Roles of Mineralogical Phases in Aqueous Carbonation of Steelmaking Slag
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Characterization
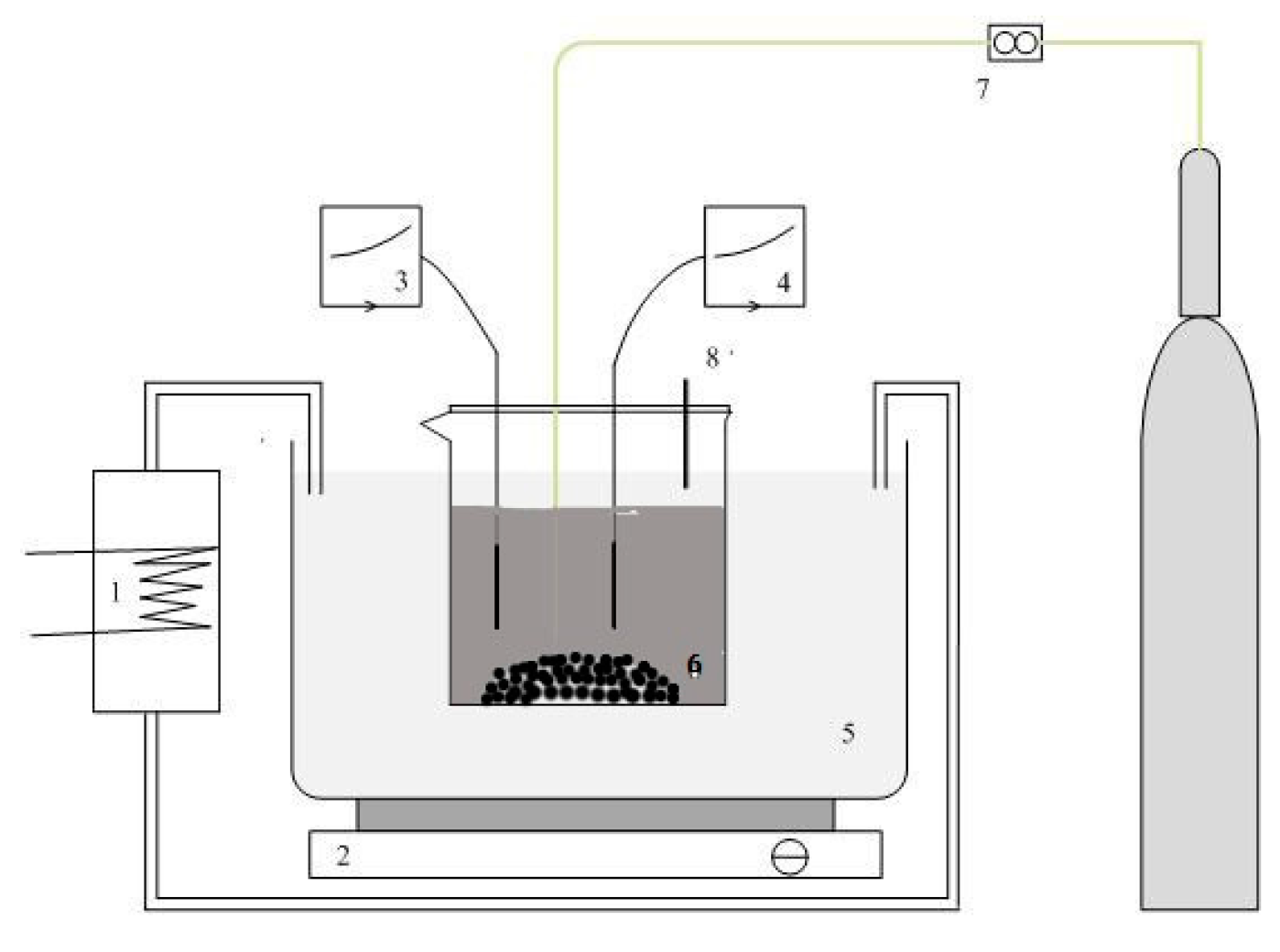
2.2. Experimental Setup
2.3. Evaluation of Carbonation Degree
3. Results and Discussion
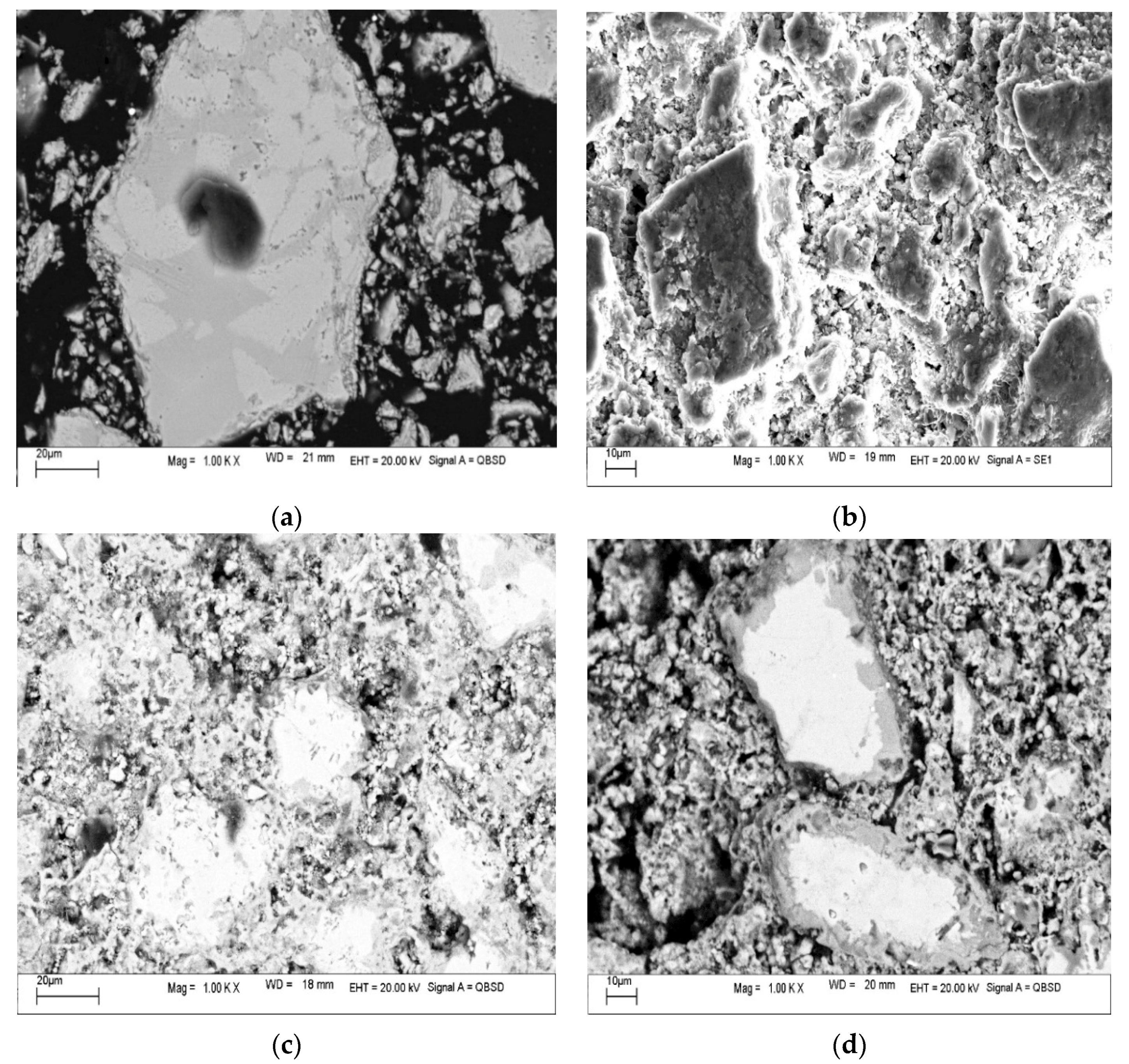
3.1. Characteristics of Slag Sample
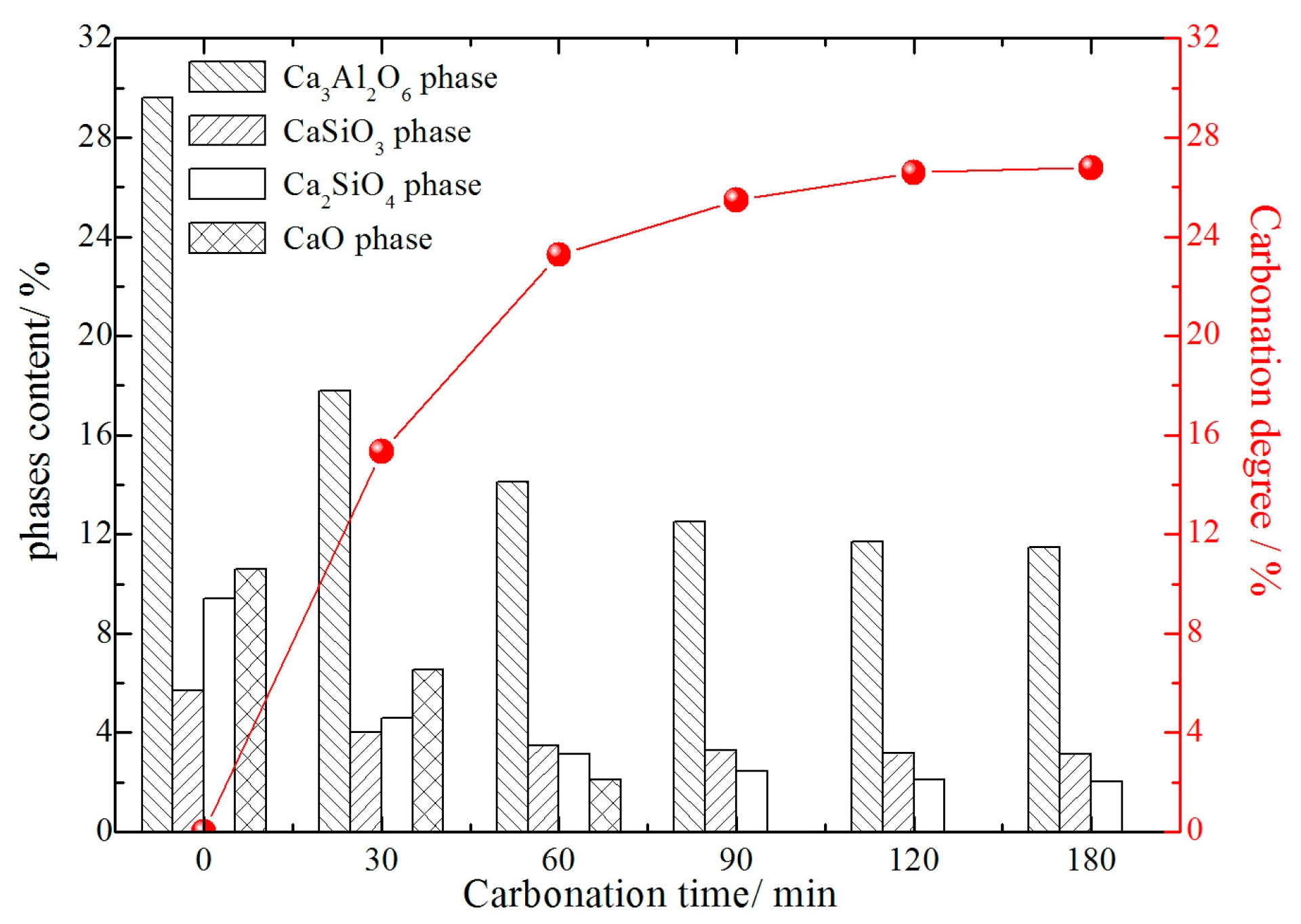
3.2. Effect of Carbonation Time on Mineralogical Phases Containing Calcium
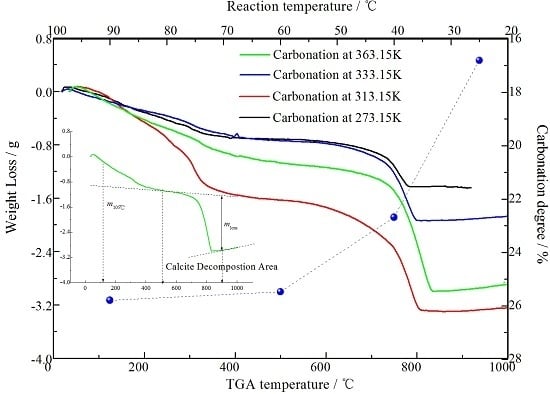
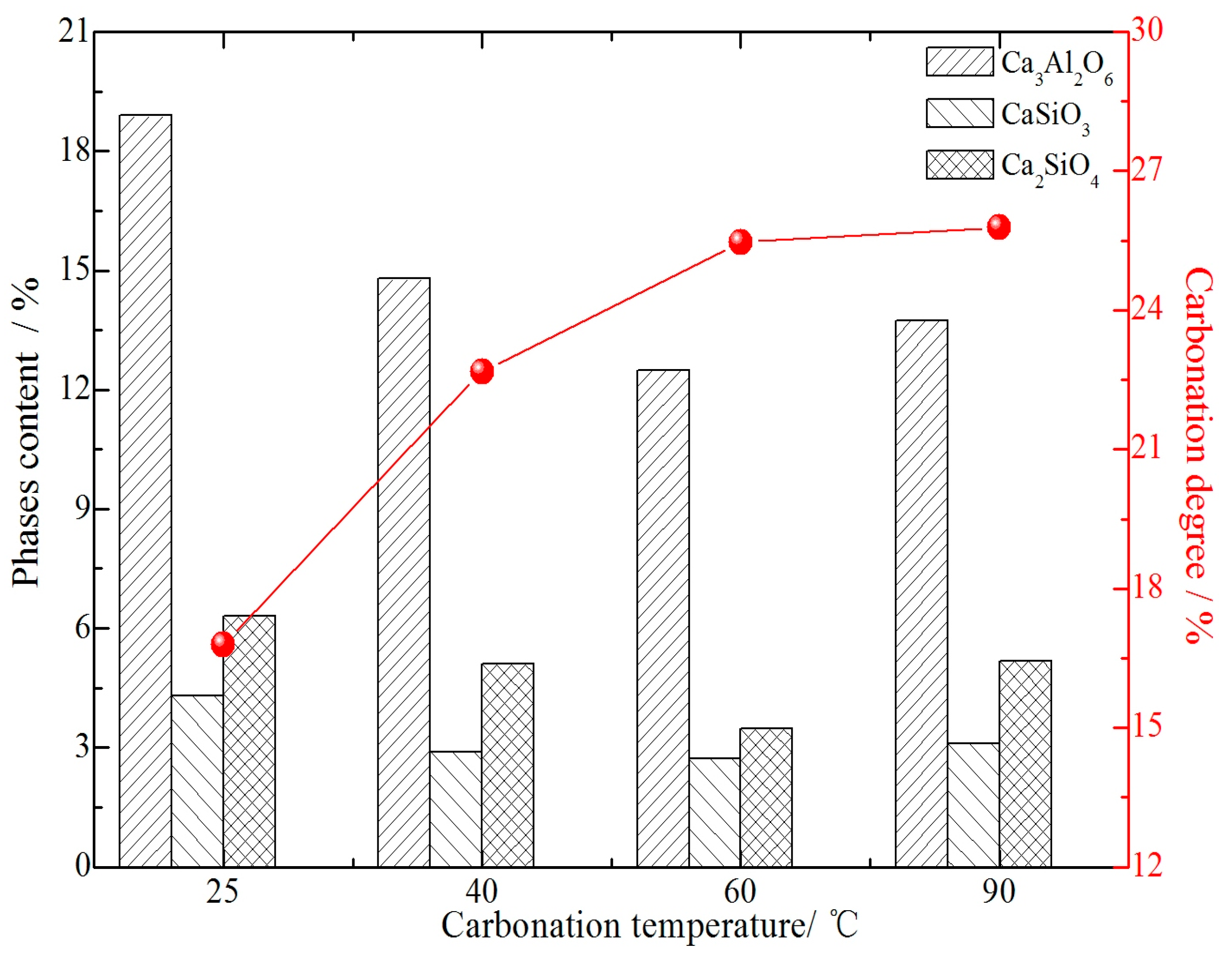
3.3. Effect of Carbonation Temperature on Mineralogical Phases Containing Calcium
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon Dioxide Disposal in Carbonate Minerals; Los Alamos National Laboratory: Los Alamos, NM, USA, 1995. [Google Scholar]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation: Literature Review; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2003. [Google Scholar]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of steel slag for CO2 sequestration: Leaching of products and reaction mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Kodamaa, S.; Nishimotob, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Huijgen, W.; Witkamp, G.; Comans, R. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.; Ruijg, G.; Comans, R.; Witkamp, G. Energy Consumption and Net CO2 Sequestration of Aqueous Mineral Carbonation. Ind. Eng. Chem. Res. 2006, 45, 9184–9194. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chiang, P.C.; Chen, Y.H.; Tan, C.S.; Chang, E.E. Ex situ CO2 capture by carbonation of steelmaking slag coupled with metalworking wastewater in a rotating packed bed. Environ. Sci. Technol. 2013, 47, 3308–3315. [Google Scholar] [PubMed]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Chu, H.W.; Wang, C.F.; Chiang, P.C. CO2 Sequestration by Carbonation of Steelmaking Slags in an Autoclave Reactor. J. Hazard. Mater. 2011, 195, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chiu, A.C.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Carbonation of Basic Oxygen Furnace Slag with Metalworking Wastewater in a Slurry Reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Van Zomeren, A.; van der Laan, S.R.; Kobesen, H.B.A.; Huijgen, W.J.J.; Comans, R.N.J. Changes in Mineralogical and Leaching Properties of Converter Steel Slag Resulting from Accelerated Carbonation at Low CO2 Pressure. Waste Manag. 2011, 31, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.; Zevenhove, R. Steel Converter Slag as a Raw Material for Precipitation of Pure Calcium Carbonate. Ind. Eng. Chem. Res. 2008, 47, 7104–7111. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Robertson, D.G.C.; Rawlins, C.H.; Richards, V.L.; Peaslee, K.D. Investigation of a Two-Stage Aqueous Reactor Design for Carbon Dioxide Sequestration Using Steelmaking Slag. Metall. Mater. Trans. B 2008, 39B, 484–492. [Google Scholar] [CrossRef]
- Yokoyama, S.; Sato, R.; Muhd, N.; Nik, H.B.M.N.; Umemoto, M. Behavior of CO2 Absorption in Wet-grinding of Electronic Arc Furnace Reducing Slag by Vibration Ball Mill. ISIJ Int. 2010, 50, 482–489. [Google Scholar] [CrossRef]
- Tan, C.; Chen, J. Absorption of carbon dioxide with piperazine and its mixtures in a rotating packed bed. Sep. Purif. Technol. 2006, 49, 174–180. [Google Scholar] [CrossRef]
- Taylor, J.C. Computer programs for standardless quantitative analysis of minerals using the full powdered diffraction profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef]
- Colin, R.W.; Taylor, C.J.; Cohen, D.R. Quantitative mineralogy of sandstones by X-ray diffractometry and normative analysis. J. Sedim. Res. 1999, 69, 1050–1062. [Google Scholar]
- Edwards, B.F.; Cai, M.; Han, H.T. Rate equation and scaling for fragmentation with mass loss. Phys. Rev. A 1990, 41, 40–43. [Google Scholar] [CrossRef]


| TFe | MFe | FeO | CaO | MgO | SiO2 | Al2O3 | MnO |
|---|---|---|---|---|---|---|---|
| 0.55 | <0.50 | <0.10 | 55.3 | 5.64 | 8.00 | 30.0 | 0.41 |
| Test No. | Temp (°C) | Time (min) | Test No. | Temp (°C) | Time (min) |
|---|---|---|---|---|---|
| 1 | 25 | 30 | 11 | 60 | 60 |
| 2 | 25 | 60 | 12 | 60 | 90 |
| 3 | 25 | 90 | 13 | 60 | 120 |
| 4 | 25 | 120 | 14 | 60 | 180 |
| 5 | 25 | 180 | 15 | 90 | 30 |
| 6 | 40 | 30 | 16 | 90 | 60 |
| 7 | 40 | 90 | 17 | 90 | 90 |
| 8 | 40 | 120 | 18 | 90 | 120 |
| 9 | 40 | 180 | 19 | 90 | 180 |
| 10 | 60 | 30 | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lu, Y.; Dong, J.; Gan, L.; Tong, Z. Roles of Mineralogical Phases in Aqueous Carbonation of Steelmaking Slag. Metals 2016, 6, 117. https://doi.org/10.3390/met6050117
Zhang H, Lu Y, Dong J, Gan L, Tong Z. Roles of Mineralogical Phases in Aqueous Carbonation of Steelmaking Slag. Metals. 2016; 6(5):117. https://doi.org/10.3390/met6050117
Chicago/Turabian StyleZhang, Huining, Yongming Lu, Jianhong Dong, Lei Gan, and Zhifang Tong. 2016. "Roles of Mineralogical Phases in Aqueous Carbonation of Steelmaking Slag" Metals 6, no. 5: 117. https://doi.org/10.3390/met6050117
APA StyleZhang, H., Lu, Y., Dong, J., Gan, L., & Tong, Z. (2016). Roles of Mineralogical Phases in Aqueous Carbonation of Steelmaking Slag. Metals, 6(5), 117. https://doi.org/10.3390/met6050117