Effects of Carboxylates on the Performance of Zn Electrode
Abstract
:1. Introduction
2. Materials
2.1. Preparation of Aqueous Electrolyte Solution
2.2. Preparation of Electrodes
2.3. Characterization
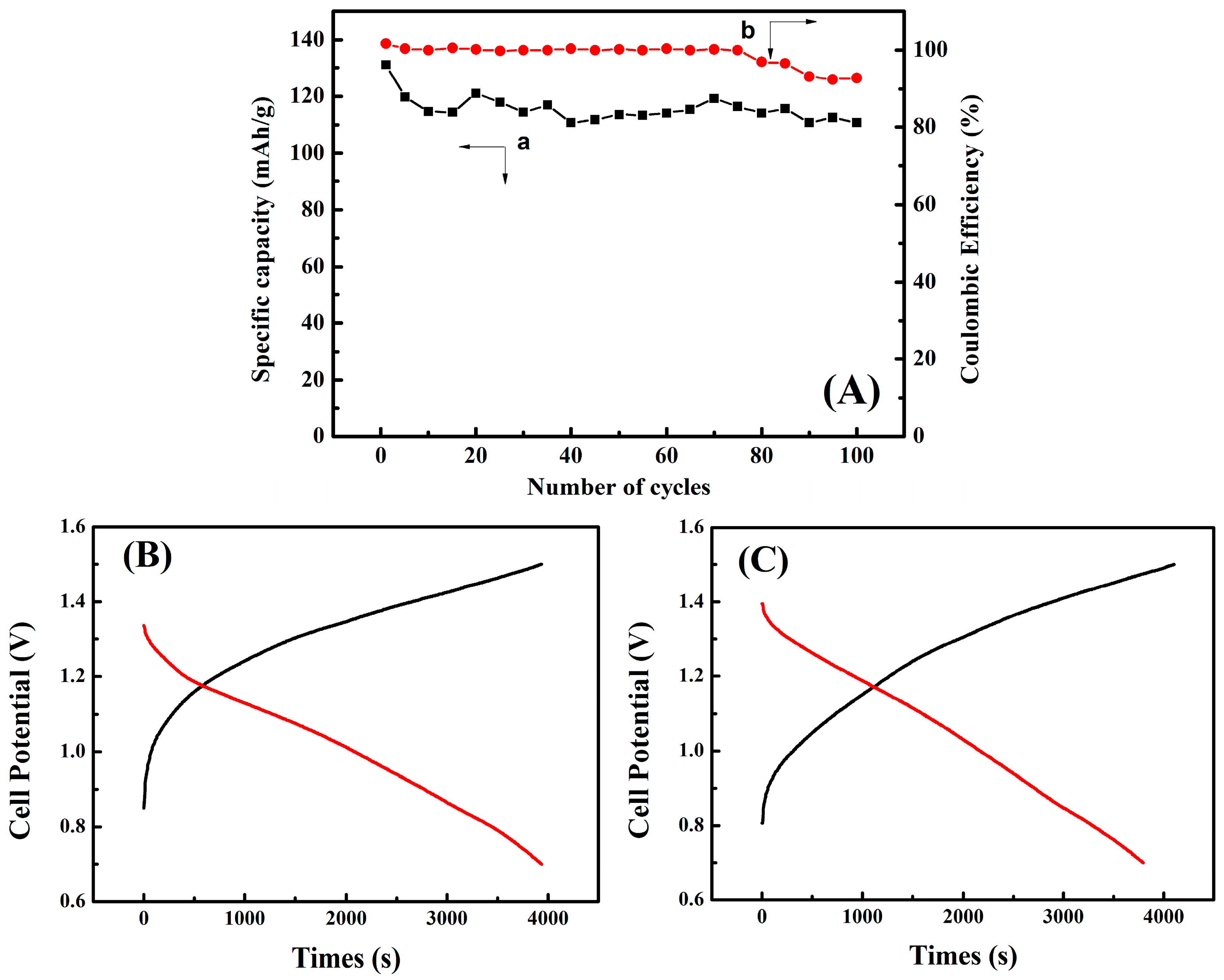
3. Results and Discussion
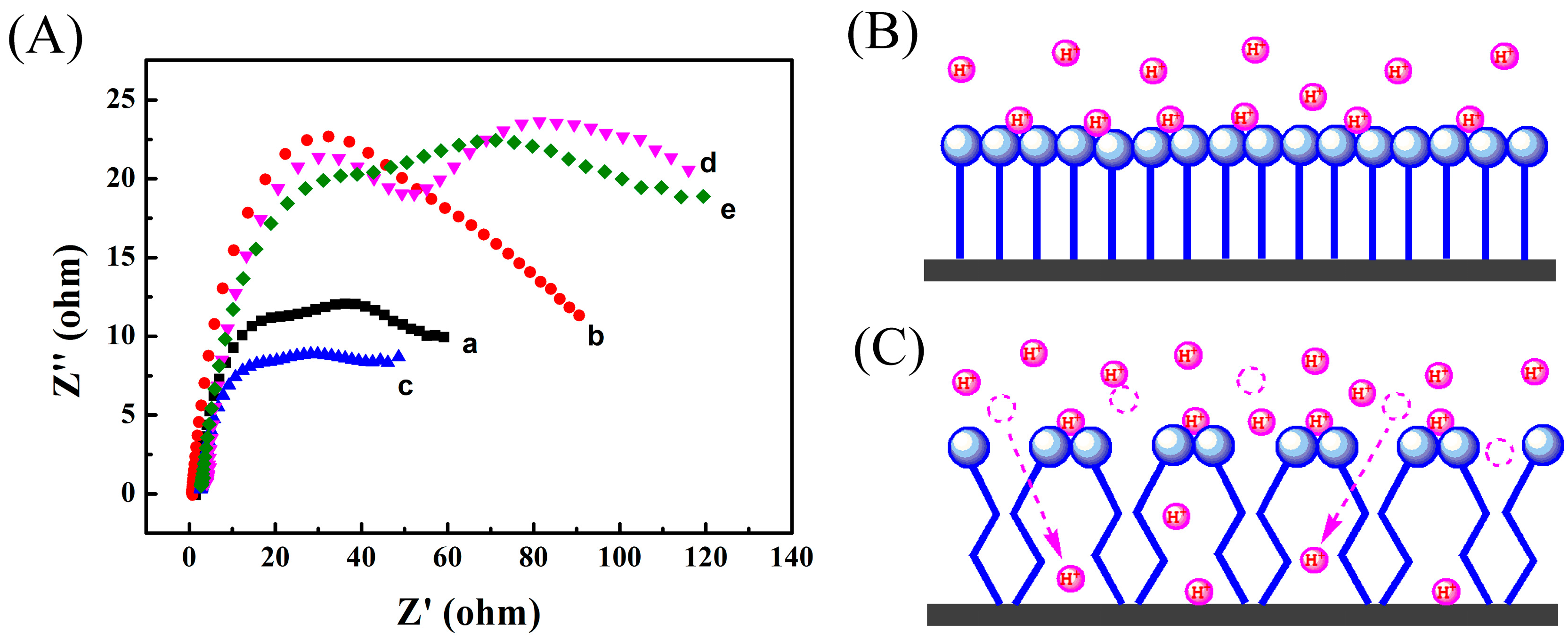
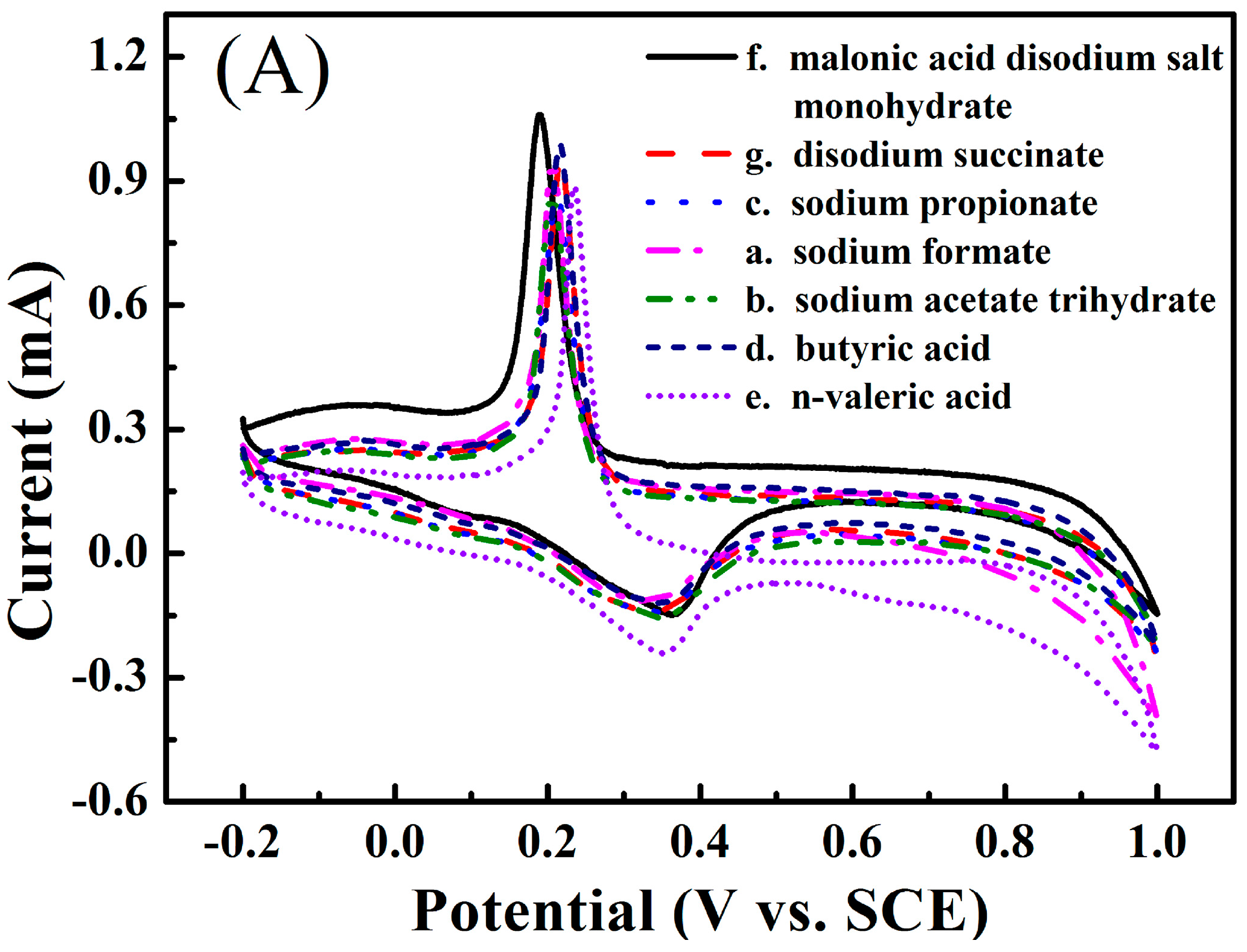
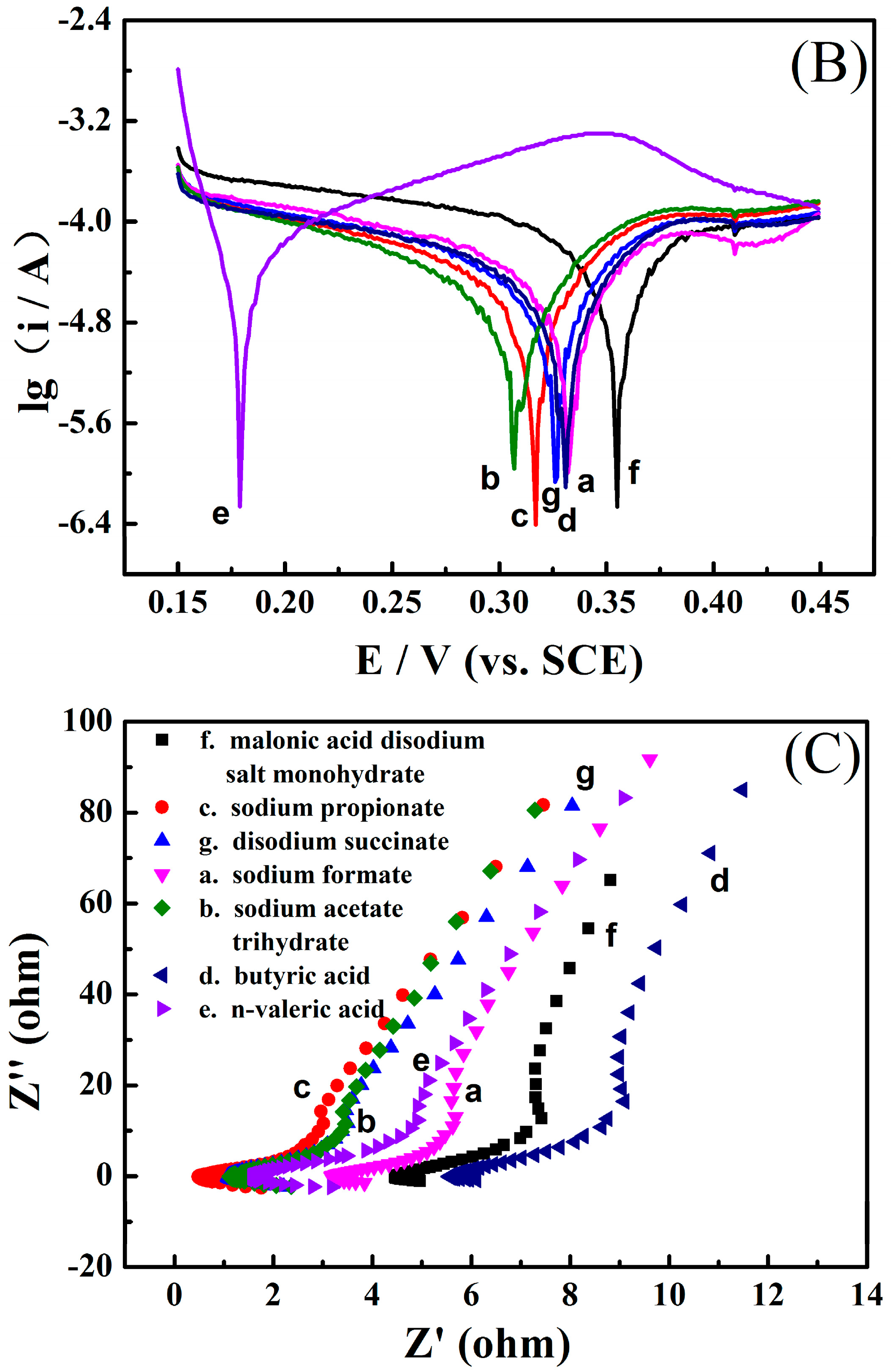
3.1. Electrochemical Performance of the Zinc Electrode in the Aqueous Electrolyte Containing Different Monocarboxylates
3.2. Electrochemical Performance of the Zinc Electrode in the Aqueous Electrolyte Containing Different Dicarboxylates
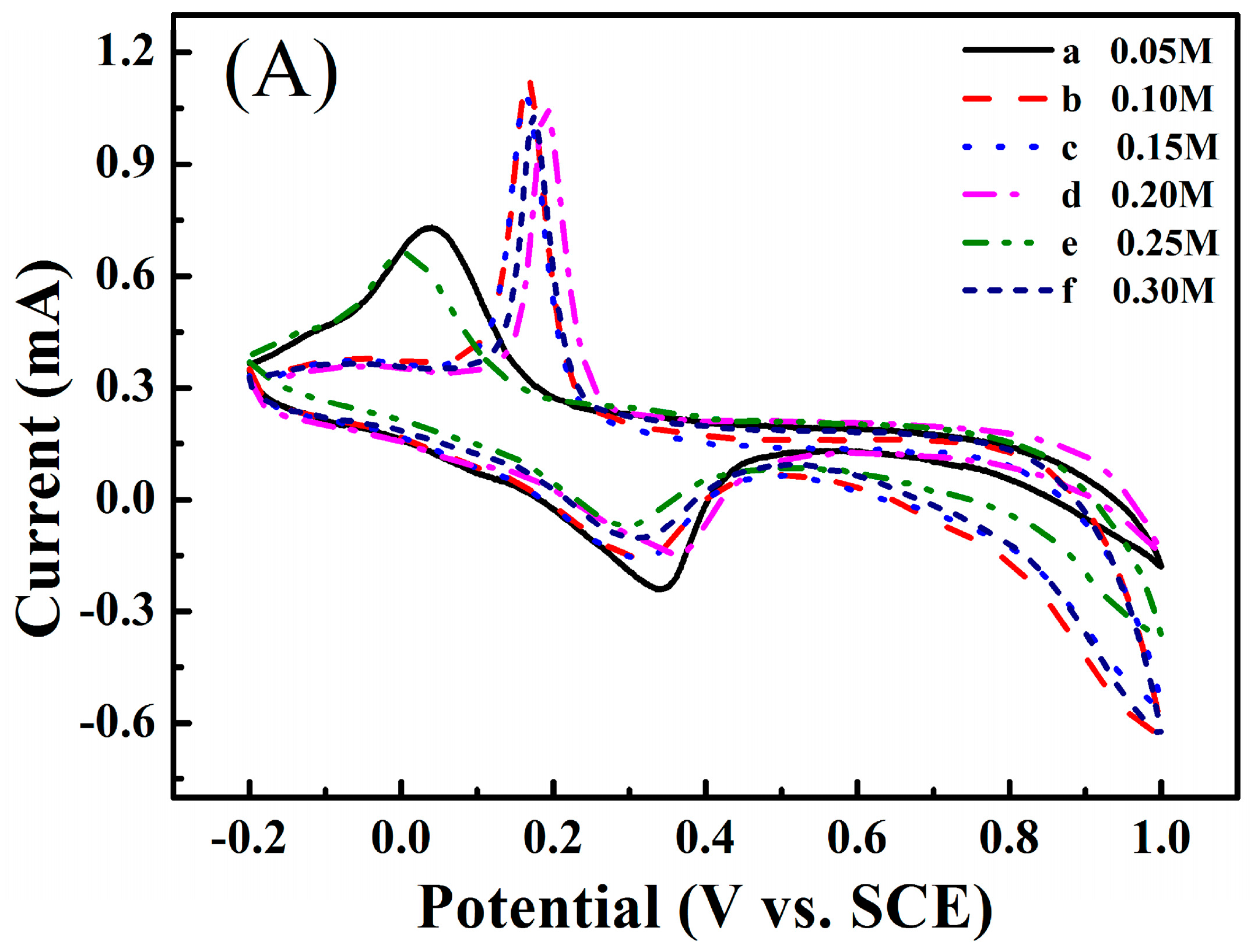
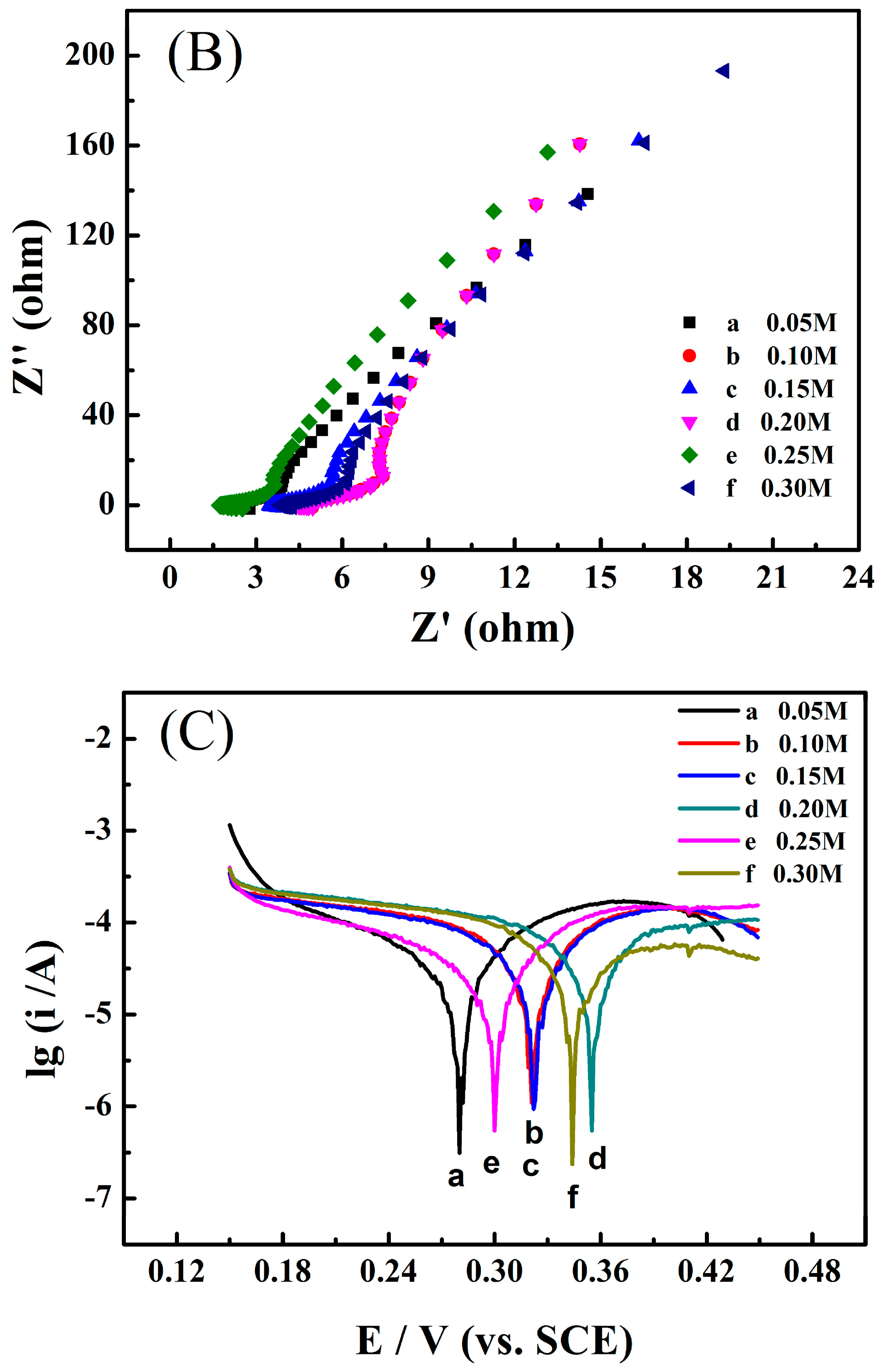
3.3. Electrochemical Performance of the Zinc Electrode in the Aqueous Electrolyte Containing Different Concentrations Sodium Propionate or Disodium Malonate
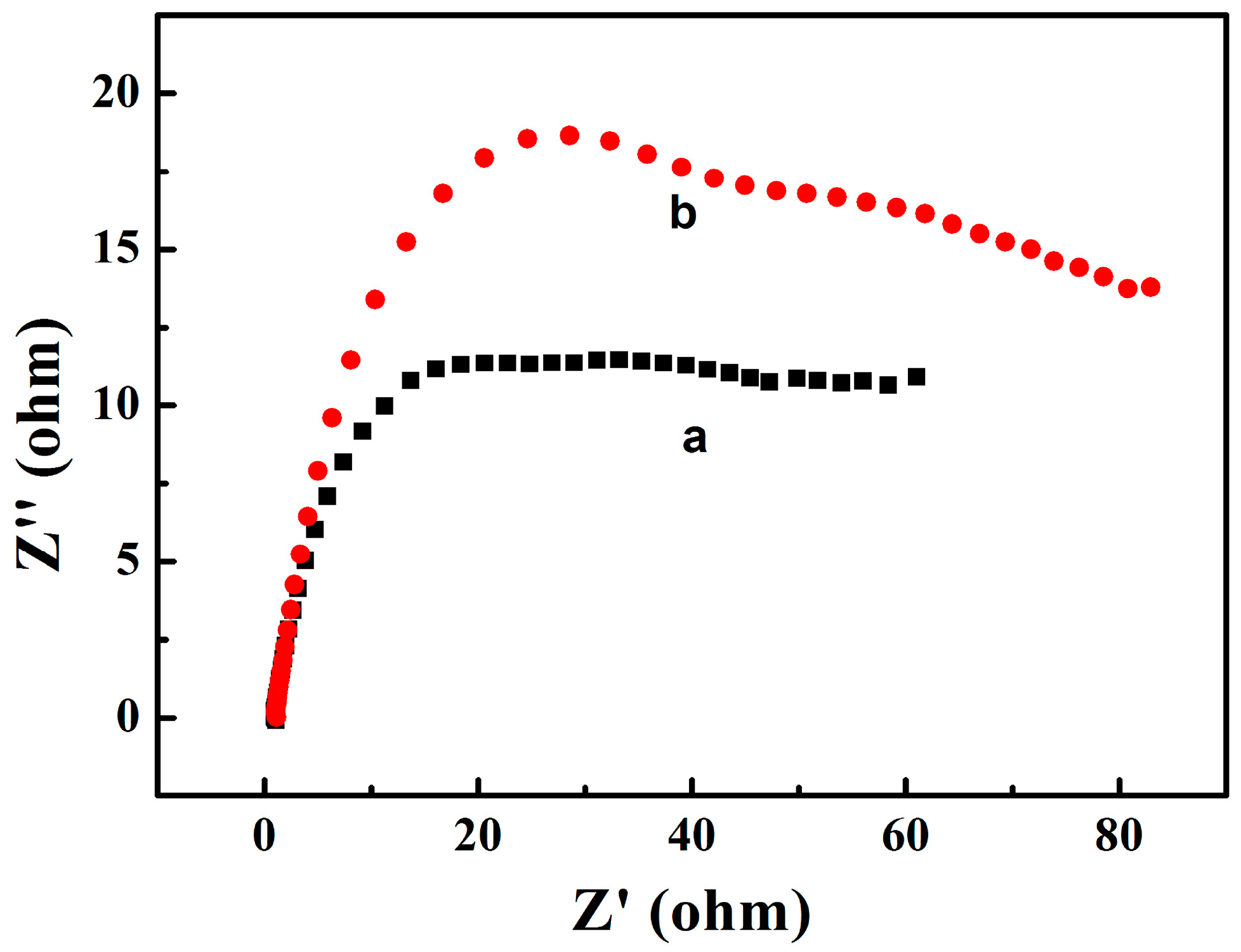
3.4. Comparing the Electrochemical Performance of the Zinc Electrodes in the Aqueous Electrolyte Containing 0.1 M Sodium Propionate or 0.2 M Disodium Malonate
3.5. Studying on the Corrosion Performance of the Zinc Sheet by SEM in the Aqueous Electrolyte Containing 0.2 M Disodium Malonate
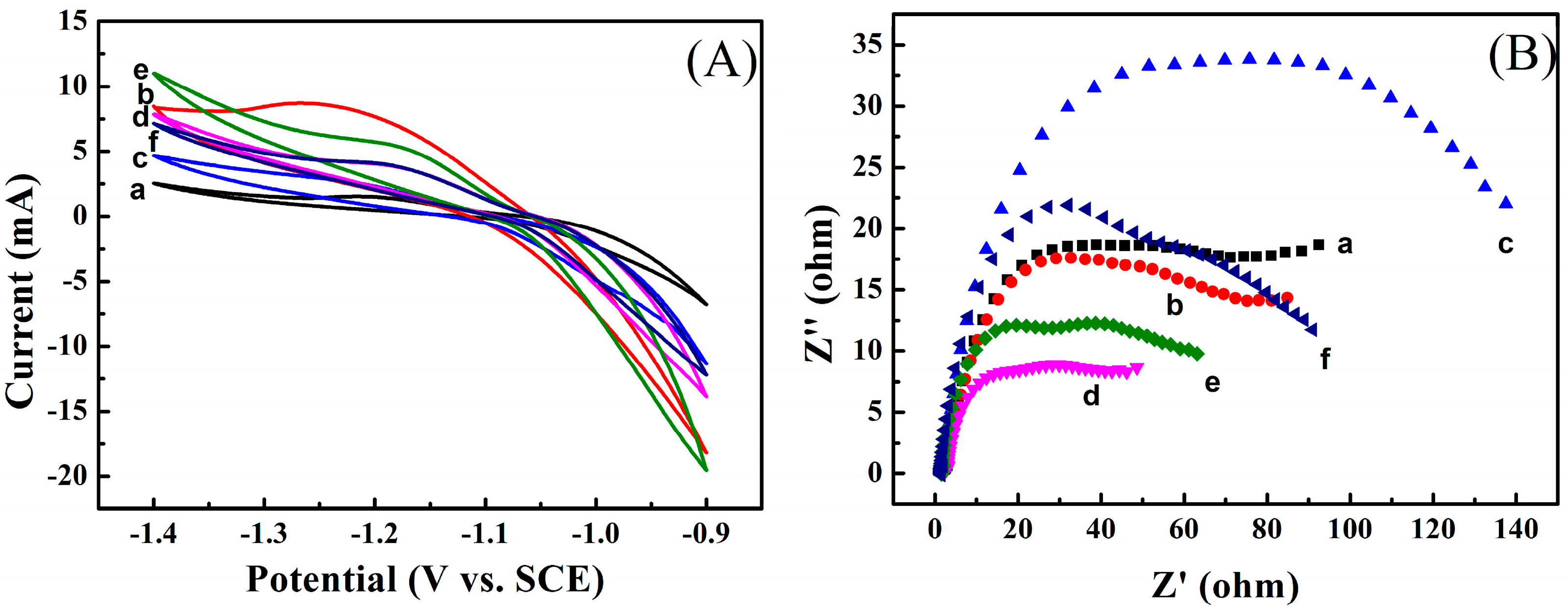
3.6. Effect of Carboxylates on Properties of PANI
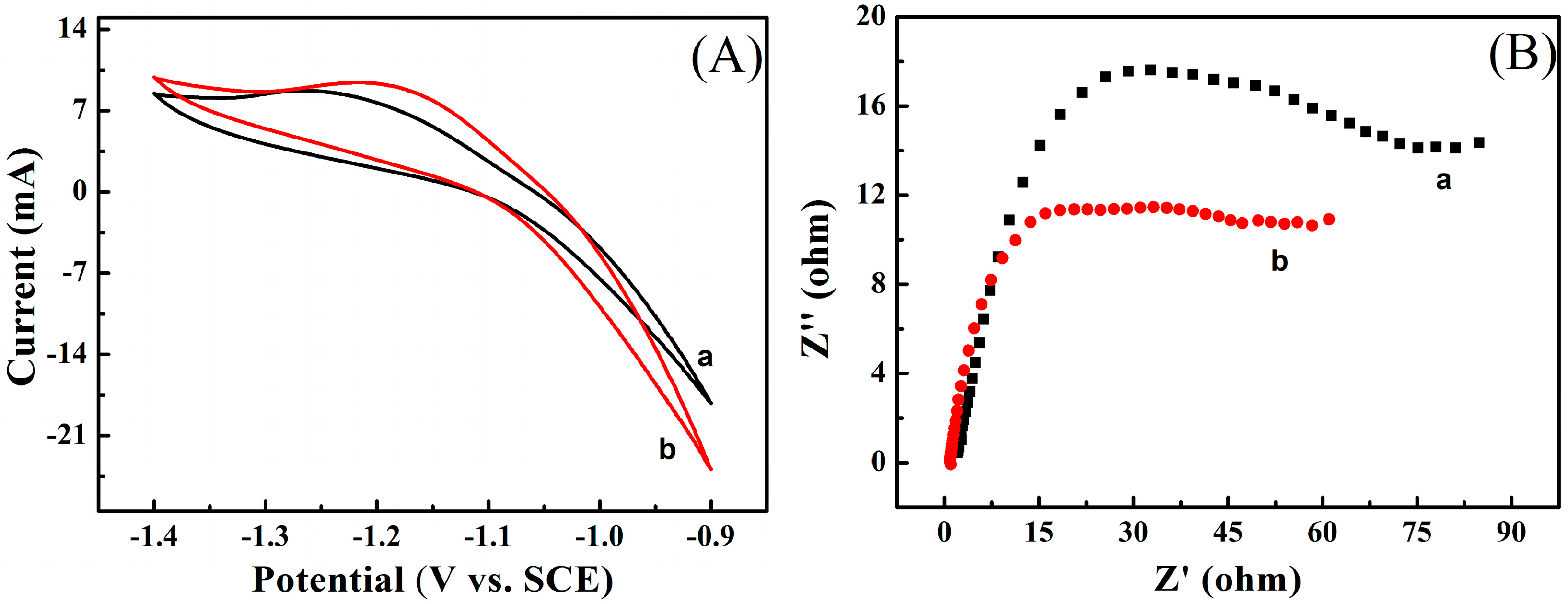
3.7. Charge-Discharge Performance Testing of Zn/PANI Secondary Battery
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ollig, J.; Kloubert, V.; Weßels, I.; Haase, H.; Rink, L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals 2016, 6, 71. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, O.; Yoo, K.; Alorro, R.D. Removal of Zn from Contaminated Sediment by FeCl3 in HCl Solution. Metals 2015, 5, 1812–1820. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, C.; Fan, S. A new type of secondary hybrid battery showing excellent performances. Nano Energy 2015, 12, 486–493. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Guo, R. Reactive polyaniline-supported sub-10 nm noble metal nanoparticles protected by a mesoporous silica shell: Controllable synthesis and application as efficient recyclable catalysts. J. Mater. Chem. 2012, 22, 5932–5935. [Google Scholar] [CrossRef]
- Li, S.; Tan, Y.; Wang, P.; Kan, J. Inhibition of benzoic acid on the polyaniline-polyphenol oxidase biosensor. Sens. Actuators B Chem. 2010, 144, 18–22. [Google Scholar] [CrossRef]
- Chen, C.; Hong, X.; Chen, A.; Xu, T.; Lu, L.; Lin, S.; Gao, Y. Electrochemical properties of poly (aniline-co-N-methylthionine) for zinc-conducting polymer rechargeable batteries. Electrochim. Acta 2016, 190, 240–247. [Google Scholar] [CrossRef]
- Mak, W.F.; Wee, G.; Aravindan, V.; Gupta, N.; Mhaisalkar, S.G.; Madhavi, S. High-energy density asymmetric supercapacitor based on electrospun vanadium pentoxide and polyaniline nanofibers in aqueous electrolyte. J. Electrochem. Soc. 2012, 159, A1481–A1488. [Google Scholar] [CrossRef]
- Dalui, B.C.; Basumallick, I.N.; Ghosh, S. Zinc-poly (aniline) rechargeable battery assembled with aqueous electrolyte. Indian J. Chem. Tech. 2008, 15, 576–580. [Google Scholar]
- Li, Y.; Hu, Z.; Ding, Y.; Kan, J. Characteristics of the electrolyte containing ethylene carbonate and dimethyl carbonate in zinc-polyaniline battery. Int. J. Electrochem. Sci. 2016, 11, 1898–1906. [Google Scholar]
- Guerfi, A.; Trottier, J.; Boyano, I.; de Meatza, I.; Blazquez, J.A.; Brewer, S.; Zaghib, K. High cycling stability of zinc-anode/conducting polymer rechargeable battery with non-aqueous electrolyte. J. Power Sources 2014, 248, 1099–1104. [Google Scholar] [CrossRef]
- Fusalba, F.; Gouérec, P.; Villers, D.; Bélanger, D. Electrochemical characterization of polyaniline in nonaqueous electrolyte and its evaluation as electrode material for electrochemical supercapacitors. J. Electrochem. Soc. 2001, 148, A1–A6. [Google Scholar] [CrossRef]
- Varela, H.; Torresi, R.M. Ionic exchange phenomena related to the redox processes of polyaniline in nonaqueous media. J. Electrochem. Soc. 2000, 147, 665–670. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, S.; Liao, C. A single flow zinc//polyaniline suspension rechargeable battery. J. Power Sources 2013, 241, 449–453. [Google Scholar] [CrossRef]
- Ghanbari, K.; Mousavi, M.F.; Shamsipur, M.; Karami, H. Synthesis of polyaniline/graphite composite as a cathode of Zn-polyaniline rechargeable battery. J. Power Sources 2007, 170, 513–519. [Google Scholar] [CrossRef]
- Rahmanifar, M.S.; Mousavi, M.F.; Shamsipur, M.; Heli, H. A study on open circuit voltage reduction as a main drawback of Zn-polyaniline rechargeable batteries. Synth. Met. 2005, 155, 480–484. [Google Scholar] [CrossRef]
- Rahmanifar, M.S.; Mousavi, M.F.; Shamsipur, M.; Ghaemi, M. What is the limiting factor of the cycle-life of Zn-polyaniline rechargeable batteries? J. Power Sources 2004, 132, 296–301. [Google Scholar] [CrossRef]
- Sima, M.; Visan, T.; Buda, M. A comparative study of zinc-polyaniline electrochemical cells having sulfate and chloride electrolytes. J. Power Sources 1995, 56, 133–136. [Google Scholar] [CrossRef]
- Liu, L.; Tian, F.; Zhou, M.; Guo, H.; Wang, X. Aqueous rechargeable lithium battery based on polyaniline and LiMn2O4 with good cycling performance. Electrochim. Acta 2012, 70, 360–364. [Google Scholar] [CrossRef]
- Manuel, J.; Kim, J.K.; Matic, A.; Jacobsson, P.; Chauhan, G.S.; Ha, J.K.; Ahn, J.H. Electrochemical properties of lithium polymer batteries with doped polyaniline as cathode material. Mater. Res. Bull. 2012, 47, 2815–2818. [Google Scholar] [CrossRef]
- Rehan, H.H. A new polymer/polymer rechargeable battery: Polyaniline/LiClO4 (MeCN)/poly-1-naphthol. J. Power Sources 2003, 113, 57–61. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Epstein, A.J. Polyanilines: A novel class of conducting polymers. Faraday Discuss. Chem. Soc. 1989, 88, 317–332. [Google Scholar] [CrossRef]
- Anand, J.; Palaniappan, S.; Sathyanarayana, D.N. Conducting polyaniline blends and composites. Prog. Polym. Sci. 1998, 23, 993–1018. [Google Scholar] [CrossRef]
- Rüetschi, P. Energy storage and the environment: The role of battery technology. J. Power Sources 1993, 42, 1–7. [Google Scholar] [CrossRef]
- Beck, F.; Rüetschi, P. Rechargeable batteries with aqueous electrolytes. Electrochim. Acta 2000, 45, 2467–2482. [Google Scholar] [CrossRef]
- Kitani, A.; Kaya, M.; Sasaki, K. Performance study of aqueous polyaniline batteries. J. Electrochem. Soc. 1986, 133, 1069–1073. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, Z.; Kan, J. Effects of Carboxylates on the Performance of Zn Electrode. Metals 2016, 6, 176. https://doi.org/10.3390/met6080176
Li Y, Hu Z, Kan J. Effects of Carboxylates on the Performance of Zn Electrode. Metals. 2016; 6(8):176. https://doi.org/10.3390/met6080176
Chicago/Turabian StyleLi, Yongli, Zhuan Hu, and Jinqing Kan. 2016. "Effects of Carboxylates on the Performance of Zn Electrode" Metals 6, no. 8: 176. https://doi.org/10.3390/met6080176





