Preparation and Characterization of Directionally Freeze-cast Copper Foams
Abstract
:1. Introduction
2. Results and Discussion
2.1. Foam Microstructure
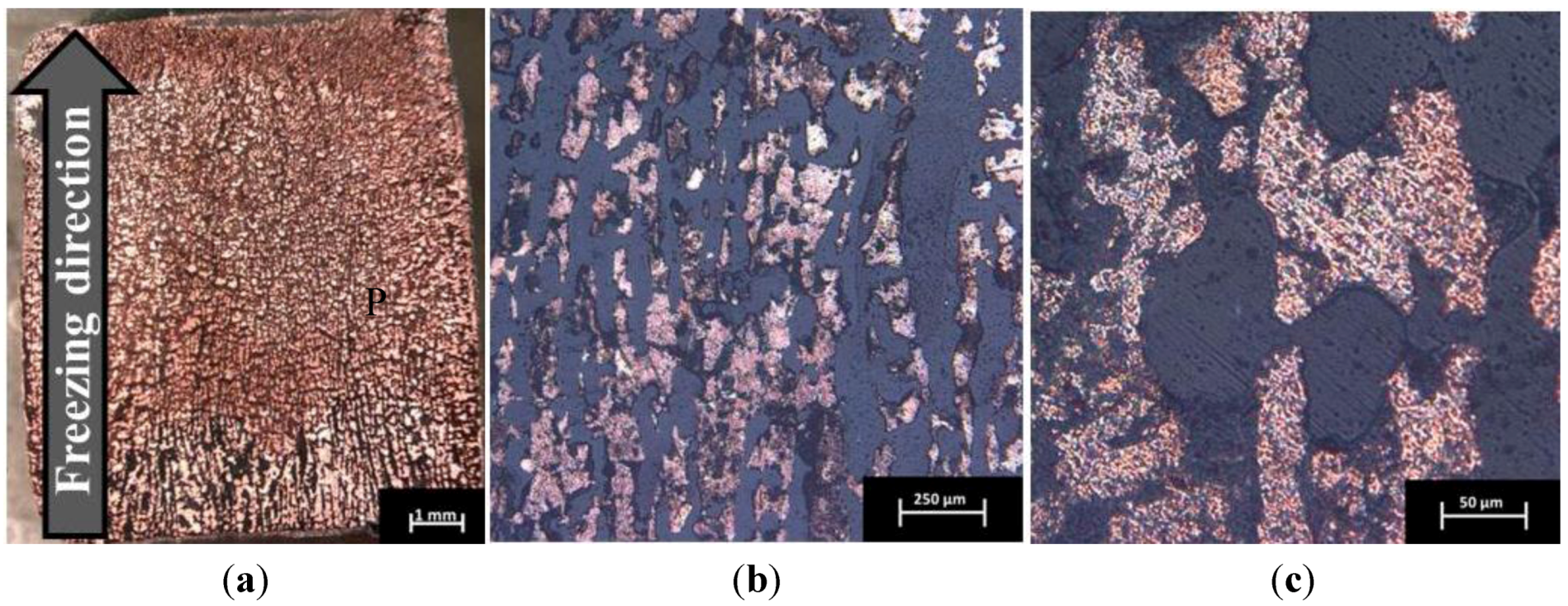
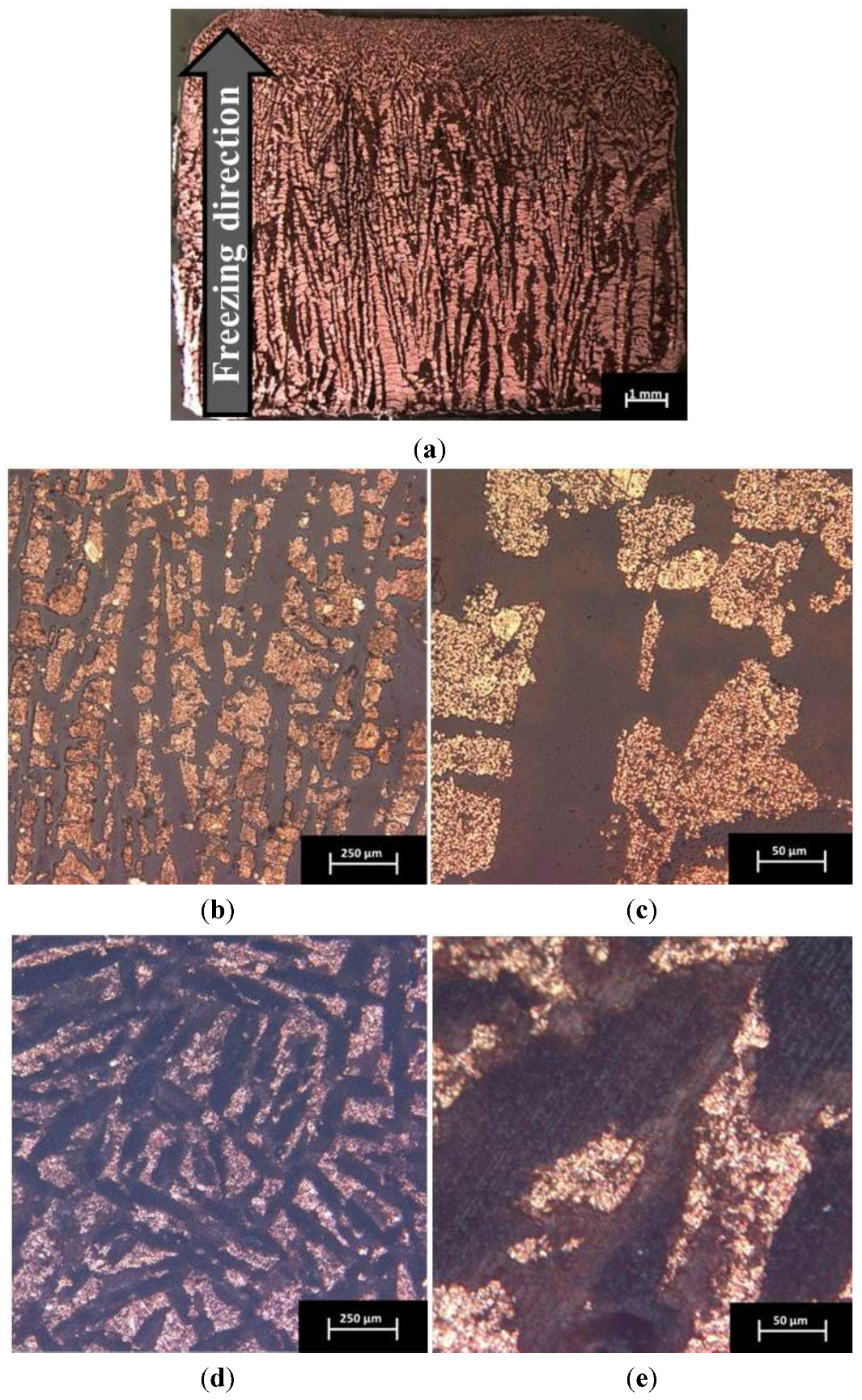
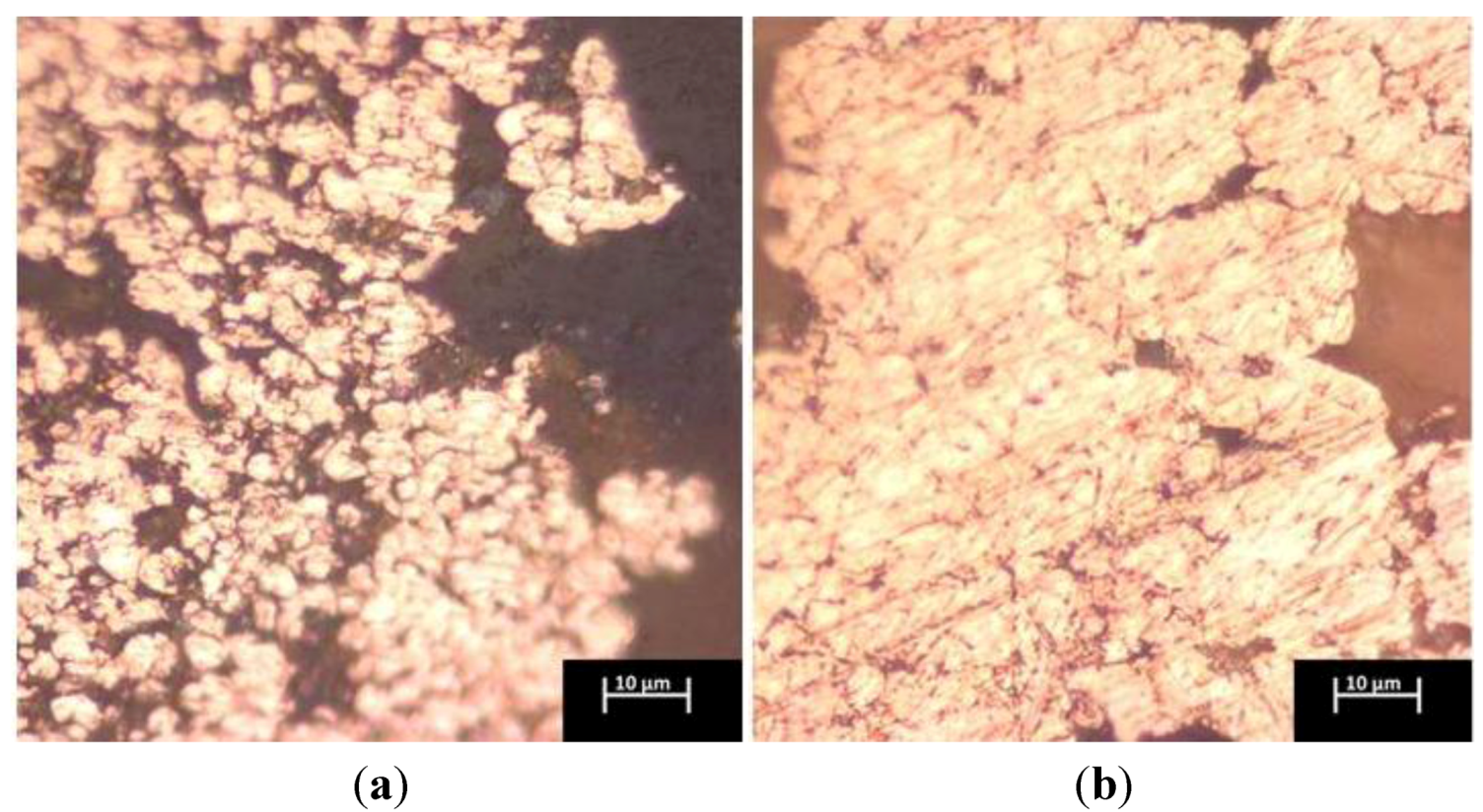
2.2. Influence of Sintering Parameters
3. Experimental Procedures
3.1. Slurry Preparation
3.2. Freeze Casting and Sintering

3.3. Microstructural Analysis
4. Conclusions
Acknowledgments
References
- Lefebvre, L.-P.; Banhart, J.; Dunand, D. Porous metals and metallic foams: Current status and recent developments. Adv. Eng. Mater. 2008, 10(9), 775–787. [Google Scholar] [CrossRef] [Green Version]
- Conde, Y.; Despois, J.-F.; Goodall, R.; Marmottant, A.; Salvo, L; San Marchi, C; Mortensen, A. Replication processing of highly porous materials. Adv. Eng. Mater. 2006, 8(9), 795–803. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Cui, C.X.; Liu, S.J.; Zhao, L.C. Open-celled porous Cu prepared by replication of NaCl space-holders. Mater. Sci. Eng. A. 2010, 527(4–5), 1275–1278. [Google Scholar]
- Hakamada, M.; Asao, Y.; Kuromura, T.; Chen, Y.; Kusuda, H.; Mabuchi, M. Density dependence of the compressive properties of porous copper over a wide density range. Acta Mater. 2007, 55(7), 2291–2299. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, B. On the compressive behavior of sintered porous coppers with low to medium porosities—Part I: Experimental study. Int. J. Mech. Sci. 2005, 47(4–5), 744–756. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fung, T.; Zhang, L.P.; Zhang, F.L. Lost carbonate sintering process for manufacturing metal foams. Scripta Mater. 2005, 52(4), 295–298. [Google Scholar] [CrossRef]
- Zhang, S.; Xing, Y.; Jiang, T.; Du, Z.; Li, F.; He, L.; Liu, W. A three-dimensional tin-coated nanoporous copper for lithium-ion battery anodes. J. Power Sources 2011, 196(16), 6915–6919. [Google Scholar]
- ERG Aerospace Corporation Homepage. Available online: http://www.ergaerospace.com (accessed on 1 July 2012).
- Nakajima, H.; Hyun, S.K.; Ohashi, K.; Ota, K.; Murakami, K. Fabrication of porous copper by unidirectional solidification under hydrogen and its properties. Colloids Surf. A 2001, 179(2–3), 209–214. [Google Scholar]
- Hyun, S.K.; Murakami, K.; Nakajima, H. Anisotropic mechanical properties of porous copper fabricated by unidirectional solidification. Mater. Sci. Eng. A 2001, 299(1–2), 241–248. [Google Scholar] [CrossRef]
- Zhang, H.; Cooper, A.I. Aligned porous structures by directional freezing. Adv. Mater. 2007, 19(11), 1529–1533. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311(5760), 515–518. [Google Scholar]
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-templated porous alumina structures. Acta Mater. 2007, 55(6), 1965–1974. [Google Scholar] [CrossRef]
- Chino, Y.; Dunand, D.C. Directionally freeze-cast titanium foam with aligned, elongated pores. Acta Mater. 2008, 56(1), 105–113. [Google Scholar] [CrossRef]
- Li, J.C.; Dunand, D.C. Mechanical properties of directionally freeze-cast titanium foams. Acta Mater. 2011, 59(1), 146–158. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ramos, A.I.C.; Dunand, D.C. Preparation and Characterization of Directionally Freeze-cast Copper Foams. Metals 2012, 2, 265-273. https://doi.org/10.3390/met2030265
Ramos AIC, Dunand DC. Preparation and Characterization of Directionally Freeze-cast Copper Foams. Metals. 2012; 2(3):265-273. https://doi.org/10.3390/met2030265
Chicago/Turabian StyleRamos, Aurelia I. Cuba, and David C. Dunand. 2012. "Preparation and Characterization of Directionally Freeze-cast Copper Foams" Metals 2, no. 3: 265-273. https://doi.org/10.3390/met2030265



