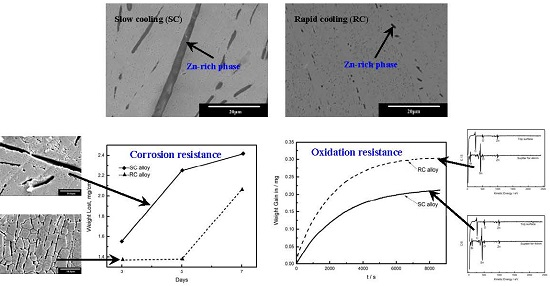
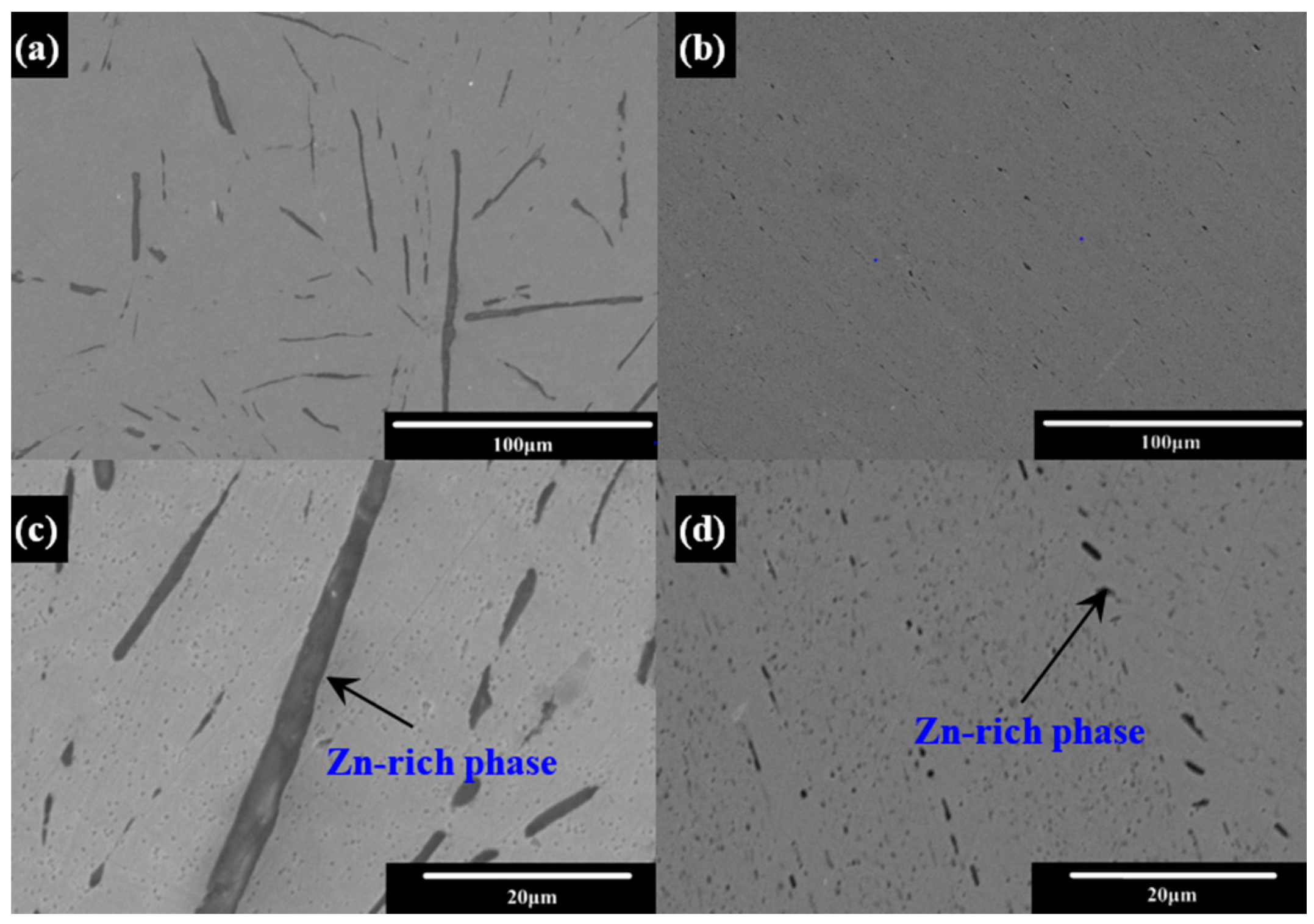
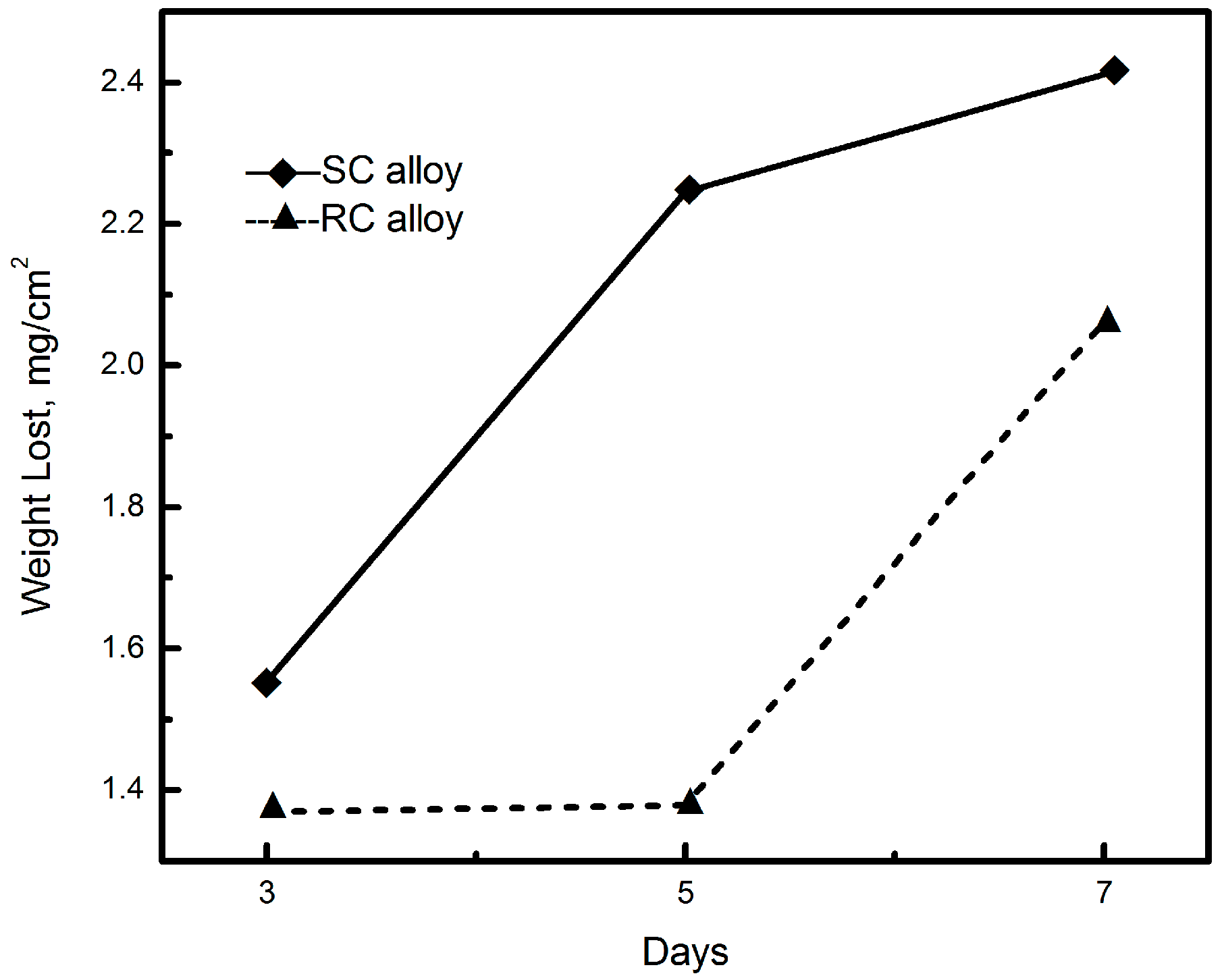
Correlation between Zn-Rich Phase and Corrosion/Oxidation Behavior of Sn–8Zn–3Bi Alloy
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tu, K.N.; Zeng, K. Tin-lead (SnPb) solder reaction in flip chip technology. Mater. Sci. Eng. R 2001, 34, 1–58. [Google Scholar] [CrossRef]
- Wood, E.P.; Nimmo, K.L. In search of new lead-free electronic solders. J. Electron. Mater. 1994, 23, 709–713. [Google Scholar] [CrossRef]
- Guang, R.; Wilding, I.J.; Collins, M.N. Alloying influences on low melt temperature SnZn and SnBi solder alloys for electronic interconnections. J. Alloy. Compd. 2016, 665, 251–260. [Google Scholar]
- Fawzy, A. Effect of Zn addition, strain rate and deformation temperature on the tensile properties of Sn–3.3 wt.% Ag solder alloy. Mater. Charact. 2007, 58, 323–331. [Google Scholar] [CrossRef]
- Pereira, P.D.; Spinelli, J.E.; Garcia, A. Combined effects of Ag content and cooling rate on microstructure and mechanical behavior of Sn–Ag–Cu solders. Mater. Des. 2013, 45, 377–383. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Desoky, W.M.; Saad, A.F.; Mansor, N.A.; Lotfy, E.H.; Abd-Elmoniem, H.M.; Hashem, H. The effect of undercooling on the microstructure and tensile properties of hypoeutectic Sn–6.5Zn–xCu Pb-free solders. Mater. Des. 2015, 80, 152–162. [Google Scholar] [CrossRef]
- Osório, W.R.; Peixoto, L.C.; Garcia, L.R.; Noël, N.M.; Garcia, A. Microstructure and mechanical properties of Sn–Bi, Sn–Ag and Sn–Zn lead-free solder. J. Alloy. Compd. 2013, 572, 97–106. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhang, G.; Wang, Z.H.; Ma, J.S.; Suganuma, K. Thermal property, wettability and interfacial characterization of novel Sn–Zn–Bi–In alloys as low-temperature lead-free solders. Mater. Des. 2015, 84, 331–339. [Google Scholar] [CrossRef]
- Spinelli, J.E.; Silva, B.L.; Garcia, A. Assessment of tertiary dendritic growth and its effects on mechanical properties of directionally solidified Sn-0.7Cu-xAg solder alloys. J. Electron. Mater. 2014, 43, 1347–1361. [Google Scholar] [CrossRef]
- Silva, B.L.; Cheung, N.; Garcia, A.; Spinelli, J.E. Sn–0.7 wt%Cu–(xNi) alloys: Microstructure–mechanical properties correlations with solder/substrate interfacial heat transfer coefficient. J. Alloy. Compd. 2015, 632, 274–285. [Google Scholar] [CrossRef]
- Garcia, L.R.; Osório, W.R.; Peixoto, L.C.; Garcia, A. Mechanical properties of Sn–Zn lead-free solder alloys based on the microstructure array. Mater. Charact. 2010, 61, 212–220. [Google Scholar] [CrossRef]
- Lin, S.; Xue, S.B.; Xue, P.; Luo, D.X. Present status of Sn–Zn lead-free solders bearing alloying elements. J. Mater. Sci. Mater. Electron. 2015, 26, 4389–4411. [Google Scholar]
- El-Daly, A.A.; Hammad, A.E.; Al-Ganainy, G.S.; Ibrahiem, A.A. Design of lead-free candidate alloys for low-temperature soldering applications based on the hypoeutectic Sn–6.5Zn alloy. Mater. Des. 2014, 56, 594–603. [Google Scholar] [CrossRef]
- Garcia, L.R.; Peixoto, L.C.; Osório, W.R.; Garcia, A. Globular to needle Zn-rich phase transition during transient solidification of a eutectic Sn–9% Zn solder alloy. Mater. Lett. 2009, 63, 1314–1316. [Google Scholar] [CrossRef]
- Garcia, L.R.; Osório, W.R.; Peixoto, L.C.; Garcia, A. Wetting behavior and mechanical properties of Sn-Zn and Sn-Pb solder alloys. J. Electron. Mater. 2009, 38, 2405–2414. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.S.; Xue, F. Properties of low melting point Sn–Zn–Bi solders. J. Alloy. Compd. 2005, 397, 260–264. [Google Scholar] [CrossRef]
- Song, J.M.; Wu, Z.M. Variable eutectic temperature caused by inhomogeneous solute distribution in Sn–Zn system. Scr. Mater. 2006, 54, 1479–1483. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Liu, N.S.; Lin, K.L. Effect of Ga on the Oxidation properties of Sn–8.5Zn–0.5Ag–0.1Al–xGa solders. Oxid. Met. 2012, 78, 285–294. [Google Scholar] [CrossRef]
- Kim, S.H.; Hui, K.N.; Kim, Y.J.; Lim, T.S.; Yang, D.Y.; Kim, K.B.; Kim, Y.J.; Yang, S. Oxidation resistant effects of Ag2S in Sn–Ag–Al solder: A mechanismfor higher electrical conductivity and less whisker growth. Corros. Sci. 2016, 105, 25–35. [Google Scholar] [CrossRef]
- Nazeri, M.F.M.; Mohamad, A.A. Corrosion resistance of ternary Sn–9Zn–xIn solder joint in alkaline solution. J. Alloy. Compd. 2016, 661, 516–525. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.W.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl− anions in pitting corrosion of Sn–Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Burstein, G.; Liu, C.; Souto, R.; Vines, S. Origins of pitting corrosion. Corros. Eng. Sci. Technol. 2004, 39, 25–30. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhang, G.; Ma, J.S.; Suganuma, K. Ti addition to enhance corrosion resistance of Sn–Zn solder alloy by tailoring microstructure. J. Alloy. Compd. 2015, 644, 113–118. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances; VCH: New York, NY, USA, 1995; Volume 2, p. 1549. [Google Scholar]
- Lee, J.E.; Kim, K.S.; Inoue, M.; Jiang, J.X.; Suganuma, K. Effects of Ag and Cu addition on microstructural properties and oxidation resistance of Sn–Zn eutectic alloy. J. Alloy. Compd. 2008, 454, 310–320. [Google Scholar] [CrossRef]
- Jiang, J.X.; Lee, J.E.; Kim, K.S.; Suganuma, K. Oxidation behavior of Sn–Zn solders under high-temperature and high-humidity conditions. J. Alloy. Compd. 2008, 462, 244–251. [Google Scholar] [CrossRef]
- Mrowec, S. Defects and Diffusion in Solids: An Introduction; Elsevier Scientific: New York, NY, USA, 1980. [Google Scholar]
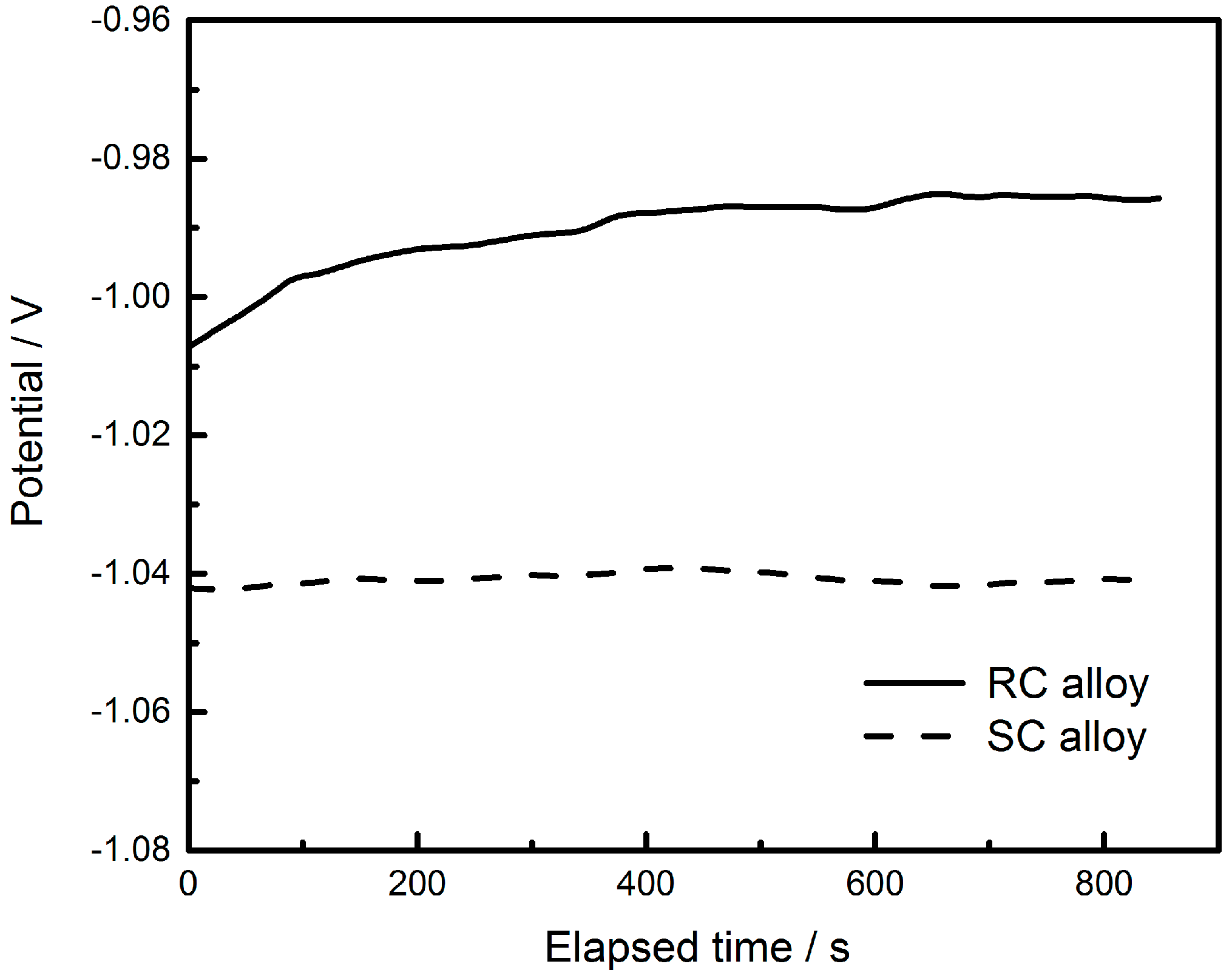
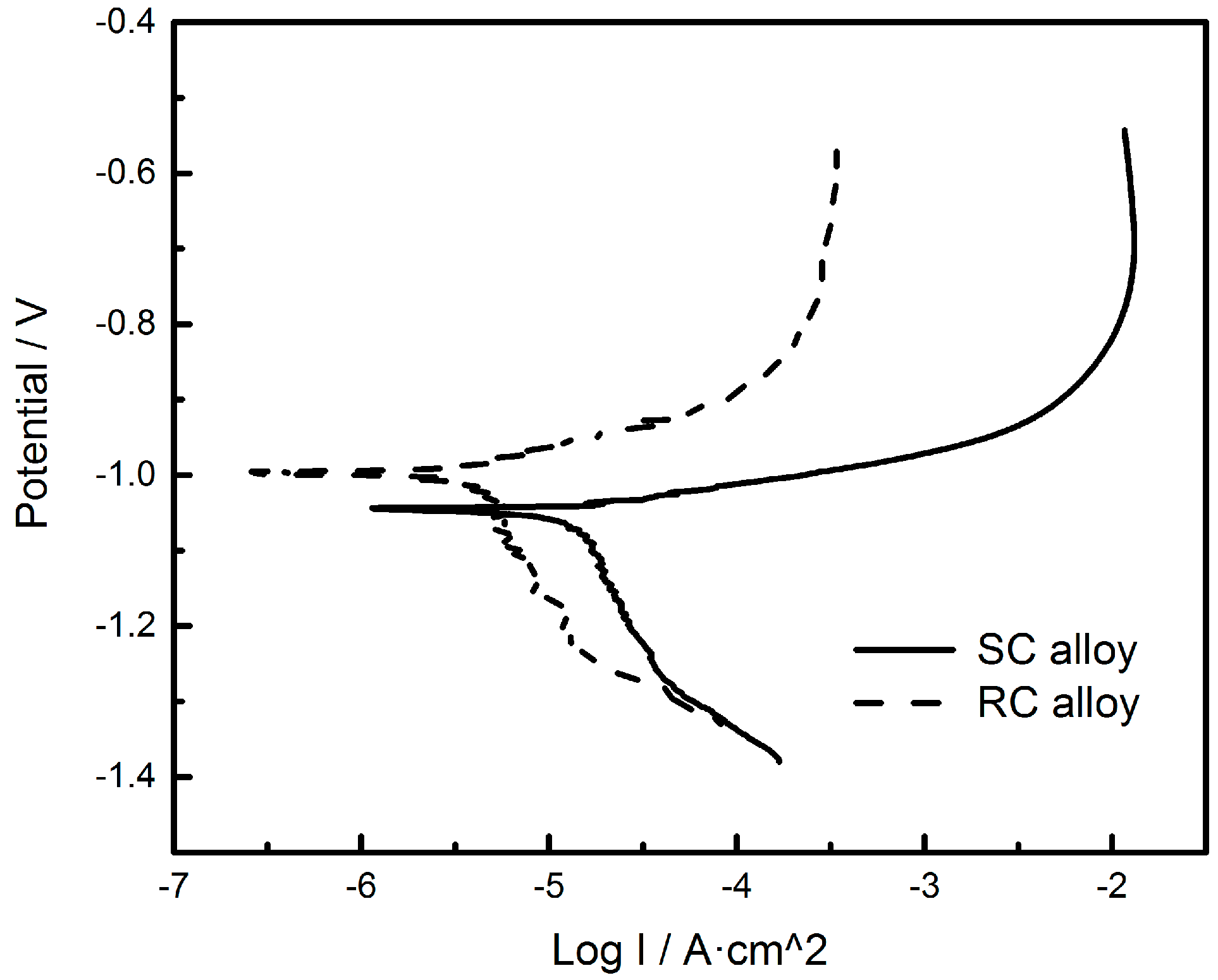
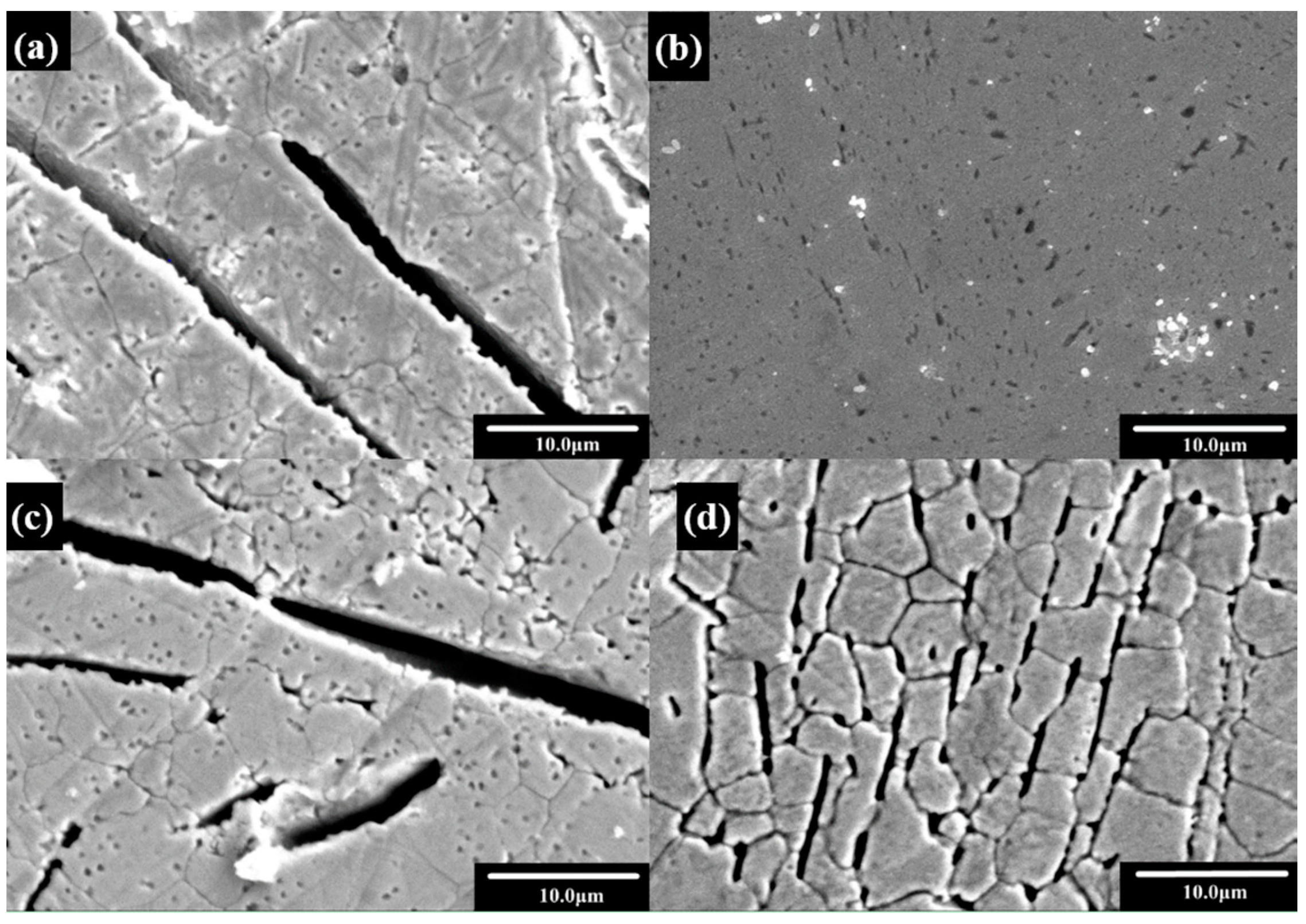
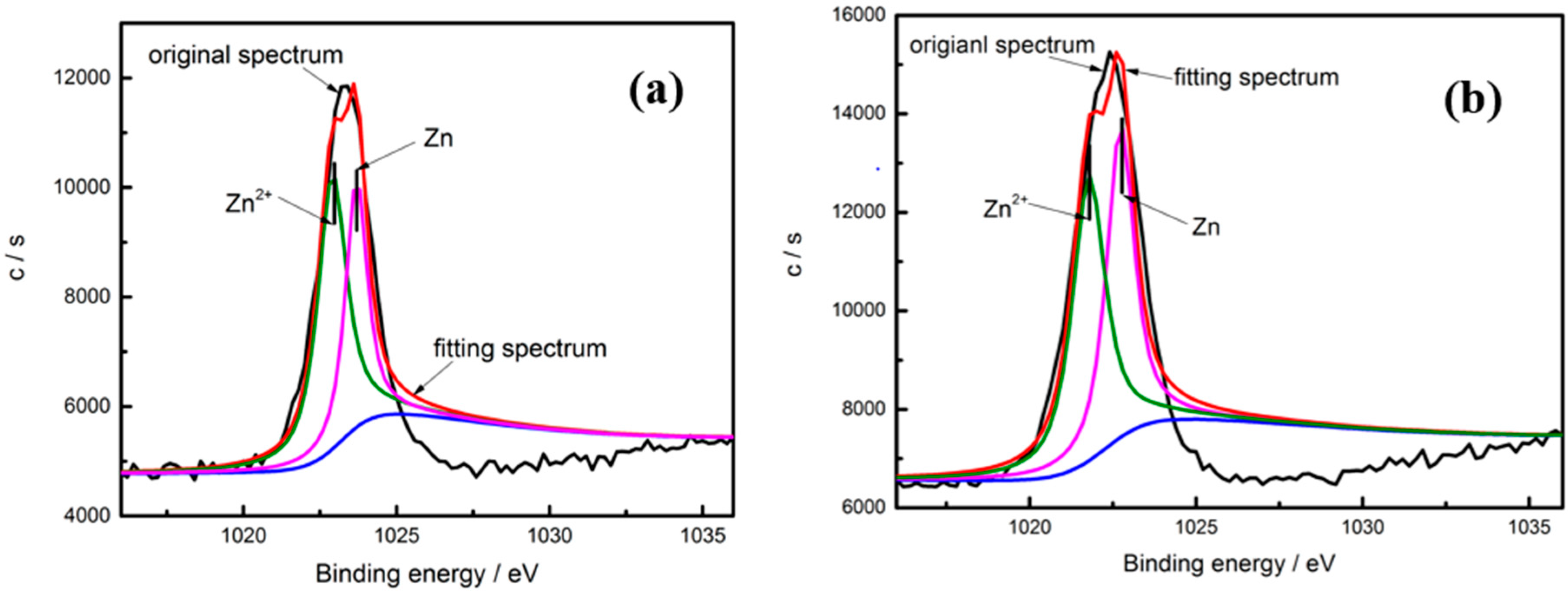


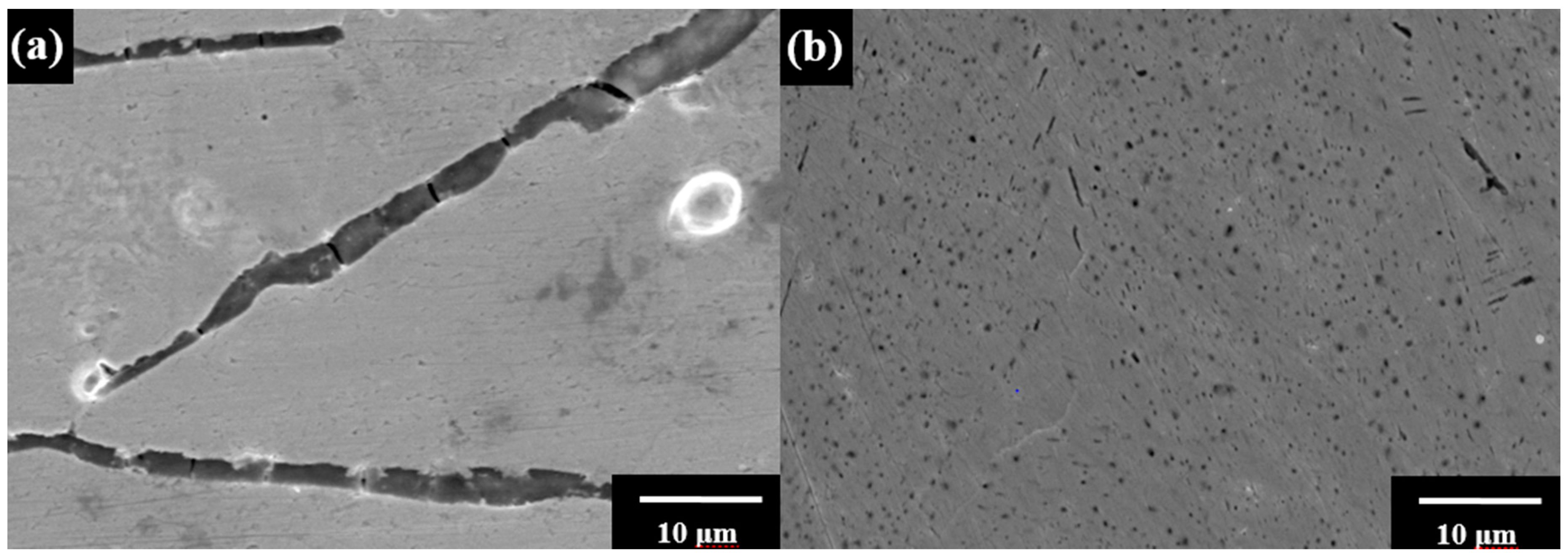
| Sample | Ecorr vs. SCE/ (V) | Icorr (A·cm−2) | Rp/(Ω·cm2) | OCP/(V) |
|---|---|---|---|---|
| SC | −1.047 | 1.298 × 10−5 | 1490.5 | −1.042 |
| RC | −0.999 | 3.907 × 10−6 | 9832.9 | −0.985 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, C.; Gao, Z.; Liu, Y.; Ma, Z.; Yu, L.; Li, H. Correlation between Zn-Rich Phase and Corrosion/Oxidation Behavior of Sn–8Zn–3Bi Alloy. Metals 2016, 6, 175. https://doi.org/10.3390/met6080175
Zhang X, Li C, Gao Z, Liu Y, Ma Z, Yu L, Li H. Correlation between Zn-Rich Phase and Corrosion/Oxidation Behavior of Sn–8Zn–3Bi Alloy. Metals. 2016; 6(8):175. https://doi.org/10.3390/met6080175
Chicago/Turabian StyleZhang, Xin, Chong Li, Zhiming Gao, Yongchang Liu, Zongqing Ma, Liming Yu, and Huijun Li. 2016. "Correlation between Zn-Rich Phase and Corrosion/Oxidation Behavior of Sn–8Zn–3Bi Alloy" Metals 6, no. 8: 175. https://doi.org/10.3390/met6080175