | 0.9 | ||
| 1.25 | 1.8 | 10.0 | 2.2 | ||
| 1.50 | 2.3 | remul (rpm) | 900 | 0.7 | |
| 1800 | 0.8 | ||||
| 2700 | 0.9 | ||||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, L.; León, G.; Senent, J.; Pérez-Sirvent, C. Optimization of Copper Removal from Aqueous Solutions Using Emulsion Liquid Membranes with Benzoylacetone as a Carrier. Metals 2017, 7, 19. https://doi.org/10.3390/met7010019
León L, León G, Senent J, Pérez-Sirvent C. Optimization of Copper Removal from Aqueous Solutions Using Emulsion Liquid Membranes with Benzoylacetone as a Carrier. Metals. 2017; 7(1):19. https://doi.org/10.3390/met7010019
Chicago/Turabian StyleLeón, Loreto, Gerardo León, Javier Senent, and Carmen Pérez-Sirvent. 2017. "Optimization of Copper Removal from Aqueous Solutions Using Emulsion Liquid Membranes with Benzoylacetone as a Carrier" Metals 7, no. 1: 19. https://doi.org/10.3390/met7010019
APA StyleLeón, L., León, G., Senent, J., & Pérez-Sirvent, C. (2017). Optimization of Copper Removal from Aqueous Solutions Using Emulsion Liquid Membranes with Benzoylacetone as a Carrier. Metals, 7(1), 19. https://doi.org/10.3390/met7010019