Glass-Forming Ability and Early Crystallization Kinetics of Novel Cu-Zr-Al-Co Bulk Metallic Glasses
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Formation of Cu-Zr-Al-Co Bulk Metallic Glasses with Good Glass-Forming Ability
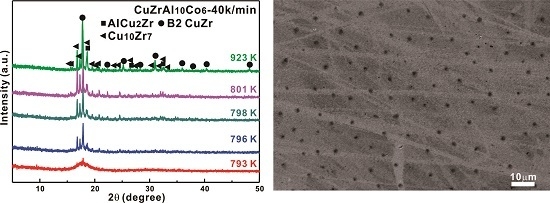
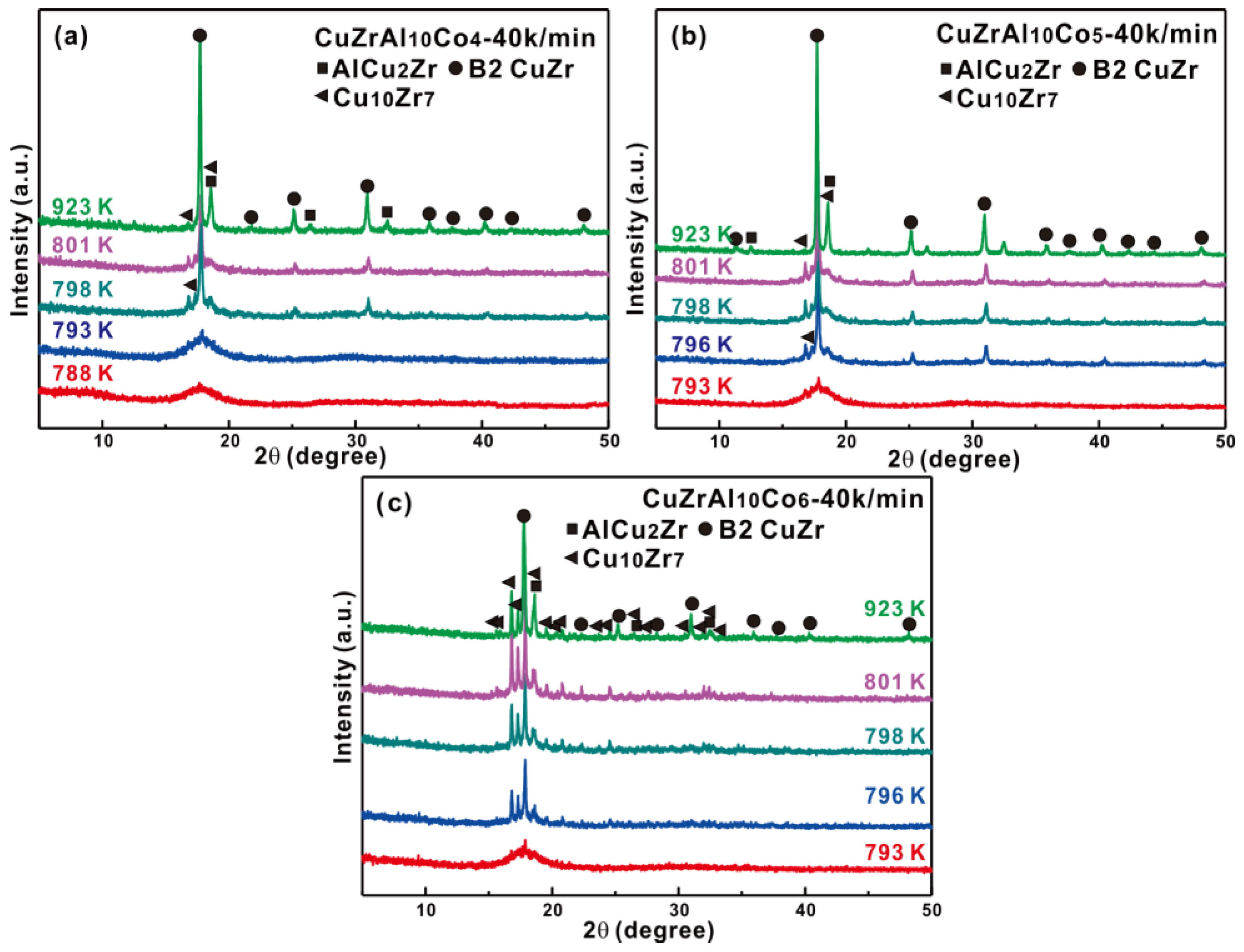
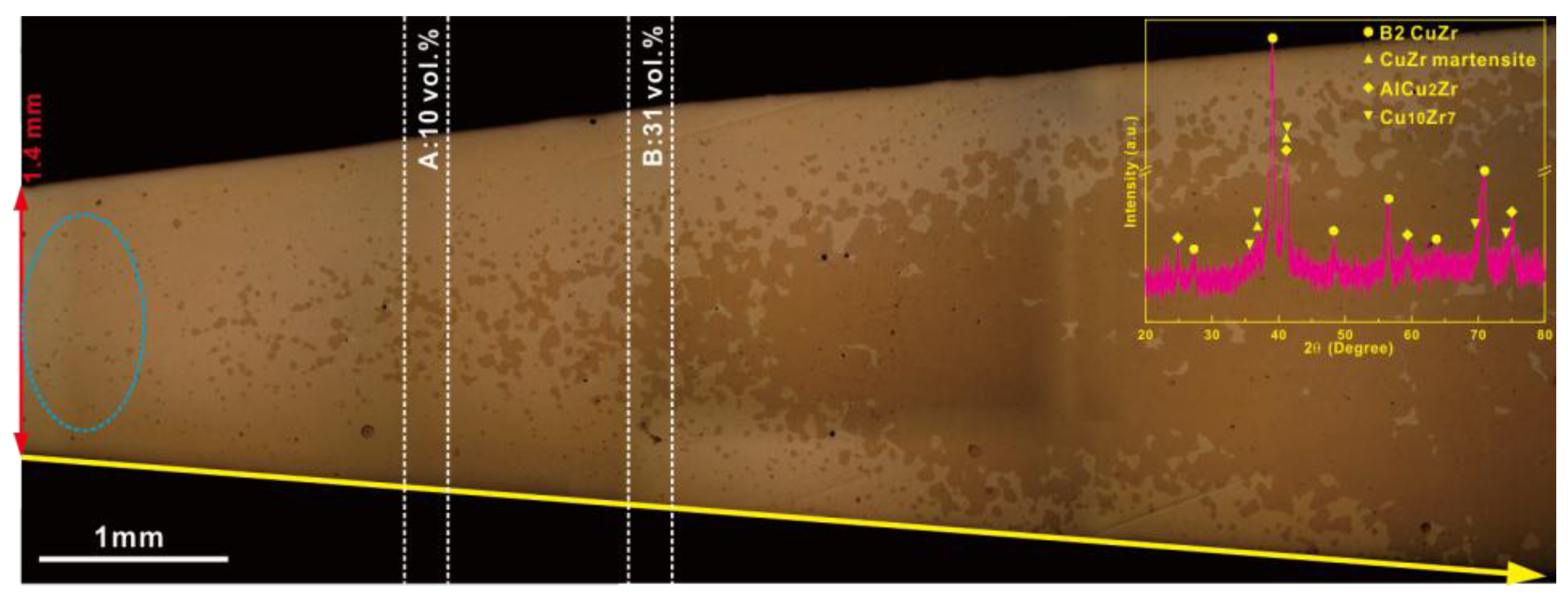
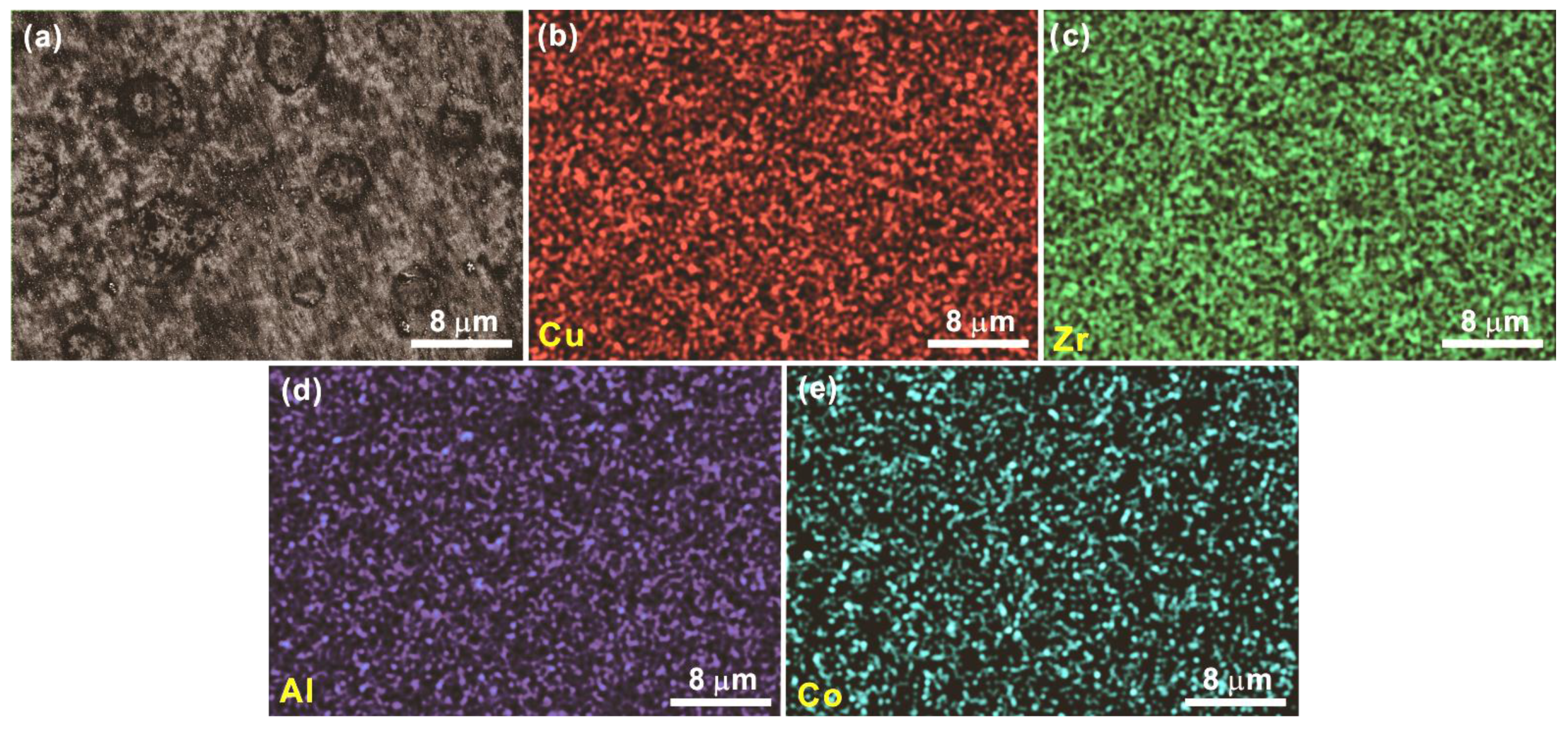
3.2. Primary Precipitates from Partially Crystallized Cu-Zr-Al-Co Bulk Metallic Glasses or from Rapid Solidification
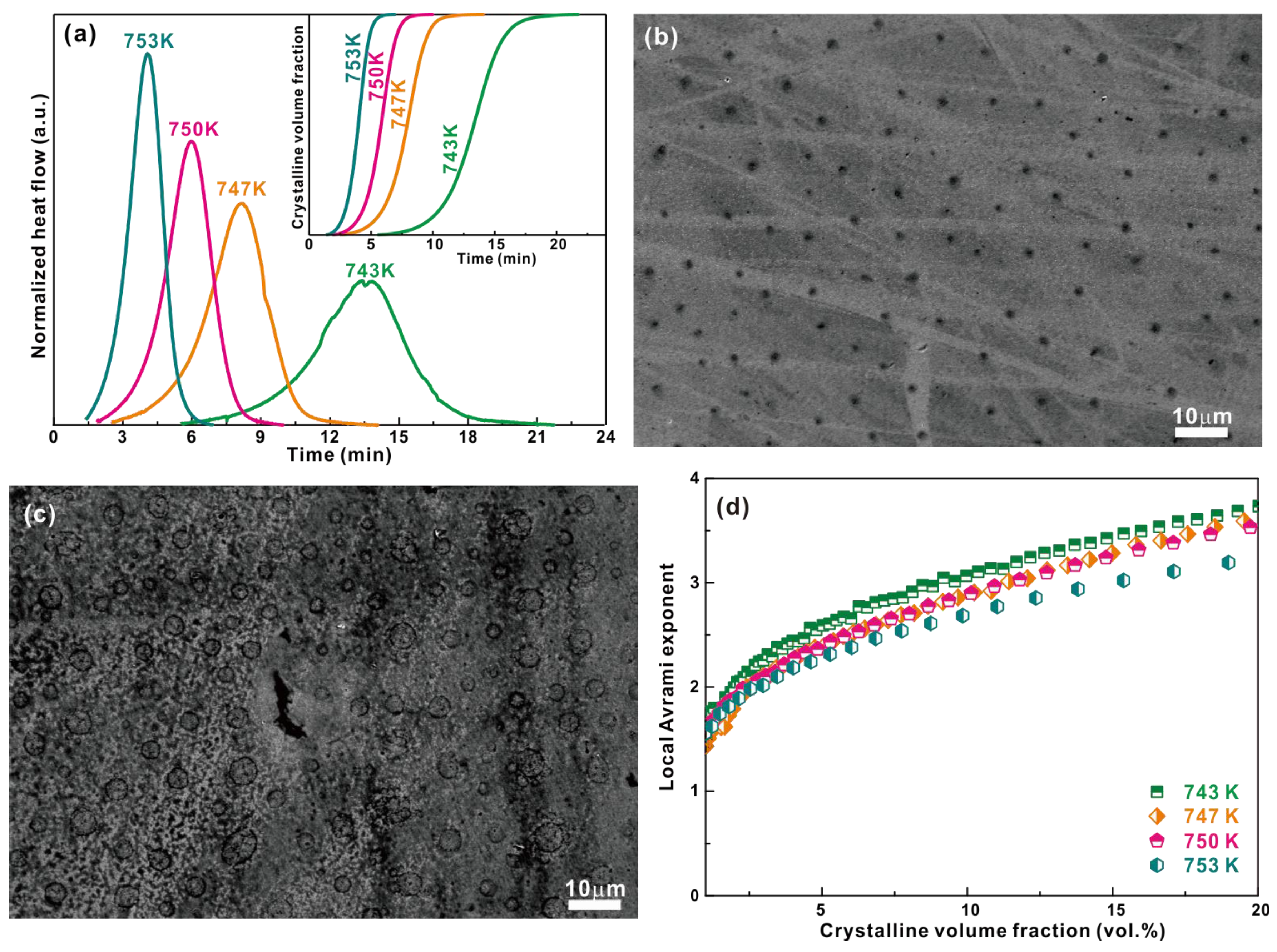
3.3. Crytallization Kinetics of Cu-Zr-Al-Co Bulk Metallic Glasses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, L.J.; Geng, L.; Peng, H.X. Microstructurally inhomogeneous composites: Is a homogeneous reinforcement distribution optimal? Prog. Mater. Sci. 2015, 71, 93–168. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65. [Google Scholar] [CrossRef]
- Wong, P.; Lee, T.; Tsai, P.; Cheng, C.; Li, C.; Jang, J.; Huang, J. Enhanced mechanical properties of MgZnCa bulk metallic glass composites with Ti-particle dispersion. Metals 2016, 6, 116. [Google Scholar] [CrossRef]
- Madge, S.; Louzguine-Luzgin, D.; Inoue, A.; Greer, A. Large compressive plasticity in a La-based glass-crystal composite. Metals 2013, 3, 41. [Google Scholar] [CrossRef]
- Chang, H.J.; Yook, W.; Park, E.S.; Kyeong, J.S.; Kim, D.H. Synthesis of metallic glass composites using phase separation phenomena. Acta Mater. 2010, 58, 2483–2491. [Google Scholar] [CrossRef]
- Choi-Yim, H.; Conner, R.D.; Szuecs, F.; Johnson, W.L. Processing, microstructure and properties of ductile metal particulate reinforced Zr57Nb5Al10Cu15.4Ni12.6 bulk metallic glass composites. Acta Mater. 2002, 50, 2737–2745. [Google Scholar] [CrossRef]
- Eckert, J.; Das, J.; Pauly, S.; Duhamel, C. Processing routes, microstructure and mechanical properties of metallic glasses and their composites. Adv. Eng. Mater. 2007, 9, 443–453. [Google Scholar] [CrossRef]
- Fan, C.; Inoue, A. Ductility of bulk nanocrystalline composites and metallic glasses at room temperature. Appl. Phys. Lett. 2000, 77, 46–48. [Google Scholar] [CrossRef]
- Pan, D.G.; Zhang, H.F.; Wang, A.M.; Hu, Z.Q. Enhanced plasticity in Mg-based bulk metallic glass composite reinforced with ductile Nb particles. Appl. Phys. Lett. 2006, 89, 261904. [Google Scholar] [CrossRef]
- Li, Y.; Poon, S.J.; Shiflet, G.J.; Xu, J.; Kim, D.H.; Loffler, J.F. Formation of bulk metallic glasses and their composites. MRS Bull. 2007, 32, 624–628. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.Y.; Wiest, A.; Duan, G.; Lind, M.L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Choi-Yim, H.; Conner, R.D.; Szuecs, F.; Johnson, W.L. Quasistatic and dynamic deformation of tungsten reinforced Zr57Nb5Al10Cu15.4Ni12.6 bulk metallic glass matrix composites. Scr. Mater. 2001, 45, 1039–1045. [Google Scholar] [CrossRef]
- Fu, H.M.; Wang, H.; Zhang, H.F.; Hu, Z.Q. In situ TiB-reinforced cu-based bulk metallic glass composites. Scr. Mater. 2006, 54, 1961–1966. [Google Scholar] [CrossRef]
- Guo, S.F.; Liu, L.; Li, N.; Li, Y. Fe-based bulk metallic glass matrix composite with large plasticity. Scr. Mater. 2010, 62, 329–332. [Google Scholar] [CrossRef]
- Qiao, J.W.; Sun, A.C.; Huang, E.W.; Zhang, Y.; Liaw, P.K.; Chuang, C.P. Tensile deformation micromechanisms for bulk metallic glass matrix composites: From work-hardening to softening. Acta Mater. 2011, 59, 4126–4137. [Google Scholar] [CrossRef]
- Abdeljawad, F.; Haataja, M. Continuum modeling of bulk metallic glasses and composites. Phys. Rev. Lett. 2010, 105, 125503. [Google Scholar] [CrossRef] [PubMed]
- Hays, C.C.; Kim, C.P.; Johnson, W.L. Microstructure controlled shear band pattern formation and enhanced plasticity of bulk metallic glasses containing in situ formed ductile phase dendrite dispersions. Phys. Rev. Lett. 2000, 84, 2901–2904. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.W.; Zhang, T.; Yang, F.Q.; Liaw, P.K.; Pauly, S.; Xu, B.S. A tensile deformation model for in-situ dendrite/metallic glass matrix composites. Sci. Rep. 2013, 3, 2816. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.F.; Chan, K.C.; Jiang, S.S.; Chen, S.H.; Wang, G. Bulk metallic glass composite with good tensile ductility, high strength and large elastic strain limit. Sci. Rep. 2014, 4, 5302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Liu, G.; Qu, R.T.; Zhang, Z.F.; Wu, S.J.; Zhang, T. Microstructural percolation assisted breakthrough of trade-off between strength and ductility in CuZr-based metallic glass composites. Sci. Rep. 2014, 4, 4167. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.C. Shape memory bulk metallic glass composites. Science 2010, 329, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Song, K.; Cao, C.; Li, R.; Wang, G.; Wu, Y.; Wan, F.; Ding, F.; Shi, Y.; Bai, X.; et al. Deformation-induced martensitic transformation in Cu-Zr-Zn bulk metallic glass composites. Metals 2015, 5, 2134. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Zhang, Y.; Sun, B.A.; He, J.; Ma, G.Z.; Kuhn, U.; Eckert, J. Thermal stability and mechanical properties of Cu46Zr46Ag8 bulk metallic glass and its composites. Mater. Sci. Eng. A 2013, 559, 711–718. [Google Scholar] [CrossRef]
- Xie, M.T.; Zhang, P.N.; Song, K.K. Thermal stability and transformation-mediated deformability of Cu-Zr-Al-Ni bulk metallic glass composite. J. Mater. Sci. Technol. 2013, 29, 868–872. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Sun, B.A.; Zhang, Y.; Tan, J.; Kühn, U.; Stoica, M.; Eckert, J. Formation of Cu-Zr-Al-Er bulk metallic glass composites with enhanced deformability. Intermetallics 2012, 30, 132–138. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Sun, B.A.; Tan, J.; Stoica, M.; Kuhn, U.; Eckert, J. Correlation between the microstructures and the deformation mechanisms of CuZr-based bulk metallic glass composites. AIP Adv. 2013, 3, 012116. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Chen, G.; Liu, C.T.; Lu, Z. Bulk metallic glass composites with transformation-mediated work-hardening and ductility. Adv. Mater. 2010, 22, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Liu, G.; Wang, G.; Kuhn, U.; Mattern, N.; Eckert, J. Microstructural heterogeneities governing the deformation of Cu47.5Zr47.5Al5 bulk metallic glass composites. Acta Mater. 2009, 57, 5445–5453. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, R.; Liu, G.; Su, W.H.; Wang, H.; Li, Y.; Shi, M.J.; Luo, X.K.; Wu, G.J.; Zhang, T. Microstructural tailoring and improvement of mechanical properties in CuZr-based bulk metallic glass composites. Acta Mater. 2012, 60, 3128–3139. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Wu, H.H.; Zhang, Z.Y.; Hui, X.D.; Chen, G.L.; Ma, D.; Wang, X.L.; Lu, Z.P. Formation of Cu-Zr-Al bulk metallic glass composites with improved tensile properties. Acta Mater. 2011, 59, 2928–2936. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, R.; Liu, G.; Song, K.K.; Pauly, S.; Zhang, T.; Eckert, J. Pronounced ductility in CuZrAl ternary bulk metallic glass composites with optimized microstructure through melt adjustment. AIP Adv. 2012, 2, 032176. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Zhang, Y.; Gargarella, P.; Li, R.; Barekar, N.S.; Kühn, U.; Stoica, M.; Eckert, J. Strategy for pinpointing the formation of B2 CuZr in metastable CuZr-based shape memory alloys. Acta Mater. 2011, 59, 6620–6630. [Google Scholar] [CrossRef]
- Javid, F.A.; Mattern, N.; Pauly, S.; Eckert, J. Effect of cobalt on phase formation, microstructure, and mechanical properties of Cu50−xCoxZr50 (x = 2, 5, 10, 20 at. pct) alloys. Metall. Mater. Trans. A 2012, 43A, 2631–2636. [Google Scholar] [CrossRef]
- Javid, F.A.; Mattern, N.; Khoshkhoo, M.S.; Stoica, M.; Pauly, S.; Eckert, J. Phase formation of Cu50−xCoxZr50 (x = 0–20 at. %) alloys: Influence of cooling rate. J. Alloy. Compd. 2014, 590, 428–434. [Google Scholar] [CrossRef]
- Kosiba, K.; Gargarella, P.; Pauly, S.; Kuhn, U.; Eckert, J. Predicted glass-forming ability of Cu-Zr-Co alloys and their crystallization behavior. J. Appl. Phys. 2013, 113, 123505. [Google Scholar] [CrossRef]
- Pauly, S.; Das, J.; Bednarcik, J.; Mattern, N.; Kim, K.B.; Kim, D.H.; Eckert, J. Deformation-induced martensitic transformation in Cu-Zr-(Al,Ti) bulk metallic glass composites. Scr. Mater. 2009, 60, 431–434. [Google Scholar] [CrossRef]
- Song, K.K.; Gargarella, P.; Pauly, S.; Ma, G.Z.; Kuhn, U.; Eckert, J. Correlation between glass-forming ability, thermal stability, and crystallization kinetics of Cu-Zr-Ag metallic glasses. J. Appl. Phys. 2012, 112, 063503. [Google Scholar] [CrossRef]
- Fu, J.; Men, H.; Pang, S.; Ma, C.; Zhang, T. Formation and thermal stability of Cu-Zr-Al-Er bulk metallic glasses with high glass-forming ability. J. Univ. Sci. Technol. B. 2007, 14, 36–38. [Google Scholar] [CrossRef]
- Fan, G.J.; Li, J.J.Z.; Rhim, W.-K.; Qiao, D.C.; Choo, H.; Liaw, P.K.; Johnson, W.L. Thermophysical properties of a Cu46Zr42Al7Y5 bulk metallic glass-forming liquid. Appl. Phys. Lett. 2006, 88, 221909. [Google Scholar] [CrossRef]
- Duan, G.; de Blauwe, K.; Lind, M.L.; Schramm, J.P.; Johnson, W.L. Compositional dependence of thermal, elastic, and mechanical properties in Cu-Zr-Ag bulk metallic glasses. Scr. Mater. 2008, 58, 159–162. [Google Scholar] [CrossRef]
- Kuo, C.N.; Huang, J.C.; Du, X.H.; Liu, X.J.; Nieh, T.G. Comparison of mechanical response in CuZrAl-V and CuZrAl-Co bulk metallic glass composites. J. Alloy. Compd. 2014, 586, S14–S19. [Google Scholar] [CrossRef]
- Gao, W.H.; Meng, X.L.; Cai, W.; Zhao, L.C. Effects of Co and Al addition on martensitic transformation and microstructure in ZrCu-based shape memory alloys. Trans. Nonferr. Met. Soc. China 2015, 25, 850–855. [Google Scholar] [CrossRef]
- Wei, Y.; Du, J.; Chen, R. Martensitic transformation induced plasticity in ZrCuAl metallic glass composites: Precipitate size and volume effects. Intermetallics 2016, 68, 1–4. [Google Scholar] [CrossRef]
- Okulov, I.V.; Soldatov, I.V.; Sarmanova, M.F.; Kaban, I.; Gemming, T.; Edstrom, K.; Eckert, J. Flash joule heating for ductilization of metallic glasses. Nat. Commun. 2015, 6, 7932. [Google Scholar] [CrossRef] [PubMed]
- Song, K.K. Synthesis, microstructure, and deformation mechanisms of CuZr-based bulk metallic glass composites. Ph.D. Thesis, TU Dresden, Dresden, Germany, November 2013. [Google Scholar]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Xie, G.Q.; Inoue, A. Glass-forming ability and mechanical properties of the ternary Cu-Zr-Al and quaternary Cu-Zr-Al-Ag bulk metallic glasses. Mater. Trans. 2007, 48, 1626–1630. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E.; Sheng, H.W. Atomic level structure in multicomponent bulk metallic glass. Phys. Rev. Lett. 2009, 102, 245501. [Google Scholar] [CrossRef] [PubMed]
- Song, K.K.; Pauly, S.; Zhang, Y.; Li, R.; Gorantla, S.; Narayanan, N.; Kuhn, U.; Gemming, T.; Eckert, J. Triple yielding and deformation mechanisms in metastable Cu47.5Zr47.5Al5 composites. Acta Mater. 2012, 60, 6000–6012. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Eckert, J.; Löser, W.; Dhindaw, B.K.; Schultz, L. Cooling rate evaluation for bulk amorphous alloys from eutectic microstructures in casting processes. Mater. Trans. 2002, 43, 1670–1675. [Google Scholar] [CrossRef]
- Ju, D.Y.; Zhang, W.M.; Zhang, Y. Modeling and experimental verification of martensitic transformation plastic behavior in carbon steel for quenching process. Mater. Sci. Eng. A 2006, 438–440, 246–250. [Google Scholar] [CrossRef]
- Nan, C.W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 1993, 37, 1–116. [Google Scholar] [CrossRef]
- Fu, X.L.; Li, Y.; Schuh, C.A. Mechanical properties of metallic glass matrix composites: Effects of reinforcement character and connectivity. Scr. Mater. 2007, 56, 617–620. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Andreeta, M.R.B. Crystallization: Science and Technology, 1st ed.; Intech: Rijeka, Croatia, 2012. [Google Scholar]
- Avrami, M. Kinetics of phase change. I general theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Johnson, W.A.; Mehl, R.F. Reaction kinetics in processes of nucleation and growth. Trans. Am. Inst. Min. Metall. Pet. Eng. 1939, 135, 416–442. [Google Scholar]
- Pauly, S.; Das, J.; Mattern, N.; Kim, D.H.; Eckert, J. Phase formation and thermal stability in Cu-Zr-Ti(Al) metallic glasses. Intermetallics 2009, 17, 453–462. [Google Scholar] [CrossRef]
- Xie, G.; Louzguine-Luzgin, D.V.; Zhang, Q.; Zhang, W.; Inoue, A. Structure and crystallization kinetics of a Cu50Zr45Ti5 glassy alloy. J. Alloy. Compd. 2009, 483, 24–27. [Google Scholar] [CrossRef]
- Qiao, J.C.; Pelletier, J.M. Crystallization kinetics in Cu46Zr45Al7Y2 bulk metallic glass by differential scanning calorimetry (DSC). J. Non Cryst. Solids 2011, 357, 2590–2594. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.H.; Qiu, K.Q.; Zhang, H.F.; Quan, M.X.; Hu, Z.Q. Crystallization kinetics and pressure effect on crystallization of Zr55Al10Ni5Cu30 bulk metallic glass. Mater. Sci. Eng. A 2003, 357, 386–391. [Google Scholar] [CrossRef]
- Park, S.O.; Lee, J.C.; Kim, Y.C.; Fleury, E.; Sung, D.S.; Kim, D.H. Crystallization kinetics of the Cu43Zr43Al7Ag7 amorphous alloy. Mater. Sci. Eng. A 2007, 449–451, 561–564. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformation in Metals and Alloys, 1st ed.; Pergamon Press: Oxford, UK, 1965. [Google Scholar]
- Martin, J.W.; Doherty, R.D.; Cantor, B. Stability of Microstructure in Metallic Systems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
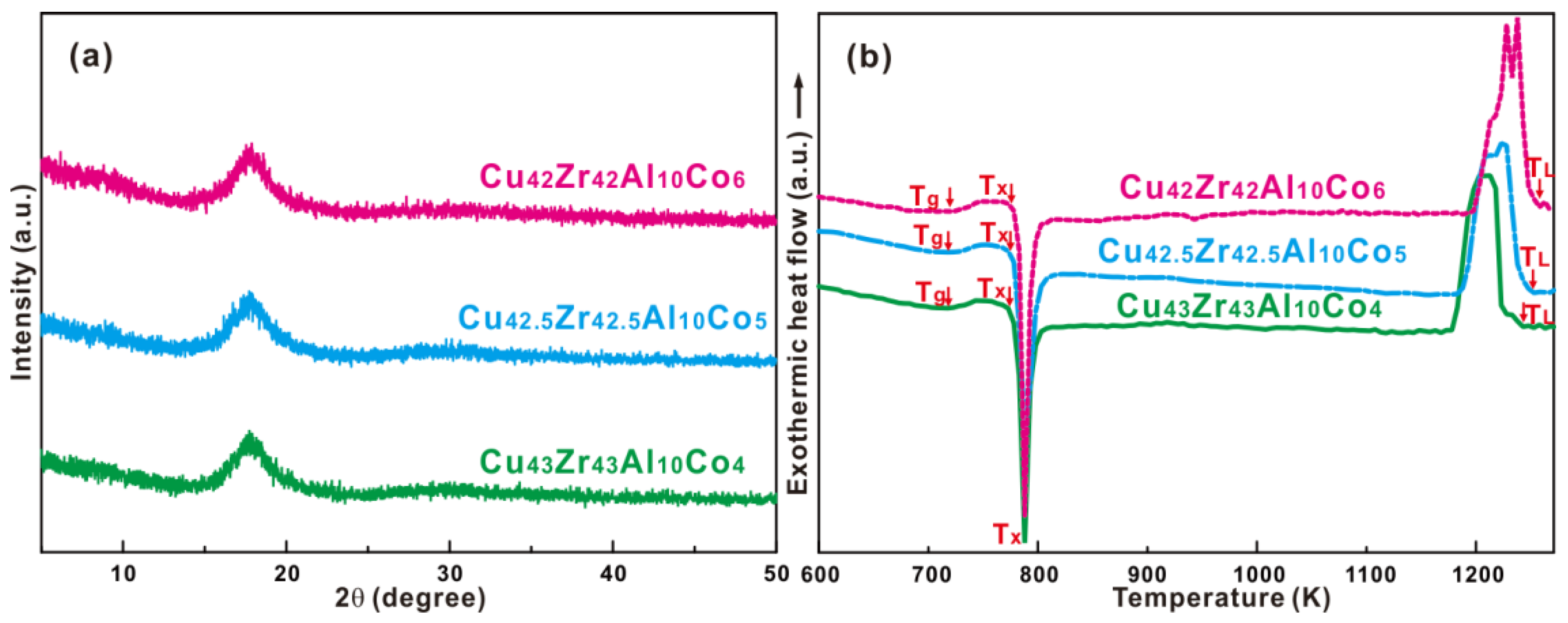

| Compositions | Tg (K) | Tx (K) | ΔTrg | TL (K) | γ |
|---|---|---|---|---|---|
| (Cu0.5Zr0.5)86Al10Co4 | 725 ± 2 | 783 ± 2 | 58 ± 4 | 1239 ± 5 | 0.399 ± 0.003 |
| (Cu0.5Zr0.5)85Al10Co5 | 726 ± 2 | 782 ± 2 | 56 ± 4 | 1241 ± 5 | 0.398 ± 0.003 |
| (Cu0.5Zr0.5)84Al10Co6 | 733 ± 2 | 789 ± 2 | 57 ± 4 | 1249 ± 5 | 0.398 ± 0.003 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Qin, Y.; Qin, K.; Li, X.; Wang, S.; Mi, J.; Song, K.; Wang, L. Glass-Forming Ability and Early Crystallization Kinetics of Novel Cu-Zr-Al-Co Bulk Metallic Glasses. Metals 2016, 6, 225. https://doi.org/10.3390/met6090225
Han X, Qin Y, Qin K, Li X, Wang S, Mi J, Song K, Wang L. Glass-Forming Ability and Early Crystallization Kinetics of Novel Cu-Zr-Al-Co Bulk Metallic Glasses. Metals. 2016; 6(9):225. https://doi.org/10.3390/met6090225
Chicago/Turabian StyleHan, Xiaoliang, Yusheng Qin, Kai Qin, Xuelian Li, Shenghai Wang, Jun Mi, Kaikai Song, and Li Wang. 2016. "Glass-Forming Ability and Early Crystallization Kinetics of Novel Cu-Zr-Al-Co Bulk Metallic Glasses" Metals 6, no. 9: 225. https://doi.org/10.3390/met6090225
APA StyleHan, X., Qin, Y., Qin, K., Li, X., Wang, S., Mi, J., Song, K., & Wang, L. (2016). Glass-Forming Ability and Early Crystallization Kinetics of Novel Cu-Zr-Al-Co Bulk Metallic Glasses. Metals, 6(9), 225. https://doi.org/10.3390/met6090225